A nomogram to preoperatively predict 1-year disease-specific survival in resected pancreatic cancer following neoadjuvant chemoradiation therapy
Ho Kyoung Hwang,Keita Wada,Ha Yan Kim,Yuichi Nagakawa,Yosuke Hijikata,Yota Kawasaki,Yoshiharu Nakamura,Lip Seng Lee,Dong Sup Yoon,Woo Jung Lee,Chang Moo Kang
1Division of HBP Surgery,Department of Surgery,Yonsei University College of Medicine,Seoul 03722,Korea;2Pancreatobiliary Cancer Clinic,Yonsei Cancer Center,Severance Hospital,Seoul 03722,Korea;3Department of Surgery,Teikyo University School of Medicine,Tokyo 173-8605,Japan;4Biostatistician,Biostatistics Collaboration Unit,Department of Biomedical Systems Informatics,Yonsei University College of Medicine,Seoul 03722,Korea;5Department of Gastrointestinal and Pediatric Surgery,Tokyo Medical University,Tokyo 160-8402,Japan;6Department of Digestive Surgery,Breast,and Thyroid Surgery,Graduate School of Medical Sciences,Kagoshima University,Kagoshima 890-0065,Japan;7Department of Gastrointestinal and Hepato-Biliary-Pancreatic Surgery,Nippon Medical School,Tokyo 890-0065,Japan;8Department of General Surgery,Changi General Hospital,Singapore 529889,Singapore
Abstract Objective:This study aimed to develop a nomogram to predict the 1-year survival of patients with pancreatic cancer who underwent pancreatectomy following neoadjuvant treatment with preoperatively detectable clinical parameters.Extended pancreatectomy is necessary to achieve complete tumor removal in borderline resectable and locally advanced pancreatic cancer.However,it increases postoperative morbidity and mortality rates,and should be balanced with potential benefit of long-term survival.Methods:The medical records of patients who underwent pancreatectomy following neoadjuvant treatment from January 2005 to December 2016 at Severance Hospital were retrospectively reviewed.Medical records were collected from five international institutions from Japan and Singapore for external validation.Results:A total of 113 patients were enrolled.The nomogram for predicting 1-year disease-specific survival was created based on 5 clinically detectable preoperative parameters as follows:age (year),symptom (no/yes),tumor size at initial diagnostic stage (cm),preoperative serum carbohydrate antigen (CA) 19-9 level after neoadjuvant treatment (<34/≥34 U/mL),and planned surgery [pancreaticoduodenectomy (PD) (pylorus-preserving PD)/distal pancreatectomy (DP)/total pancreatectomy].Model performance was assessed for discrimination and calibration.The calibration plot showed good agreement between actual and predicted survival probabilities;the the Greenwood-Nam-D’Agostino (GND) goodness-of-fit test showed that the model was well calibrated (χ2=8.24,P=0.5099).A total of 84 patients were used for external validation.When correlating actual disease-specific survival and calculated 1-year disease-specific survival,there were significance differences according to the calculated probability of 1-year survival among the three groups (P=0.044).Conclusions:The developed nomogram had quite acceptable accuracy and clinical feasibility in the decisionmaking process for the management of pancreatic cancer.
Keywords:Pancreatic cancer;neoadjuvant treatment;pancreatectomy;survival;nomogram
Introduction
Pancreatic cancer is among the most fatal cancers of the gastrointestinal tract.The overall 5-year survival rate of pancreatic cancer is only approximately 6%,and it is the fourth leading cause of cancer-related deaths in the United States (1) as well as in Korea (2).Although surgical resection with clear safe margin is the only modality for curative treatment,only 20% of patients are eligible for surgical resection at the time of initial diagnosis because majority of patients are often diagnosed in the late stage and thus have poor general condition.Moreover,more than 50% of patients who undergo curative surgery develop recurrence within 1 year,and the 5-year survival rate of resected pancreatic cancer is less than 20% (1,3,4).
According to the National Comprehensive Cancer Network (NCCN) guideline (5),pancreatic cancers requiring neoadjuvant treatment are those that involve the major vascular structures and adjacent organs.When margin-negative resection is considered as the most effective treatment (6),extended pancreatectomy including combined major vascular resection and adjacent organ resection may be necessary to achieve complete tumor removal in borderline and locally advanced pancreatic cancers.However,extended pancreatic resections following combined preoperative chemotherapy and radiotherapy before surgery are known to increase the rates of postoperative morbidity and mortality.
Several randomized control studies (7-10) that compared oncologic outcomes and short-term perioperative outcomes between extended pancreatectomy and standard pancreatectomy showed that extended pancreatectomy is associated with severe complications without improving oncologic outcomes.Bhayaniet al.(11) analyzed 273 extended pancreatoduodenectomies from the National Surgical Quality Improvement Project database and reported a threefold increase in major perioperative morbidity and mortality rates even after adjusting for comorbidity in such surgical strategy.Hartwiget al.(12)also recently assessed the outcome of extended pancreatectomy for borderline resectable and locally advanced pancreatic cancer and showed that extended resections were associated with increased perioperative morbidity and mortality.
Surgeons need to consider many clinical and practical factors when deciding the need for pancreatectomy,such as potential oncologic benefit of surgical approach,patients’existing co-morbidity,expected life span of the patients,quality of life after surgery,potential postoperative complications,and surgery-related mortality.If possible,it was a good strategy to avoid unnecessary surgery in patients without surgical benefits.
In this study,we aimed to develop a nomogram to predict the 1-year survival of patients with pancreatic cancer who underwent pancreatectomy following neoadjuvant treatment with preoperatively detectable clinical parameters to ultimately provide a basis for decision-making for the appropriate management of pancreatic cancer following neoadjuvant treatment.
Materials and methods
Study population
This study protocol was approved by the Institutional Review Board of Yonsei University College of Medicine(Approval number:4-2018-0374).The medical records of the patients who underwent pancreatectomy following neoadjuvant treatment from January 2005 to December 2016 were retrospectively reviewed.Neoadjuvant treatment was routinely performed for the patients with borderline resectable or locally advanced pancreatic cancer.Among patients in the resectable stage,neoadjuvant treatment was performed preoperatively,even if there was no major vascular involvement,but the tumor size was large and there was invasion to the surrounding organs.
In this study,we included patients who underwent neoadjuvant treatment before surgery and were able to perform curative resection in pancreatic cancer.Patients who did not attempt pancreatic resection due to distant metastasis found during surgery and patients who did not undergo curative resection due to severe major vascular invasion were excluded.
Data collection
Data on clinicopathologic variables,such as sex,age,serum carbohydrate antigen (CA) 19-9,tumor location,operation type,tumor size,tumor grade (differentiation),pathologic tumor (pT) stage,presence of lymph node metastasis,lymph node ratio (i.e.,total number of metastatic lymph nodes divided by the total number of retrieved lymph node),microscopic perineural invasion and lymphovascular invasion,and disease-specific survival were collected.Surgeons followed up all the patients,usually every 3−4 months and the follow-up continued until recurrence or death was confirmed.
External validation
External validation to test the accuracy of developed nomogram to predict 1-year disease-specific survival was conducted by reviewing the medical records of the patients who underwent pancreatectomy following neoadjuvant treatment for pancreatic cancer from five international institutions from Japan and Singapore.
Statistical analysis
When developing the prediction model,preoperatively available clinical parameters were included in the final model that achieved statistical significance in the univariate Cox model and were also considered clinically important.Based on the results of the multivariate Cox model,a nomogram was created by using the packagermsin R software (Version 3.1.3;R Foundation for Statistical Computing,Vienna,Austria) (http://www.r-project.org/).Serum CA19-9 levels were divided into two based on a cutoff of 34 U/mL,which was based on the proposal of Contal and O’Quigley (13) as determined via log-rank test.
To quantify the discriminative capability of the final model,Harrell’s C-index was measured.Calibration was evaluated via a calibration plot,which is a graphic representation of the relationship between the observed and predicted survival,and the Greenwood-Nam-D’Agostino (GND) goodness-of-fit test (14).Internal validation was performed using 1,000 bootstrapped resamples and also generated the calibration plot.To further assess the calibration,we plotted Kaplan-Meier curves over the risk-stratified groups by the predicted probability by the nomogram.Finally,we tested the performance of the nomogram in the external validation cohort.The formula derived in the development cohort was applied to all patients in the validation cohort.To further validate the nomogram,patients were categorized into three risk groups according to the nomogram-based calculated 1-year disease-specific survival probability,and survival was compared between the three groups using logrank test.Continuous variables were described as,and categorical variables were described as a frequency (%).Student’st-test,Chi-square test,or Fisher’s exact test were performed as appropriate.Statistical analyses were performed using SAS software (Version 9.4,SAS Institute,Cary,NC,USA),and P<0.05 was considered statistically significant.
Results
Patient characteristics
During the study period,113 patients underwent pancreatectomy following neoadjuvant chemoradiation therapy.Of these,64 were men and 49 were women.The mean age was 51.5±9.4 years old.A total of 42 (37.2%)patients were asymptomatic.In terms of resectability,46(40.7%) patients had resectable pancreatic cancer,46(40.7%) had borderline resectable pancreatic cancer,and 21 (18.6%) had locally advanced pancreatic cancer.The most common location of the tumor was the pancreatic head (86/113,76.1%),followed by the pancreatic body/tail(27/113,23.9%).Pylorus-preserving pancreaticoduodenectomy (PPPD) was performed in 83 (73.5%) patients,distal pancreatosplenectomy (DPS) in 27 (23.9%) patients;and total pancreatectomy (TP) in 3 (2.7%) patients.Combined venous vascular resection including superior mesenteric vein (SMV)-splenic vein (SV)-portal vein (PV)confluence was performed in 40 (35.4%) patients.The maximum diameter of the resected pancreatic cancer was 2.1±1.5 cm.Lymphovascular and perineural invasion were observed in 10 (8.8%) and 50 (44.2%) patients,respectively.Lymph node metastasis was found in 36(31.9%) patients.The median disease-free survival and disease-specific survival was 11.0 [95% confidence interval(95% CI):8.8−13.2]months and 29.0 (95% CI:1.6−38.4)months,respectively.Twenty-seven (23.9%) patients were found to have cancer-related mortality within 1 year postoperatively.
Preoperative factors to predict 1-year disease-specific survival
In univariate analysis,age (P=0.0165),serum CA 19-9 (after neo-Tx) following neoadjuvant treatment (P=0.0023),and preoperative image-based tumor size (P=0.0469) were statistically significant preoperative parameters to predict less than 1-year disease-specific survival in patients with pancreatic cancer who underwent pancreatectomy following neoadjuvant treatment.Subsequent multivariate analysis showed that age [hazard ratio (HR)=1.062,95%CI:1.008−1.118,P=0.024]and CA 19-9 ≥34 U/mL (after neo-Tx) following neoadjuvant treatment (HR=2.863,95%CI:1.123−7.300,P=0.0276) were independent prognostic factors to predict less than 1-year disease-specific survival(Table 1).
Developing nomogram to predict 1-year disease-specific survival and model performance
Of the 113 patients,the variables of“preoperative serum CA 19-9 after neoadjuvant treatment”were missing in 9 patients,we excluded this data and 104 patients were finally analyzed.Based on the complete survival-specific data and preoperatively available parameters of the 104 patients,the nomogram to predict 1-year disease-specific survival of the patients who underwent pancreatectomy following neoadjuvant treatment for pancreatic cancer was created(Figure 1).The nomogram was created based on 5 clinically detectable preoperative parameters:age (year),symptom(no/yes) on initial diagnosis,image-based tumor size at initial diagnostic stage (cm),preoperative serum CA 19-9(after neo-Tx) (<34/≥34 U/mL),and planned surgery [PD(PPPD)/DPS/TP].The model performance was quantified via Harrells’ c-statistics,which was 0.736 [standard error(SE)=0.064].
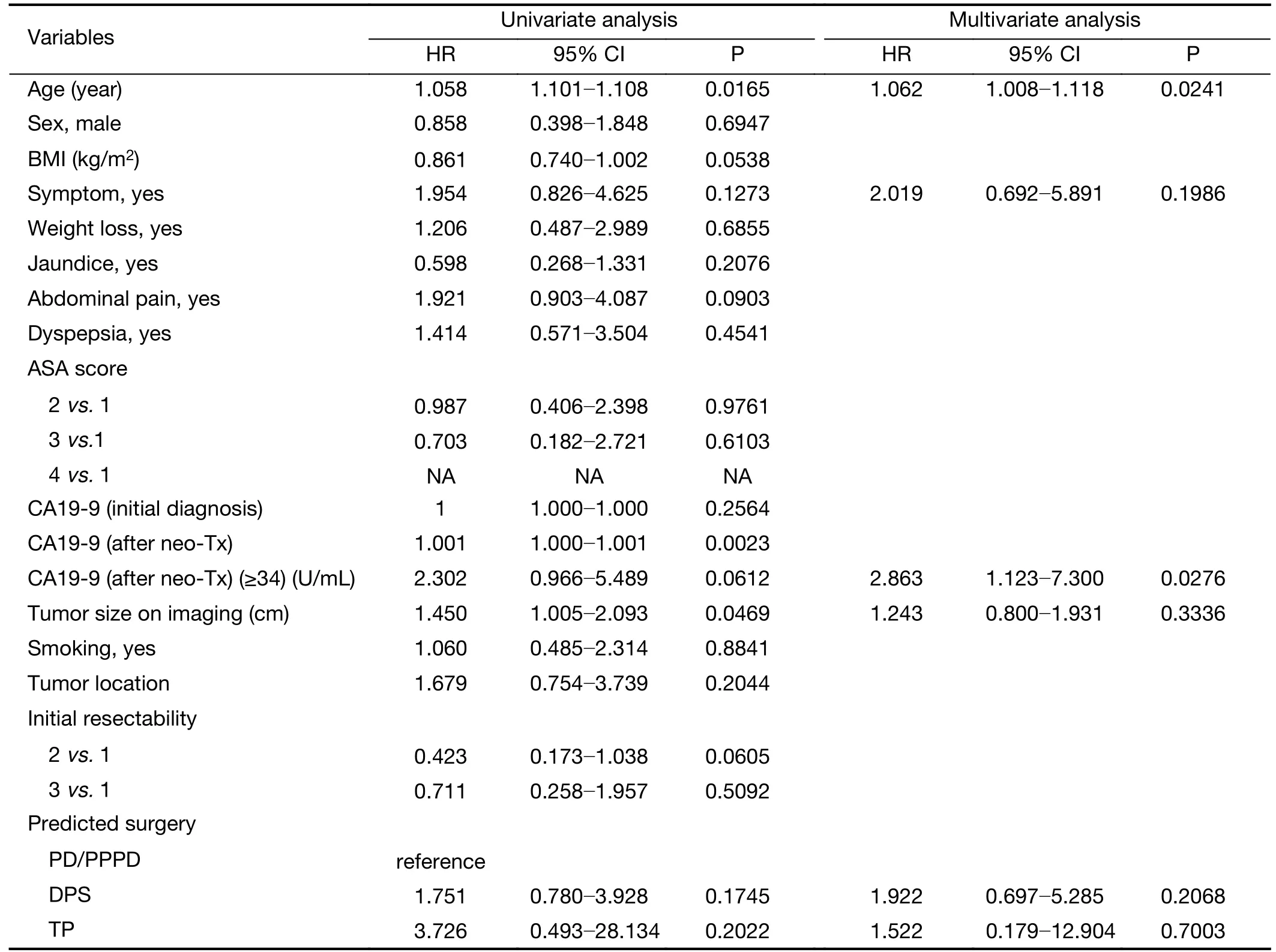
Table 1 Univariate and multivariate analyses showing association between preoperative clinical parameters and 1-year disease-specific survival
Validation of nomogram
Model performance for discrimination and calibration was assessed.Discrimination was validated using the bootstrap validation with 1,000 resamples.The calibration plot(Figure 2) showed good agreement between actual and predicted survival probabilities,and the GND goodness-offit test showed that the model was well calibrated (χ2=8.24,P=0.5099).To further assess the discriminating capability of the nomogram,according to nomogram-based estimated 1-year disease-specific survival probability,patients were categorized into three risk groups as follows:the low-risk group comprised patients with a nomogram-based calculated 1-year disease-specific survival probability of<33.3%;the intermediate-risk group,33.3%−66.6%;and the high-risk group,>66.6%.Statistically significant differences in the survival rates were found among the three groups (P<0.001,Figure 3).
External validation to assess nomogram performance in predicting 1-year disease-specific survival
Institutional data of the patients who underwent resection following neoadjuvant treatment were obtained from five international hospitals.A total of 84 patients were used for external validation to assess the nomogram performance.Patients’ age (61.4±9.6vs.66.9±9.7,P=0.0001) and serum CA 19-9 (after neo-Tx) (≥34 U/mL) following neoadjuvant treatment [48 (46.2%)vs.64 (74.4%),P<0.0001]were statistically significant between the study cohort and the validation cohort (Table 2).Moreover,when correlating actual disease-specific survival and calculated 1-year disease-specific survival,there was statistically significant differences according to the calculated probability of 1-year survival among the three groups (P=0.044,Figure 4).
Discussion
Pancreatic cancer is the fourth leading cause of cancerassociated mortality,with approximately 43,090 deaths recorded in 2017 in the United States (15).The median survival is 26 months,and the 5-year survival rate is 30%(6).Margin-negative pancreatectomy followed by adjuvant chemotherapy is the standard treatment modality for resectable pancreatic cancer.However,only 15%−20% of the patients are eligible for resection at the time of diagnosis.In addition,patients who underwent marginpositive pancreatectomy show poor survival outcomes similar to those with locally advanced and unresectable pancreatic cancer (6).Therefore,neoadjuvant treatment has been utilized for borderline resectable and locally advanced (unresectable) pancreatic cancer to improve eligibility for surgical resection,with the ultimate aim to optimize the treatment outcomes and minimize the risk of treatment-related morbidity.
Studies (16,17) have shown the importance of neoadjuvant treatment in pancreatic cancer.A recent metaanalysis (18) of studies reporting median overall survival by intention to treat in patients with resectable or borderline resectable pancreatic cancer treated with or without neoadjuvant treatment showed that neoadjuvant treatment appears to improve overall survival (18.8 monthsvs.14.8 months) despite lower overall resection rates (66.0%vs.81.3%).In addition,Choiet al.(19) conducted a prospective randomized controlled trial on the oncologic benefits of neoadjuvant treatment in borderline resectable pancreatic cancer and showed that neoadjuvant treatment provided oncologic benefit over upfront surgery in patients with borderline resectable pancreatic cancer.Even in the metastatic setting,FOLFIRINOX and nab-paclitaxelgemcitabine (20,21) have shown a survival advantage over previously standard gemcitabine monotherapy;thus,they have become the standard treatment option in patients with good performance status.Based on these clinical evidences,neoadjuvant treatment is expected to be actively used in managing pancreatic cancer.
Deciding on the necessity of radical pancreatectomy following neoadjuvant for pancreatic cancer can be complicated.Most patients who underwent neoadjuvant treatment have borderline resectable and locally advanced pancreatic cancer and usually require combined vascular resection with extensive surgical dissection for marginnegative resection.Several studies have reported that extended radical pancreatectomy is associated with increased morbidity and mortality (10,12).In addition,except for resectable pancreatic cancer,other tumors are biologically aggressive.Thus,patients are at high risk of early recurrence and early cancer-related mortality despite successful curative surgical resection.Therefore,the patients and their families should be well-informed of these risks before surgery.Moreover,the balance between potential risk and oncologic benefit should be considered in deciding the need for surgery.However,many researches on survival analysis in patients with pancreatic cancer who underwent surgery following neoadjuvant treatment are based on pathological examinations after surgical intervention,such as tumor grade (22),pathological complete response (23),lymph node status (24),perineural invasion (25),and margin-status (6).The response of neoadjuvant chemotherapy is thought to be an important prognostic factor in pancreatic cancer with neoadjuvant treatment (23).However,in our clinical practice,it is true that response to neoadjuvant treatment cannot be always measured objectively due to inflammatory changes and previous biliary stent.Therefore,in order to develop nomogram to be applied in every case of pancreatectomy following neoadjuvant treatment,we did not include the response of neoadjuvant chemotherapy,but include preoperative serum CA 19-9 after neoadjuvant treatment.Methods to preoperatively predict the survival outcome for these patients are thus important in clinical practice.
In the present study,only preoperative clinical parameters,such as,age,symptom,image-based tumor size at diagnosis,preoperative serum CA 19-9 after neoadjuvant treatment,and planned surgery,were considered in developing a nomogram (internet calculator;http://40.121.207.11:8080/home1.jsp) to predict 1-year survival outcomes after pancreatectomy following neoadjuvant treatment for pancreatic cancer.In univariate analysis,variables with P<0.2 were age,body mass index (BMI),symptom,abdominal pain,CA19-9 (after neo-Tx),CA19-9(≥34 U/mL,) tumor size,initial resectability,and predicted surgery.The three parameters (symptom,tumor size,and planned surgery) were of no significance in multivariate analysis but were used in the development of the nomogram.The reason for this was to consider the concept of events per predictor variable (EPV) when constructing the nomogram and to include variables with clinical importance among variables with P<0.2.Although there are some variables that are not significant when multiple logistic regression analysis is performed,the reason why we built the prediction model with these five variables was because we wanted to find a model with better overall model performance than the significance of each variable.We selected this model as the final model because it showed the best performance when modeled using these five variables in this data.Harrell’s c-index was 0.684(SE=0.064) when the nomogram was created with age,tumor size,and CA19-9 after neoadjuvant treatment with P<0.05 in univariate analysis and when the nomogram was created with age,abdominal pain,and CA19-9 after neoadjuvant treatment,which were selected by the stepwise selection method,the Harrell’s c-index was 0.711(SE=0.064).Internal validation of the current nomogram via Harrells’ c-statistics (0.736) and the calibration plot demonstrated its quite acceptable clinical accuracy.In addition,external validation of the clinical availability and feasibility of the current nomogram in predicting survival outcome in patients who underwent pancreatectomy following neoadjuvant treatment was conducted using dataset from 5 international institutions.Similar to the primary observation from the development dataset,the predicted 1-year disease-specific survival was successfully correlated with actual survival outcomes in external validation set,which is among the strong points of the current nomogram.However,when correlating actual disease-specific survival and calculated 1-year diseasespecific survival,the survival curves between high-risk group and intermediate-risk group crossed in the external validation data (Figure 4).We think the statistical power is a bit lower due to the small number of event cases in the high-risk group in the external data.Even though the p-value of external validation set increased compared to development set,considering P-value less than 0.05,the prediction effect seems to be useful.It was thought to be meaningful that we performed external validation with multinational data to evaluate generalizability as well as internal validation for our prediction model.
However,when we tried the GND goodness-of-fit test in the survival setting,the number of events was too small to calculate,therefore,the P-value calculation for accuracy between predicted value and actual value seems to be a limitation in this study.The accuracy and clinical application of this nomogram should be re-assessed in prospective large volume-patient cohorts.To the best of our knowledge,the preoperatively detectable parameterbased nomogram to predict 1-year disease-specific survival is the first to be developed in the era of pancreatectomy following neoadjuvant treatment for managing pancreatic cancer.We hope that the present nomogram could provide crucial information in the decision-making process for the management of pancreatic patients who underwent neoadjuvant treatment.
There are several limitations in the present study.First,this was a retrospective study with a limited number of patients.In addition,initial radiological resectability (26)and various different neoadjuvant protocols were not considered in the analysis.Recently,preoperative comorbidity index (27,28) and nutritional parameters (29)have been reported to be independent prognostic factors in patients who underwent pancreatectomy following neoadjuvant treatment.The accuracy and clinical application of this nomogram should be re-assessed in prospective large volume-patient cohorts.Further,more accurate and reliable methods to calculate and predict survival need to be developed to improve patient-oriented surgical approach to pancreatic cancer.Particularly,in the medical field influenced by the 4th Industrial Revolution,such methods are predicted to be developed via data-based artificial intelligent algorithms.The continued increase in available data and emerging computational methods indicate that precision public health is highly likely to be available in the future (30).
Conclusions
The nomogram to predict 1-year disease-specific survival for patients with pancreatic cancer who are eligible for surgery following neoadjuvant treatment showed accuracy and clinical feasibility for decision-making in the management of pancreatic cancer.
Acknowledgements
This study was supported by a faculty research grant of Yonsei University College of Medicine for 6-2015-0053.
Footnote
Conflicts of Interest:The authors have no conflicts of interest to declare.
 Chinese Journal of Cancer Research2020年1期
Chinese Journal of Cancer Research2020年1期
- Chinese Journal of Cancer Research的其它文章
- Advances and challenges in immunotherapy of small cell lung cancer
- Spectral CT imaging parameters and Ki-67 labeling index in lung adenocarcinoma
- PGC-MG7 combination could be used as a follow-up panel for monitoring dynamical progression of gastric precancerous diseases
- Tumor-associated macrophages regulate gastric cancer cell invasion and metastasis through TGFβ2/NF-κB/Kindlin-2 axis
- A CT-based radiomics nomogram for prediction of human epidermal growth factor receptor 2 status in patients with gastric cancer
- Prognostic impact of D2-plus lymphadenectomy and optimal extent of lymphadenectomy in advanced gastric antral carcinoma:Propensity score matching analysis
