Effect of Liraglutide Combined with Nephritis Rehabilitation Tablets for Type 2 Diabetic Nephropathy
Xiao-Hui Cao, Li-Yan Jia, Yan-Yun Hu
Baoding first central hospital, 071000, Baoding, Hebei
Keywords:Type 2 Diabetic Nephropathy Liraglutide Nephritis Rehabilitation Tablets Glucose Metabolism Index Renal Function Inflammatory Factor
ABSTRACT Objective: To explore the effect of liraglutide combined with Nephritis Rehabilitation Tablets in the treatment of type 2 diabetic nephropathy. Methods: Ninety patients with type 2 diabetic nephropathy who had received treatment in the Hospital from January 2018 to March 2019 were enrolled, and then randomly divided into a Liraglutide group, a Nephritis Rehabilitation Tablets group and a combined treatment group, with 30 cases in each group. All patients were given routine treatment. Besides, the Liraglutide group was treated with subcutaneous injection of liraglutide, the Nephritis Rehabilitation Tablets group was treated with oral Nephritis Rehabilitation Tablets, and the combined treatment group was given subcutaneous injection of liraglutide and oral administration of Nephritis Rehabilitation Tablets. The three groups were treated continuously for 12 weeks to compare the changes of glucose metabolism index, renal function and inflammatory factors. Results: After treatment, the levels of FPG, 2hPG and HbALc were decreased in the three groups, and the levels in the combined treatment group were lower than the liraglutide group and the Nephritis Rehabilitation Tablets group, and the liraglutide group lower than the Nephritis Rehabilitation Tablets group. The difference was statistically significant (P<0.05). After treatment, the levels of serum creatinine and urea nitrogen decreased in the three groups, and the levels in the combined treatment group were lower than the liraglutide group and the Nephritis Rehabilitation Tablets group, and the Nephritis Rehabilitation Tablets group lower than the liraglutide group. The difference was statistically significant (P<0.05) After treatment, the levels of IL-6, TNF-α, and hs-CRP decreased in the three groups, and the levels in the combined treatment group were lower than the liraglutide group and the Nephritis Rehabilitation Tablets group. The difference was statistically significant (P<0.05). Conclusions: For patients with type 2 diabetic nephropathy, Liraglutide combined with Nephritis Rehabilitation Tablets can help reduce the blood sugar, relieve renal inflammation and improve their renal function.
1. Introduction
Type 2 diabetic nephropathy, as one of the progressive renal diseases, is caused by capillaries of the kidney glomerulus which is a result of continuous hyperglycemia in the body. After developing to the terminal stage, it will induce most serious lifethreatening complication, renal failure [1]. At present, type 2 diabetic nephropathy cannot be cured clinically, but clinical studies have confirmed that by controlling the blood sugar in a proper level, the kidney damage and incidence of complications can be reduced [2]. Liraglutide, a human glucagon-like peptide-1 analogue, can stablize blood sugar level. Shenyan Kangfu Tablets is a Chinese patent medicine that can nourish the kidney and strengthen the spleen, improve renal function, and relieve clinical symptoms. The combined use of the two mediciens is with satifactory effect since it can reduce kidney damage and boost renal function recovery [3]. In this study, Liraglutide and Shenyan Kangfu Tablets were combined in the treatment of type 2 diabetic nephropathy, and the results are satisfactory. It is reported as follows.
2. Materials and Methods
2.1 Patients Data
90 patients with type 2 diabetic nephropathy who had received treatment in the Hospital from January 2018 to March 2019 were enrolled. Inclusion criteria: those meet the diagnostic criteria for primary hypertension defined in the Expert Consensus for the Prevention and Treatment of Diabetic Nephropathy (2014 Edition) [4]; those taking any similar drugs in the past 2 weeks; those who signed an informed consent letter voluntarily; this study was conducted with the approval of the Medical Ethics Committee. (2) Exclusion criteria: those who were allergic to one of the medicines used in the study and those who participated in other clinical trials in the past month; those who had serious cardiovascular, cerebrovascular, liver and kidney, or hematopoietic system primary diseases; those with mental system disease. According to the random number table, the patients were divided into a Liraglutide Group, a Shenyangkangfu Tablet Group, and a Combined Medication Group. There was no statistically significant difference among the two groups in general information (P>0.05), with comparability among the two groups. See Table 1.
2.2 Methods
Group: Oral administration of Shenyangkangfu Tablet (produced by Tianjin Tongrentang Group Co., Ltd., SFDA Approval Number: Z10940034, specification: 0.48g/tablet) 5 tablets/times, 3 times/d. The Combined Medication Group was given Liraglutide combined with Shenyangkangfu Tablet, and the usage and dosage were the same as above. Patients in the three groups were treated continuously for 12 weeks.
2.3 Observation Indexes
Before treatment, and after 12 weeks of treatment, 3 ml of elbow venous blood was drawn under fasting state and 2 hours after the meal, and centrifuged at 3000 r/min for 5 min in a centrifuge, with the upper serum layer taken and store in -70 ℃ environment within a refrigerator for future use. (1) Glucose metabolism indicators: fasting plasma glucose (FPG) and 2-hours plasma glucose (2hPG) were measured by Roche blood glucose meter, and the level of glycated hemoglobin (HbALc) was measured by glycated hemoglobin analyzer. (2) Renal function: the serum creatinine level was measured by standard picric acid colorimetric method; 10 ml of fresh morning urine was collected from the patients, and the urea nitrogen level was detected by the isotope method. (3) Inflammatory factors: Enzyme Linked Immunosorbent Assay was adopted to determine the levels of interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α) and high-sensitive C-reactive protein (hs-CRP).
2.4 Statistical Methods
All patients received routine treatments such as diet control, exercise therapy, and subcutaneous insulin injections. The Liraglutide Group: subcutaneously injection of Liraglutide (produced by Novo Nordisk (China) Pharmaceutical Co., Ltd., SFDA Approval Number: J20160037, specifications: 3ml; 18mg), with an initial dose of 0.6mg/d, increased to 1.2 mg/d after 7 days. Shenyangkangfu Tablet
The statistical software SPSS 21.0 version was used in the study to process data. Measurement data were described as x±s, and independent sample t test was used between different groups and paired sample t test was used for comparison within groups. The counting data was expressed as a percentage (%) and χ2test was applied. P<0.05 suggests the difference is statistically significant.

Table 1 Comparison of general information(x±s)(n)

Table 2 Comparison of glucose metabolism indicators among the three groups(x±s)
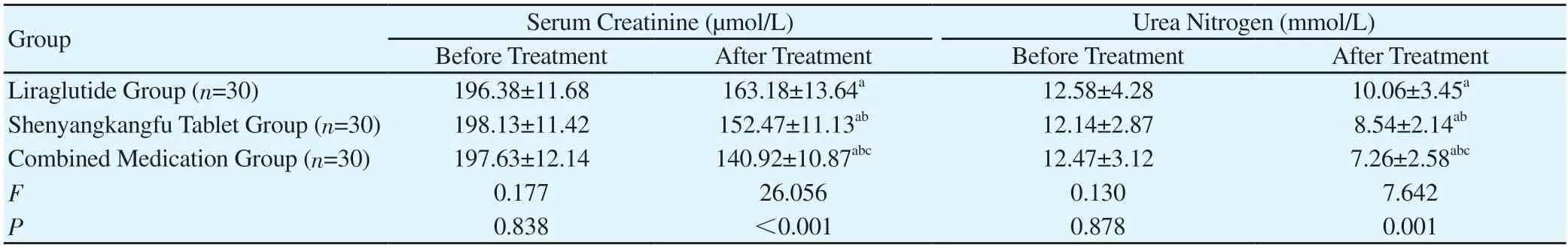
Table 3 Comparison of renal function levels among the three groups(x±s)

Table 4 Comparison of inflammatory factor levels among the three groups (x±s)
3. Results
3.1 Glucose metabolism indexes
Before treatment, there was no significant difference among the three groups in terms of FPG, 2hPG, and HbALc (P>0.05). After treatment, however, the levels of FPG, 2hPG, and HbALc were decreased in all of the three groups, and the levels in the Combined Medication Group were shown to be lower than those in the Liraglutide Group and the Shenyangkangfu Tablet Group, and the levels in the Liraglutide Group lower than those in the Shenyangkangfu Tablet Group; the difference was statistically significant (P<0.05). See Table 2.
3.2 Renal Function
Before treatment, there was no significant difference in serum creatinine and urea nitrogen among the three groups (P>0.05). After treatment, however, the levels of serum creatinine and urea nitrogen decreased in all of the three groups, and the levels in the Combined Medication Group were shown to be lower than those in the Liraglutide Group and the Shenyangkangfu Tablet Group, and the levels in the Shenyangkangfu Tablet Group lower than those in the Liraglutide Group; the difference was statistically significant (P<0.05). See Table 3.
3.3 Inflammatory Factors
Before treatment, there was no significant difference in IL-6, TNF-α, and hs-CRP among the three groups (P>0.05). After treatment, however, the levels of IL-6, TNF-α, and hs-CRP decreased in all of the three groups, and the levels in the Combined Medication Group were shown to be lower than those in the Liraglutide Group and the Shenyangkangfu Tablet Group; the difference was statistically significant (P<0.05). See Table 4.
4. Discussion
Modern medicine believes that the major pathogenesis of diabetic nephropathy is chronic hyperglycemia and advanced glycation end products in patients with type 2 diabetes, which change the renal microvascular circulation and lead to the thickening of the glomerular basement membrane and the increase of the mesangial matrix, eventually leading to renal function damage or even failure. Therefore, the key to clinical treatment of diabetic nephropathy is to regulate blood glucose metabolism and improve renal function [5-6]. There are many clinical hypoglycemic drugs, but few western medicines can help improve renal function or with ideal effects. Traditional Chinese medicine classifies diabetic nephropathy into the category of "thirst-quenching", regarding it a disease caused by qi and yin deficiency, with blood stasis and obstruction as the symptoms, and its main pathogenesis is the stagnant heat caused by deficiency of body fluid, sputum obstruction caused by waterliquid metabolism disorders, and obstruction of renal collaterals. Kidney function can be regulated by drugs that can help nourish qi and yin, or those promoting blood circulation and removing blood stasis. To conclude, study on integrated traditional Chinese and western medicine in the treatment of diabetic nephropathy is of great significance [7-8].
The results of this study show that, the levels of FPG, 2hPG, and HbALc in the Combined Medication Group are lower than those in the Liraglutide Group and the Shenyan Kangfu Tablets Group, and the levels in the Liraglutide Group is lower than those in the Shenyan Kangfu Tablets Group, indicating that Liraglutide combined with Shenyan Kangfu Tablets can help reduce blood glucose level. Through analysis, we assume the reasons are as follows: ① Liraglutide, as a glucagon nutrient peptide-1 analogue, can help increase insulin secretion while inhibiting the body’s secretion of glucagon, reducing the body’s digestion speed of food, thus ensuring that insulin β cells can fully paly its function;② Shenyan Kangfu Tablets is a Chinese patent medicine, whose main ingredients include American Ginseng, Ginseng, Rehmannia, Eucommia, Yam, Hedyotis diffusa, Black Bean, rhizoma smilacis glabrae, leonurus, Salvia, Alisma, lalang grass rhizome, and Platycodon grandiflorum. The catalol contained in Rehmannia has hypoglycemic effect; Eucommia is rich with catalpol, which can effectively inhibit α-glucosidase activity, thereby reducing blood glucose; Salvia miltiorrhiza can regulate body metabolism and balance blood sugar in both directions [9-11]. Additionally, the levels of serum creatinine and urea nitrogen in the Combined Medication Group are lower than those in the Liraglutide Group and the Shenyang Kangfu Tablets Group, and the levels in the Shenyang Kangfu Tablets Group lower than the Liraglutide Group, indicating that Liraglutide combined with Shenyang Kangfu Tablets can help boost the patients’ renal function recovery. Through analysis, we believe the reasons are as follows: ① The American ginseng and ginseng contained in the Shenyan Kangfu Tablets can nourish qi and yin, clear heat and promote hydration; rehmannia can clear heat and cool blood, nourish yin and boost hydration; eucommia ulmoides can nourish kidney qi; yam can nourish kidney for relieving desertion, and nourish qi and yin; Oldenlandia diffusa can clear heat and promote blood circulation; black beans can invigorate kidney and yin; rhizoma smilacis glabrae can clear dampness and heat; motherwort and salvia can promote blood circulation and dissipate blood stasis; rhizoma alismatis can relieve heat; lalang grass rhizome can clear heat and cooling blood; platycodon can expel phlegm. With all these components combined, this medicine can help clear heat and expectorant, promote blood circulation and remove blood stasis with the target at pathogenesis of nephropathy in patients with diabetes. Modern pharmacology believes that Eucommia ulmoides, motherwort, and salvia miltiorrhiza can promote adrenal cortex function, improve the blood flow of small blood vessels in the kidney, thus reducing kidney damage. ② Liraglutide can rapidly reduce the level of blood glucose in patients, reduce further damage to the kidneys caused by hyperglycemia, thus improving the renal function [12-13]. The study showed that, the levels of IL-6, TNF-α, and hs-CRP in the combined medication group were lower than those in the Liraglutide Group and the Shenyang Kangfu Tablets Group, indicating that the Liraglutide and Shenyang Kangfu Tablets can help reduce inflammation response in the body. Through analysis of the underlying reasons, we attribute it to:① Liraglutide can help reduce the oxidative stress response in the body caused by hyperglycemia, alleviate inflammatory response of the kidney, and thereby reduce the level of inflammatory factors in the serum;② Shenyang Kangfu Tablets contains the Oldenlandia diffusa., lalang grass rhizome, Platycodon, which can regulate the patients’ immune function and fight inflammations; the ingredient salvia is rich in tanshinone, therefore, it can inhibit lipid oxidation and improve oxidative stress response [14-15].
In summary, Liraglutide combined with Shenyan Kangfu Tablets can help reduce blood sugar in patients with diabetic nephropathy, alleviate inflammation in their kidney, and improve their renal function.
 Journal of Hainan Medical College2020年1期
Journal of Hainan Medical College2020年1期
- Journal of Hainan Medical College的其它文章
- Screening and bioinformatics analysis of diabetic peripheral neuropathyrelated genes in women
- Study on TCM Syndrome Types and Syndromes of Knee Osteoarthritis Based on Modern Literature
- Study on the medication rule of Wang Xun in the treatment of dysentery based on Data Mining
- A Systematic Review and Meta-analysis for Duhuo Jisheng Decoction Combined with Manipulation in Treatment of Lumbar Disc Herniation
- Correlation analysis of osteonecrosis of the femoral head with blood lipid and coagulation indexes
- Patterns of Eye-Infiltrating Cells between Chronic and Acute Experimental Autoimmune Uveitis
