Establishment of a Rapid Propagation Protocol of GLRaV-3,GFKV,and GRSPa Free Grapevine Rootstocks
WANG Li-min,FU Yan,LI Yong-zhou,HE Fei,WANG Jun,ZHU Yuan-di
Department of Pomology,China Agricultural University,Beijing 100193,PRC
Abstract Nursery plant propagation by grafting has been widely used in modern viticulture to minimize the damage caused by biotic and abiotic stresses.In grapevine(Vitis spp.),an effective way to control disease damage is to provide producers and growers with pathogen-free stock plants.In this study,five grapevine rootstock varieties,‘SO4’,‘101-14’,‘5BB’,‘110R’ and ‘1103P’,were selected as explants to establish an in vitro culture protocol,and three species of grapevine viruses were tested by real-time RT-PCR.The results showed that MS medium supplemented with 0.2 mg/L IBA,1.0 mg/L 6-BA,0.5 mg/L KT,4.0 mg/L adenine for culture initiation,and WPM supplemented with 0.2 mg/L IBA for subculture were suitable for all five rootstock varieties,with multiplication coefficients ranging from 1.6 to 4.4.Virus testing showed that single RT-PCR was more effective for detecting the three viruses compared to double or triple RT-PCR.Only plantlets free from the aforementioned viruses were retained for subculture.Plantlets were hardened at room temperature under natural lighting in Hoagland solution for 2 weeks and transplanted to pots filled with mixed media in a greenhouse.This protocol is applicable for rapid propagation of the five grapevine rootstock varieties and can be used for commercial production of virus-free grapevine stocks.
Key words Grapevine rootstock;Rapid propagation;Virus testing;Virus-free plantlet
1.Introduction
Grapes are one of the world’s most commonly cultivated fruit crops,with approximately 75 million tonnes produced in 2016 according to statistics reported by the Food and Agriculture Organization.The production and crop productivity of grapes are affected by biotic and abiotic stresses.Virus resistant varieties of rootstocks are being developed to increase industrial output and reduce labor input[1-2].Therefore,the production and application of clean grapevine stocks is essential for healthy vineyards[3].
Grapevines can be propagated sexually (through seeds) or vegetatively (through cuttings,grafting,and micro-propagation).Seed propagation is used in breeding programs and rootstock production.However,commercial propagation of grapevine stocks is commonly done vegetatively,through cuttings and grafting in nurseries.Compared with cuttings,grafted cultivars have been used widely in modern viticulture for rootstocks that exhibit a broad resistance to abiotic stresses,such as drought and salt stress[4],and biotic stresses like the knot nematode[5].The rootstock varieties ‘SO4’,‘5BB’,‘101-14’,‘1103P’,and ‘110R’,which originated mainly from crosses of three wild species,Vitis riparia,V.rupestris,andV.berlandieri,have been shown to adapt well to diverse locations and have a good affinity with scion cultivars[6].Additionally,high-quality virus-free grapevine rootstocks have been produced through meristem culture alone or in combination with heat treatment to break physiological dormancy[7].The advantage of tissue cultures is the rapid multiplication using fewer explants without seasonal constraints[8].The important factors forin vitroculture include explant inoculation,basal medium selection,and exogenous growth substance ratios that have been extensively explored to establish specific protocols for micro-propagation of ‘deGrasset’,‘110R’,‘5BB’,and ‘41B’[4].
Viruses and related pathogens are incurable and impose large costs on plant production.Grapevine leaf roll associated virus 3 (GLRaV-3)[9],Grapevine fleck virus (GFKV)[10],and Grapevine rupestris stem pitting associated virus (GRSPaV)[10]are the major diseases worldwide,and need to be eliminated to produce certified virus-free grapevine stocks[11-12].These viruses are typical RNA viruses and can be detected at very low concentrations by the reverse transcriptionpolymerase chain reaction (RT-PCR).
Clean planting stocks can minimize the spread of viral infection in the field.Tissue culture is an essential way of producing clean stocks.However,an efficient protocol needs to be established forin vitroculture of grapevine rootstocks.In this study,five rootstock varieties were used as materials to develop a rapid propagation protocol,and virus testing was performed by RT-PCR.The protocol developed here for the production of GLRaV-3,GFKV,and GRSPaV free grapevine rootstocks is intended to be universally applicable to micropropagation of grapevine rootstocks,and could be used for the commercial production of virus-free stock plants.
2.Materials and Methods
2.1.Plant materials
Dormant grapevine wood samples of ‘5BB’(V.berlandieri×V.riparia),‘SO4’ (V.berlandieri×V.riparia),‘1103P’ (V.berlandieri×V.riparia),‘101-14’(V.riparia×V.rupestris) and ‘110R’ (V.berlandieri×V.rupestris) were collected from the experiment station of the China Agricultural University in Beijing,China(40°08′9.75″N,116°10′45.77″E) in November,2016.‘Summer Black’ plantlets containing GLRaV-3,GFKV and GRSPaV were used as a positive control for virus testing.
2.2.RNA extraction and virus detection
Grapevine plants contain high concentrations of phenolic compounds,hence,total RNA was extracted using the CTAB method as previously described in the literature[13]and purified using an RNeasy Mini Elute Cleanup kit (Qiagen,Dusseldorf,Germany).Complementary DNA was synthesized using a PrimeScript™ 1stStrand cDNA Synthesis Kit (TaKaRa,Dalian,China) and stored at -20 °C.Single RT-PCR,double RT-PCR and triple RT-PCR were used to detect viruses in grapevine plantlets.The primers are listed in Table 1.
2.3.Breaking of dormancy and sterilization of explants
The dormant grapevine wood samples were cut into one-bud stem nodes and immersed in 45 °C water for 90 min to break dormancy.The cuttings were then cultured in clean water at room temperature in a container covered with plastic film to promote bud break for 3 to 4 weeks.Once the new shoots grew 10 leaves,with several semi-lignified nodes,the shoots were sampled as explants.To determine the optimum sterilization time and concentration of sodium hypochlorite (NaClO),explants of 5~10 cm in length were surface sterilized using three treatments(T1:2% NaClO,10 min;T2:1% NaClO,10 min;T3:0.4% NaClO,16~18 min).The sterilized explants were cultured on initiation medium (MS medium supplemented with 0.2 mg/L IBA,1.0 mg/L BAP,0.5 mg/L KT and 4.0 mg/L adenine,sucrose agar) for 30 d.After the initial 30 d culture,the shoot apexes(~0.5 mm) were collected from the propagules under a microscope,and cultured on the same medium for 30 d to get adventitious shoots for subculture.

Table 1 List of primers disigned for RT-PCR amplification of three grape viruses and the VvACTIN.
2.4.Subculture
Four media,M1,M2,M3,and M4,were tested for optimum grapevine subculture (M1:WPM medium supplemented with 0.2 mg/L IBA and 0.5 mg/L BAP;M2:WPM medium supplemented with 0.2 mg/L IBA and 1 mg/L BAP;M3:WPM medium supplemented with 0.2 mg/L IBA and 1.5 mg/L BAP;M4:WPM medium supplemented with 0.2 mg/L IBA and 2 mg/L BAP).All media contained 30 g/L sucrose and 8 g/L agar,and were at a pH between 5.8~5.9.Plantlets were kept at 24±2 °C and under a 16 h/8 h light/dark cycle at a light intensity of 30~40 μmol/(m2·s).Ten plantlets were included in each treatment,and each treatment was run in triplicate.
2.5.Hydroponic hardening and transplantation
After the third subculture,when the plantlets had grown to a height of 7~9 cm with 2~3 adventitious roots of 3~5 cm in length each,the plantlets were hydroponically cultured in modified Hoagland solution with partial nutrients for 1 week and in normal Hoagland solution for another 1 to 2 weeks to determine the appropriate hydroponic culture time.During the hydroponic hardening,the plantlets were stored in a container initially covered with plastic film to retain moisture,and the container was aired often,before the plastic film was gradually removed.After hydroponic hardening,the plantlets were transferred to 12×12 cm pots containing garden soil,compost,and vermiculite in a volume ratio of 1 ∶1 ∶1.The plantlets were acclimatized in a greenhouse for 40 d,and a simple arch shed was built to keep in moisture.
2.6.Statistical analysis
All samples were analyzed in triplicate,and the data were expressed as means±standard error unless noted otherwise.Statistical significance was determined using SPSS 17.0.A difference atP<0.05 was considered significant,andP<0.01 was considered extremely significant.
3.Results
3.1.Grapevine explant sampling
To break dormancy,dormant wood samples of grapevines were incubated in a water bath at 45 °C for 90 min.Breaking bud dormancy was further promoted by keeping the dormant wood samples in clean water at room temperature.After 30 d,the buds of ‘SO4’,‘110R’,and ’1103P’ started to break,while ‘5BB’ and‘101-14’ showed no response.The rates of bud break for ‘SO4’,‘110R’,and ‘1103P’ were 100%,57%,and 44% respectively.After 50 d,the five tested rootstock varieties showed high rates of bud break,with ‘SO4’,‘110R’,’1103P’,‘5BB’,and ‘101-14’ showing 100%,85.7%,97.3%,65.4%,and 80% sprouting,respectively (Fig.S1).Compared with the other four varieties,‘110R’ had weak growth of new shoots.Explants were sampled when the new shoots became semi-lignified after hydroponic culture after 40~50 d.
3.2.Different treatments for explant surface disinfection
To obtain aseptic explants,three surface disinfection treatments were conducted on explants on a clean bench before initiating tissue culture.The results showed that treatment using a low concentration of a sodium hypochlorite solution (0.4%)for 16~18 min was more successful than a higher concentration (1% or 2%) of disinfectant in terms of explant survival.‘101-14’,‘SO4’,‘5BB’,’1103P’,and‘110R’ showed different survival rates under 2%,1%,and 0.4% sodium hypochlorite solution,respectively(Table 2).A high concentration of sodium hypochlorite(1% or 2%) stimulated explant browning,and axillary bud abortion.Conversely,explants sterilized with sodium hypochlorite at a low concentration (0.4%)exhibited less browning and were not prone to fungal contamination.After sterilization,the explants were inoculated onto the initiation medium.The axillary buds of the explants started to grow at 20~25 d after the start of the culture (Fig.1).New shoots were subcultured 28~30 d after the start of the initiation culture.
3.3.Subculture of propagules
To select a suitable medium for grapevine rootstocks,basic WPM medium was supplemented with different proportions of plant growth regulators(IBA and BAP).In M1 medium,‘SO4’,‘101-14’,‘5BB’,‘1103P’,and ‘110R’ grew and rooted well,with root lengths of 4.19±0.73 cm,4.58±0.82 cm,5.12±0.86 cm,2.22±0.68 cm,and 2.71±1.28 cm,respectively,after 30 d (Table 3 and Fig.S2).‘SO4’,‘101-14’ and ‘5BB’ plantlets in M1 medium were significantly taller than those in the other three media,whereas the plantlet height of ‘1103P’ and ‘110R’showed no obvious differences among the four media(Table 3).The proliferation coefficients of ‘SO4’,‘101-14’,‘1103P’,and ‘5BB’ in M1 medium were 4.1,4.4,1.6,and 2.3,respectively,after one generation of subculture.The multiplication efficiency were superior in M1 compared with the other three media.‘SO4’,‘101-14’,and ‘5BB’ plantlets in M1 medium displayed robust growth with longer shoots and more adventitious roots than ‘1103P’ and ‘110R’ (Table S1)did.These results suggested that the broad cultivar range of the M1 medium may be suitable for different rootstocks at the subculture stage.
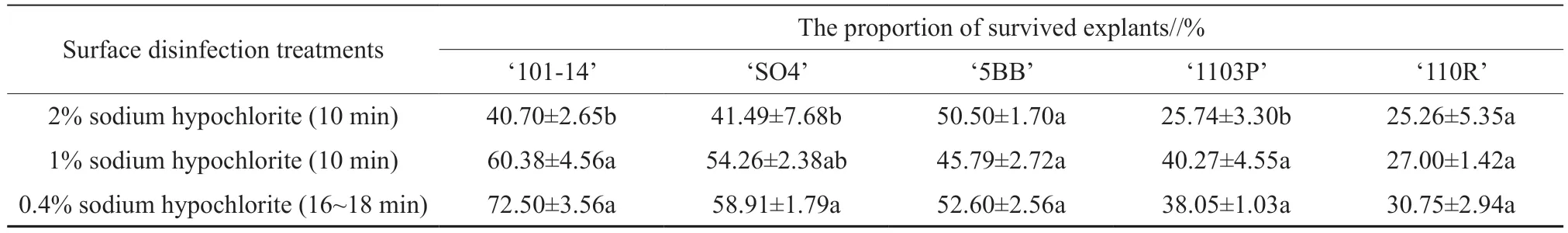
Table 2 The proportion of survived grapevine explants after different

Fig.1 Growth status of 4 grape varieties in initiation media for 25 d

Table 3 The heights of grapevine plantlets grown in different media
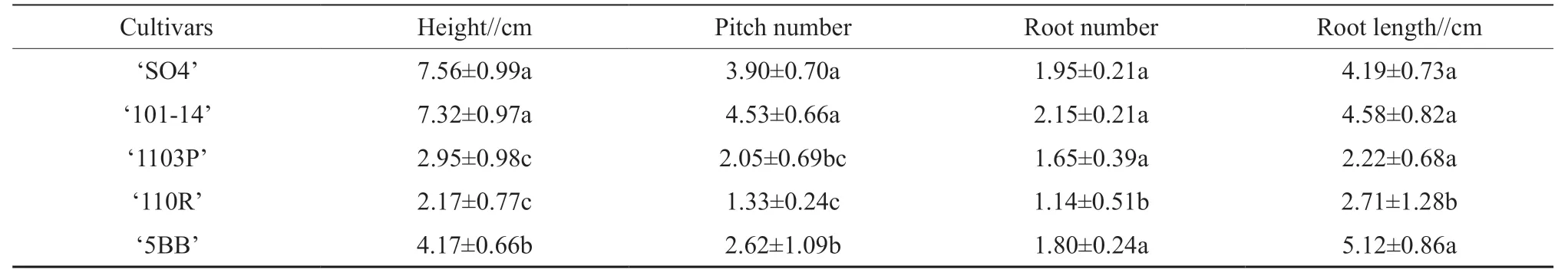
Table S1 The growth status of grapevine plantlets in the M1 medium for 30 days
3.4.Virus testing by RT-PCR
Single and duplex RT-PCR was used to detect the presence of GFKV,GLRaV-3,and GRSPaV in all five rootstocks.These viruses were detected at template concentrations of 0.11×10-4,1.2,and 0.019 ng/μL by single RT-PCR,respectively (Fig.2a-c).In duplex RT-PCR,GLRaV-3 and GFKV were detected simultaneously at a concentration of 1.2 ng/μL,GRSPaV and GFKV were detected at 120 ng/μL and GLRaV-3 and GRSPaV at 12 ng/μL (Fig.2d-f).Single RT-PCR was more sensitive than duplex RT-PCR for the detection of the viruses.Thus,plantlets of the five varieties of grapevine rootstocks were tested for the three viruses by single RT-PCR.‘101-14’,‘SO4’,‘5BB’,and ‘1103P’ contained all three species of the viruses.GFKV was not detected in the tested plantlets of ‘110R’,whereas it was detected in all tested plantlets of ‘1103P’ (Table S2).The detection rates of GLRAV-3,GRSPaV and GFKV was 46.2%~66.7%,12.5%~42.9% and 20%~35.5% respectively.Any plantlets containing at least one of the viruses were discarded.Finally,eight plantlets of ‘110R’,eight plantlets of ‘101-14’,and seven plantlets of ‘SO4’,in which GLRaV-3,GFKV,and GRSPaV were not detected by single RT-PCR,were retained for the next round of subculture.

Fig.S2 Growth status of different mediums after substituting for 30 d in
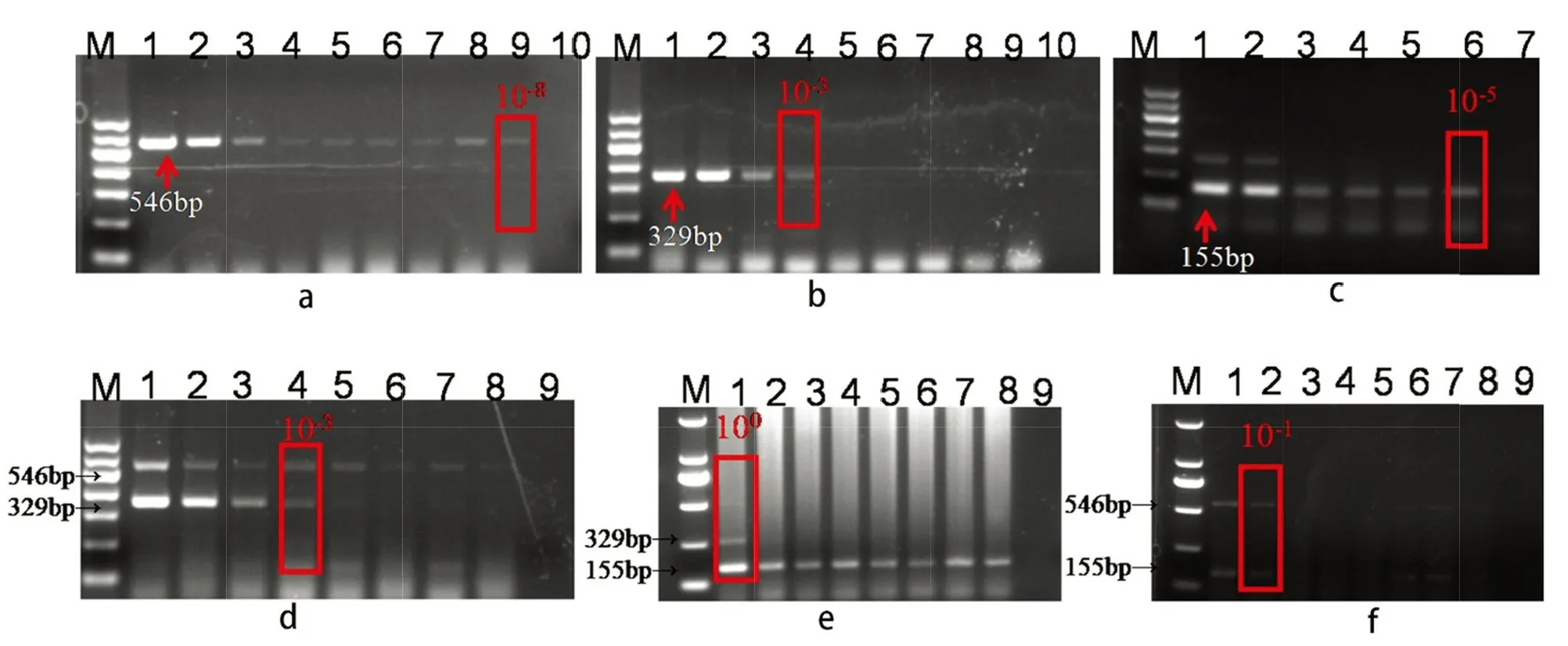
Fig.2 The electropherogram of GLRaV-3(a),GFKV(b) and GRSPaV(c) sensitivity detection
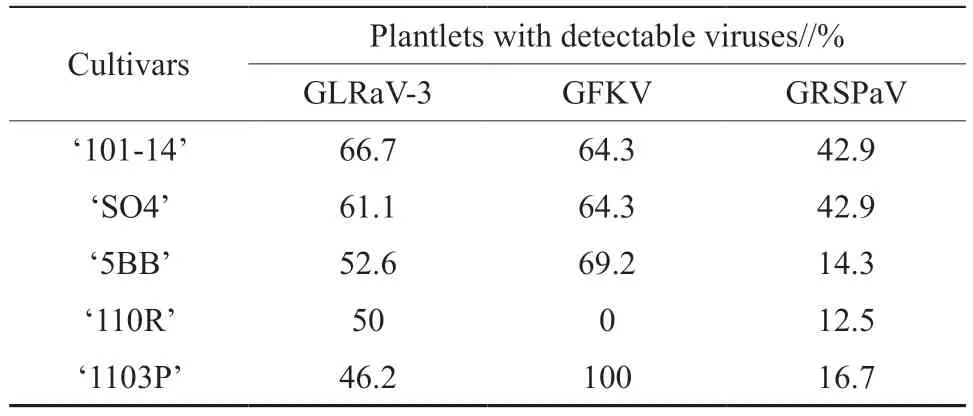
Table S2 The proportion of grapevine plantlet with detectable viruses
3.5.Hardening and transplanting of virus-free plantlets
Only plantlets free from the aforementioned viruses were used for multiplication.After three or four generations of subculture in M1 medium (Table S3),the plantlets were hardened in a hydroponic culture of Hoagland solution.After 2 weeks of hardening,the plantlets displayed dark green leaves and numerous roots (Fig.3).Surviving plantlets of‘101-14’,‘SO4’,and ‘110R’ accounted for 97.05%,96.15%,and 73.7%,respectively (Table 4).The plantlets were transplanted into pots with mixed media in a greenhouse for further acclimatization (Fig.S3).After one month,approximately 97% of plants survived.The five rootstock varieties adapted well to the process of hydroponic hardening and transplantacclimatization.
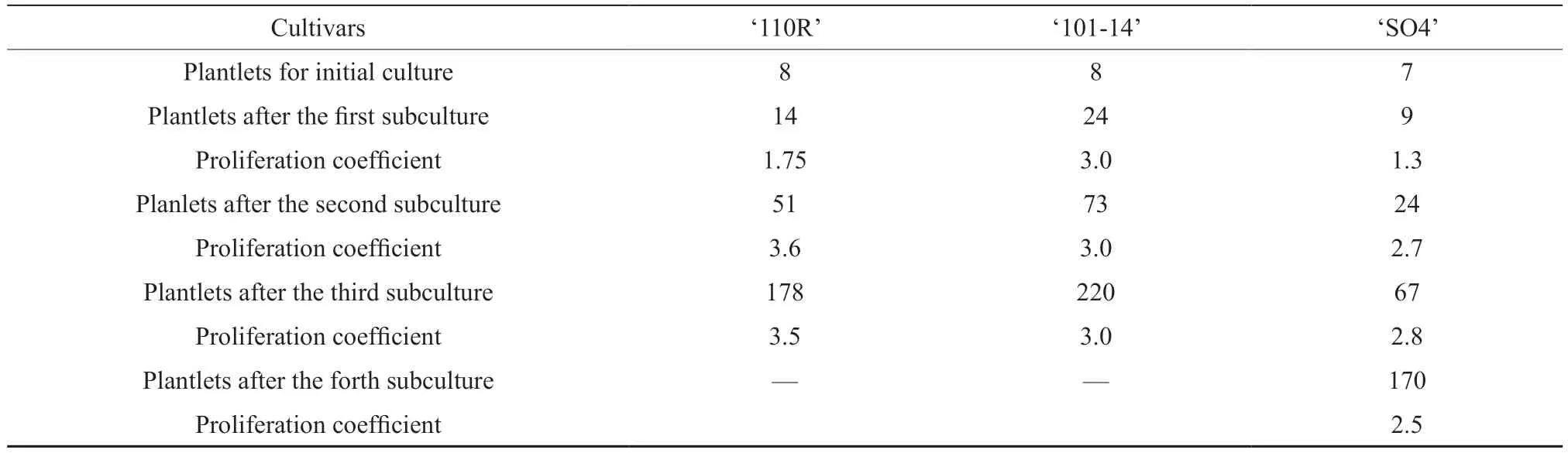
Table S3 Proliferation coefficient of subculturing GLRaV-3,GFKV,and GRSPaV free gravevine plantlets

Fig.3 Growth status of hydroponic seedlings of 3 rootstock grape varieties for 2 weeks.

Fig.S3 Different stages of transplanted grape plantlets
4.Discussion
4.1.The establishment of aseptic explants
The establishment of aseptic explants is a key step in plant tissue culture,during which browning and contamination of explants occur frequently[14].The survival of explants in the initiation culture is affected by the sampling time,type of explants,and subsequent surface sterilization.In this study,explants were obtained by indoor hydroponic culture of dormant grapevine wood,not young shoots grown in the field as previously reported by MUKHERJEE Pet al.[15].The physiological dormancy of grapevine wood can be broken by heat treatment[16].While the heat treatment kills the endogenous microorganisms in dormant shoots,physiological dormancy protects the buds from damage caused by high temperature stress[17].
It has been reported that superficial fungi was completely removed from samples of ‘Paul Garr’and ‘Princess Seedless’.The bud break rate of dormant buds and the rooting rate were improved by a continuous water bath at 50 °C for 0.5~1 h[18].We found that heat treatment in water bath at 45 °C for 90 min broke the dormancy of grapevine samples (‘5BB’,’SO4’,’1103P’,’101-14’ and ’110R’),promoted bud break,and reduced pathogen contamination in the initial culture step[19].Thus,new shoots grown indoors were cleaner than shoots grown outdoors in terms of pathogen infection.
Sodium hypochlorite has broad-spectrum antimicrobial activity and can rapidly kill vegetative spores,bacteria,fungi,and protozoa through the irreversible oxidation of sulfhydryl groups of essential enzymes,and has deleterious effects on DNA and membrane-associated enzyme activity[20-21].Sodium hypochlorite has been widely used for the surface disinfection of explants.A high concentration of sodium hypochlorite (5%) and a short treatment time (5 min) has been used to sterilize young shoots of ‘Shiraz’and ‘Cabernet Sauvignon’ grown in the field,and 90%of explants survived during primary culture[19].Our experiments indicated that a lower concentration of disinfectant (0.4%) and a longer treatment time (16~18 min) were more efficient than the previously reported method.
4.2.Optimizing the subculture medium
Subculture is the second step in the process of tissue culture,in which vitrification of propagules can occur in various media[14].The micronutrient compositions are markedly different among MS,B5,and WPM media.The variation of micronutrients between the media may cause differences in shoot proliferation among different species[22].For example,WPM medium contains higher contents of vitamins,lower contents of nitrate and ammonium nitrogen,and has proven to be suitable for several woody plants when compared to MS medium[23].In addition to basic medium,the plant growth regulators auxin and cytokinin are important factors during tissue culture.C2D medium was suitable for mass micropropagation of adventitious shoots of ‘Spero’ when supplemented with 1 mg/L BA,and 1 mg/L IBA for rooting induction[24].When a high concentration of (2.5 mg/L cytokinin)was applied,vitrification occurred duringin vitroculture of ‘Dogridge’ (Vitis champini),‘SO4’,‘H-144’(V.vinifera×V.labrusca) and ‘3309 C’ (V.riparia×V.rupestris)[25].When WPM medium was supplemented with 0.5 mg/L BAP,no vitrification was observed inin vitroculture of ‘Canonannon’ and ‘Cheninblanc’[26].We tested WPM medium supplemented with 0.2 mg/L IBA,which simultaneously induced shoot growth and adventitious roots,whereas it did not induce adventitious shoots.This subculture medium is quite different from previously reported media,and is suitable for multiplication of ‘SO4’,‘101-14’,‘110R’,‘1103P’ and ‘5BB’.However,a specific medium needs to be developed for ‘SO4’ to increase the coefficient of multiplication and enable rapid propagation.
4.3.Virus detection of plantlets
GFKV,GLRaV-3,and GRSPaV affect the normal growth and development of grapevines,leading to a reduction of yield and quality[27-28].The most common method used to eliminate viruses and viruslike pathogens is meristem culture combined with heat treatment,which has been shown to effectively remove GLRaV-1 and GRSPaV-1[29].It has been hypothesized that viruses are transmitted in plants through the vascular bundle and plasmodesmata[30-31]and thus,the meristems of shoot tips are less effected when plants are infected by viruses.Tissue culture of the shoot apex in combination with virus detection was conducted in our study.Single RT-PCR for virus testing is a simple,quick and sensitive method[32].Although multiplex-PCR is superior to single RTPCR in the time required for detection,conventional single RT-PCR is still widely used.This is due to the technical difficulties in combining several compatible primers in a single amplification system;the specific primer design required for each target DNA;and the difference in DNA amplification accuracy for genes of different sizes[33-34].Our results showed that single RTPCR was more sensitive for the detection of GFKV,GLRaV-3,and GRSPaV than duplex RT-PCR.
5.Conclusion
In this study,explants of grapevine rootstocks were collected from new shoots grown indoors by hydroponic culture of wood samples.Heat treatment in a water bath at 45 °C for 90 min broke the dormancy of the grapevine samples.Treatment with a low concentration of sodium hypochlorite (0.4%)for 16~18 min was used to sterilize the explants to aide in survival.WPM medium supplemented with 0.2 mg/L IBA is suitable for multiplication of ‘SO4’,‘101-14’,‘5BB’,‘110R’,and ‘1103P’.Single RTPCR is sensitive for GFKV,GLRaV-3,and GRSPaV detection.Hydroponic hardening of plantlets for 2 weeks and transplant acclimatization of plants in a greenhouse for 40 d are beneficial to improve the survival rates of plantlets.
 Agricultural Science & Technology2020年4期
Agricultural Science & Technology2020年4期
- Agricultural Science & Technology的其它文章
- Genetic Analysis of Main Plant Type-related Traits in Bitter Gourd (Momordica charantia L.)
- Changes of Soil nutrients and Tobacco Quality in Linli County and Some Suggestions on Fertilization
- Effects of Altitude on Polyphenols Contents in Cigar Leaves
- Micromorphology of Leaf Epidermal Cells of Different Species of Cynodon dactylon in Pastoral Soil
- Study on Seed Dormancy Characteristics of Acer miaotaiense Tsoong in the Qinling Mountains
- Comparative Analysis of Bioactive Components of Polygonatum cyrtonema Hua from Different Areas
