Effects of Different Carbon Sources on the Water of Aristichthys nobilis Richardson Aquaculture by Biofloc
JIANG Yan-xia ,YANG Jun ,GUAN Gui-ping ,LIU Bo-cheng ,WANG Hui ,WANG Hong-bing
1.Hunan Animal and Veterinary Science,Changsha 410131,PRC;2.College of Bioscience and Biotechnology,Hunan Agricultural University,Changsha 410128,PRC
Abstract In order to explore the effects of different carbon sources on the water of Aristichthys nobilis Richardson culture by biofloc as well as regular,glucose,sucrose,dextrin,brown sugar and molasses 5 soluble carbon source were added into the water of Aristichthys nobilis Richardson culture containing a certain amount of ammonia nitrogen,nitrite nitrogen to research their effects on pH value,dissolved oxygen,ammonia-nitrogen,nitrite nitrogen value.The results showed that sucrose had the best effect in converting ammonia nitrogen and nitrite nitrogen;glucose and dextrin had significant effect in converting ammonia nitrogen.However,compared with sucrose,glucose and dextrin had bad effect in converting nitrite nitrogen molasses had bad effect in converting ammonia nitrogen and nitrite nitrogen,and had little effect on pH value and dissolved oxygen;glucose,sucrose and dextrin had significant effect in decreasing pH value and dissolved oxygen;brown sugar had good effect in converting nitrite nitrogen,whereas it had little effect in converting ammonia nitrogen.Therefore,sucrose was the best carbon source,and if the cost factor was taken into account,glucose also had a high cost performance.
Key words Biofloc;Carbon source;pH value;Dissolved oxygen;Ammonianitrogen;Nitrite nitrogen
1.Introduction
In intensive high-density aquaculture water,massive feed residues and excrement of the aquaculture objects are discharged,resulting in excessive ammonia nitrogen,nitrite and other harmful substances in the water.If not solved in time,it will lead to a large outbreak of disease and even death of farmed animals[1].Traditional farms often change water frequently to solve the problem of water quality deterioration,and aquaculture wastewater is discharged untreated,which can cause environmental pollution.In China,72.72% of the river reach has water pollution,thereby it is extremely urgent to solve the water pollution of aquaculture and realize the recycling of aquaculture water[2].
The biofloc technology (BFT) is that using heterotrophic bacteria to transform the abundant nitrogen and phosphorus in the aquaculture water into bacterial proteins by adding carbon source to the aquaculture water.Biofloc can not only solve the retention problem of plankton and various organic detritus,but also can be used for feeding fish and shrimp,so as to improve the utilization rate of feed protein.This technology can achieve the purpose of changing less or even no water,greatly reducing the production of aquaculture wastewater,but also can degrade the excess bait and excrement in the water,and convert to phytoplankton for use.Compared with traditional aquaculture model,BFT can not only improve the water quality,but also can reduce the input of feed and cost.Meanwhile,it can change the aquatic bacteria system from autotrophic bacteria to heterotrophic bacteria,which can inhibit the growth of pathogenic microorganisms and reduce the occurrence of fish and shrimp diseases[3].Some studies showed that when C/N was 10~15,it had a good effect on the ammonia nitrogen and nitrite nitrogen in aquaculture water[4-5],which can promote the synthesis of bacterial protein by heterotrophic bacteria and effectively remove ammonia nitrogen and nitrite nitrogen in aquaculture water.The selection of C/N and carbon source types in current studies mostly stays at the empirical level.There are few systematic studies on carbon source supply,and harmless transformation of pollutants in the system under different carbon source types and gradient conditions.The application of BFT inOreochromis sppandPenaeus vannameihas been relatively mature[6].Aristichthys nobilis,one of the main freshwater aquaculture fish,is well received by consumers in China's economic aquatic products,with an annual consumption of more than 2 million t[7].Aristichthys nobilisis a kind of economic filteringfeeding fish.LI Chao-bing used bioflocs as feed forAristichthys nobilis,and found that the growth rate and content of flavor substances in the bioflocs group were better than those in the compound feed group[8].Therefore,it is of great value to apply BFT toAristichthysnobilisaquaculture.
In this study,glucose,sucrose,dextrin,brown sugar and molasses 5 soluble carbon source were added into the water ofAristichthys nobilis Richardsonaquaculture to determine their effects on pH value,dissolved oxygen,ammonia-nitrogen,nitrite nitrogen,so as to explore the effects of different carbon sources on the water ofAristichthys nobilis Richardsonculture by biofloc as well as regular.
2.Materials and Methods
2.1.Culture of microorganisms
Bacillus subtilisandBacillus licheniformis,isolated and preserved by research group of this project,were taken as experimental strains.All the media were LB medium:tryptone 10 g/L,yeast extract 5 g/L,NaCl 10 g/L,preparation with distilled water,pH value 7.4,sterilization at 121℃ for 20 min,then subpackage into 500 mL conical flask,200 mL medium per bottle.After inoculation,it was placed in a constant temperature culture oscillator at 37 ℃ and shaken at 200 r/min for 18 h.
2.2.Culture of biofloc technology
300kg tap water were added into 6 fishbowls with the same size,respectively.The water temperature was kept at 28℃ with the same ventilatory capacity.In each fishbowl,50 mL bacteria solution and 25 bigheads with a average weight of 2 g were added.1 g fish feed with 30% protein was fed at forenoon and afternoon everyday.After 4 d,biofloc began to form.
2.3.Determination of indicators
3 d after the formation of biofloc,a certain amount of ammonia nitrogen and nitrite were accumulated in the aquaculture water.5 g glucose,sucrose,dextrin,brown sugar and molasses were added in the 5 fishbowls,respectively.Nothing was added in the control.Then the indicators in the aquaculture water started to determine.The pH value and dissolved oxygen were determined by portable pH test pen and portable dissolved oxygen analyzer every 10 min,respectively.The contents of ammonia nitrogen and nitrite nitrogen were determined by spectrophotometry every 1 h until the value kept stable.
The water samples were centrifuged by a highspeed centrifuge at 12 000 r/min for 2 min to remove large particles of impurities and organic matter such as biofloc.
The contents of ammonia nitrogen were determined referring to HJ535-2009 and the contents of nitrite nitrogen were determined by diazo-coupling spectrophotometry.
2.4.Data processing
Excel was used for statistical analysis,and GraphPad Prism 8.0 software was used for plotting.
3.Results and Analysis
3.1.Effects of carbon source on the pH value in the water
As shown in Fig.1,the pH value in the treatment with carbon sources all decreased.Of which,there were significant differences in the pH value between glucose,sucrose,dextrin,brown sugar treatment group and the control,but there was no significant difference in the pH value between molasses treatment group and the control.After treatment with glucose,sucrose,dextrin and brown sugar for 70 min,pH value decreased rapidly.Glucose is a rapidly available carbon sources.The pH value in the treatment with glucose declined the fastest,decreasing from 7.68 to 7.25.The pH value in the treatment with sucrose and dextrin decreased slowly,this may be related to the fact that glucose can be directly and rapidly metabolized by microorganisms in the biofloc into small molecular organic acids such as carbonic acid,acetic acid and lactic acid.Therefore,the pH value in the treatment with glucose was also the first one that reached the lowest value.While sucrose,brown sugar,dextrin,etc.need to be further degraded into glucose,fructose and other monosaccharides to further participate in metabolism,therefore its influence on pH value was relatively gentle.

Fig.1 Effects of different carbon sources on pH value
3.2.Effects of carbon sources on the dissolved oxygen in the water
As shown in Fig.2,the dissolved oxygen in the treatment with carbon sources all decreased,which was significantly different from that in the control.Of which,the dissolved oxygen value in the treatment with glucose,sucrose,dextrin decreased to below 3.0 mg/L with a reduction of more than 25%.The dissolved oxygen value in the treatment with brown sugar and molasses remained above 3.0 mg/L.After treatment with carbon sources for 40 min,the dissolved oxygen value decreased rapidly,and then gently until they returned to the normal value.In the treatment with glucose,the dissolved oxygen value decreased from 3.9 to 2.8 at the fastest rate within 30 min.This was because glucose can be directly and rapidly metabolized by microorganisms under the participation of oxygen,resulting in a rapid decline in pH value and dissolved oxygen value.
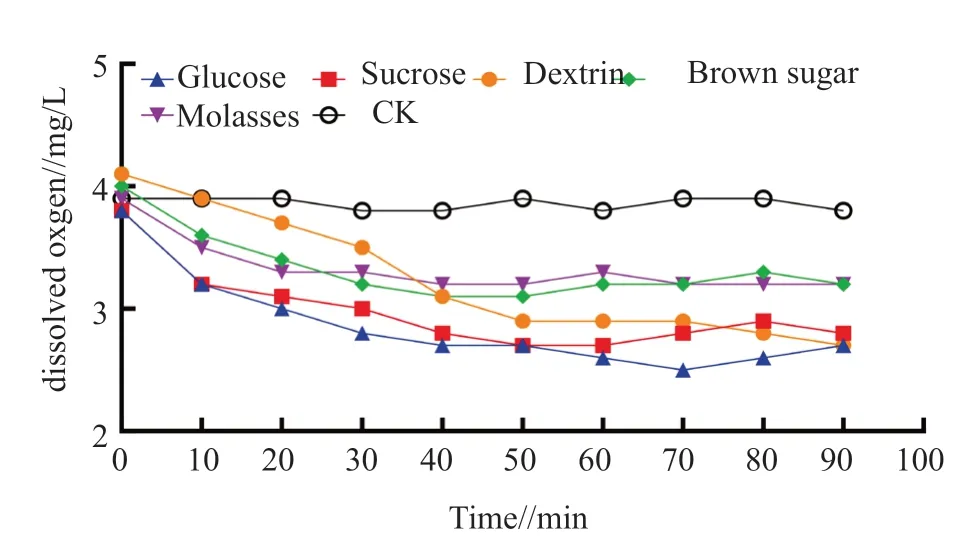
Fig.2 Effects of different carbon sources on dissolved oxgen
3.3.Effects of carbon sources on the ammonia nitrogen content in the water
As shown in Fig.3,the ammonia nitrogen content in the treatment with glucose,sucrose,dextrin,brown sugar all decreased,which was significantly different from that in the control.While the molasses had no effect on the ammonia nitrogen in the water,and the difference was not significant compared to the control.Of which,the effect of sucrose,glucose and dextrin on ammonia nitrogen was obvious,the ammonia nitrogen content decreased from 1.8 mg/L to 0.1 mg/L within 5 h.The effect of sucrose on ammonia nitrogen was the most significant one,followed by glucose with a reduction of 90% in the ammonia nitrogen content within 3 h,and finally brown sugar with a gently decrease in the ammonia nitrogen content.Molasses had no obvious effect on ammonia nitrogen,which was consistent with that on pH value and dissolved oxygen value.This may be due to the complex composition of molasses and its relatively low carbon content.
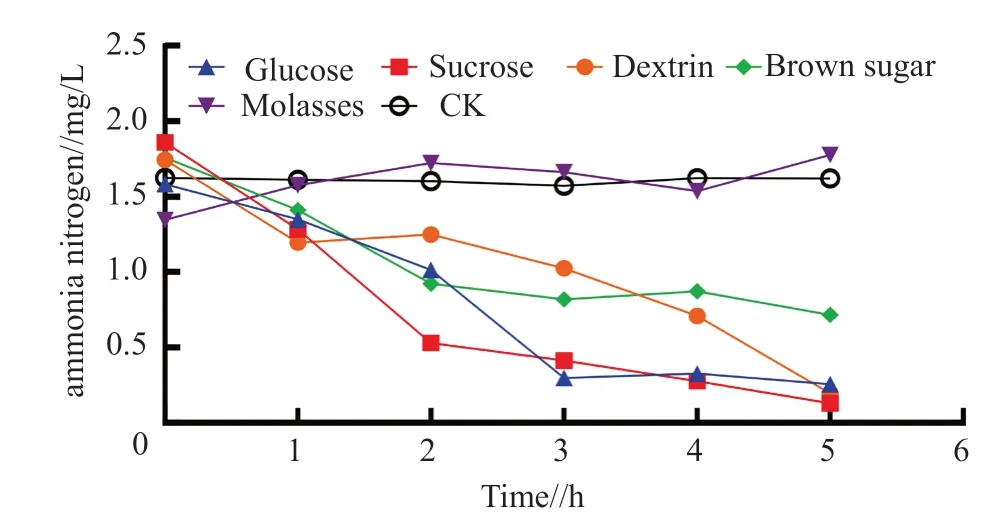
Fig.3 Effects of different carbon sources on ammonia nitrogen content
3.4.Effects of carbon sources on the nitrite nitrogen content in the water
As shown in Fig.4,the nitrite nitrogen content in the treatment with carbon sources all decreased,and there were significant differences between treatments.Of which,the sucrose had obvious effect on the nitrite nitrogen content,which decreased by 85% from 2.7 mg/L to 0.4 mg/L within 5 h,followed by brown sugar with a reduction of more than 50% in the nitrite nitrogen content.This was related to the fact that the brown sugar contained more than 88% sucrose,so it is speculated that the molecular structure or metabolite of sucrose is conducive to the conversion of nitrite nitrogen into mycoprotein.Judging from the establishment of aquaculture water system only one week,the nitrification and denitrification pathways should not be established yet.

Fig.4 Effects of different carbon sources on nitrite content
4.Conclusion and Discussion
With comprehensive analysis,it was found that carbon sources could decrease ammonia nitrogen,nitrite nitrogen in the water,this was because carbon sources could improve the C/N in the water,promote the growth of heterotrophic bacteria,increase assimilation and biofloc microorganism could effectively utilize carbon,phosphorus,ammonia nitrogen and nitrite nitrogen to synthesize their own mycoprotein and reduce the accumulation of ammonia nitrogen and nitrite nitrogen[9].In this study,carbon sources also could decrease dissolved oxygen,this was because heterotrophic bacteria need to consume a lot of oxygen in the process of rapid metabolism,resulting in the decrease of dissolved oxygen in water.Meanwhile,the small molecular organic acids produced by the metabolism of carbon source and the rapid consumption of ammonia nitrogen will also lead to the decline of water pH value.
In this study,sucrose had the best effect in converting ammonia nitrogen and nitrite nitrogen;glucose and dextrin had significant effect in converting ammonia nitrogen;however,compared with sucrose,glucose and dextrin had bad effect in converting nitrite nitrogen;molasses had bad effect in converting ammonia nitrogen and nitrite nitrogen,and it had little effect on pH value and dissolved oxygen;glucose,sucrose and dextrin had significant effect in decreasing pH value and dissolved oxygen.It is worth noting that molasses,which is widely used in aquaculture today,is not the optimal choice.LIU K Met al.found that compared with molasses,brown sugar had good effect in converting ammonia nitrogen and nitrite nitrogen,which was consistent with the results of this study[10].
In conclusion,sucrose was the best carbon source,and if the cost factor was taken into account,glucose also had a high cost performance.
 Agricultural Science & Technology2020年4期
Agricultural Science & Technology2020年4期
- Agricultural Science & Technology的其它文章
- Genetic Analysis of Main Plant Type-related Traits in Bitter Gourd (Momordica charantia L.)
- Changes of Soil nutrients and Tobacco Quality in Linli County and Some Suggestions on Fertilization
- Effects of Altitude on Polyphenols Contents in Cigar Leaves
- Establishment of a Rapid Propagation Protocol of GLRaV-3,GFKV,and GRSPa Free Grapevine Rootstocks
- Micromorphology of Leaf Epidermal Cells of Different Species of Cynodon dactylon in Pastoral Soil
- Study on Seed Dormancy Characteristics of Acer miaotaiense Tsoong in the Qinling Mountains
