Microbiota and Shelf Life of Whole and Gutted Pacific Saury (Cololabis saira) During Refrigerated Storage
CAO Rong , LIN Ruihuan, SUN Huihui and LIU Qi
1) Yellow Sea Fisheries Research Institute, Chinese Academy of Fishery Sciences, Qingdao 266071, China
2) Laboratory for Marine Drugs and Bioproducts of Qingdao National Laboratory for Marine Science and Technology, Qingdao 266071, China
3) College of Food Science and Technology, Shanghai Ocean University, Shanghai 200090, China
Abstract Pacific saury (Cololabis saira) is usually sold as whole fish in wholesale markets, or in its gutted form which is easy for consumers to cook in retail markets. In order to assess the effect of gutting on the shelf life of Pacific saury during refrigerated storage and reveal the microbial community, fish samples grouped into (I) whole fish and (II) gutted fish were analyzed periodically for sensory, biochemical and microbiological characteristics, and high-throughput sequencing technology was used to investigate the microbiota. Results showed that the sensory score for the gutted fish became unacceptable on day 8, while the whole fish score remained acceptable for 10 days. The total volatile basic nitrogen (TVB-N) value of the gutted fish reached 30 mg N (100g) -1 on day 6,while that of the whole samples surpassed 30 mgN(100g) -1 on day 10. The thiobarbituric acid reactive substances (TBARS) value of the gutted fish got close to 5.0 mg kg-1 on day 10, while that of the whole samples surpassed 5.0 mg kg -1 on day 14. The aerobic plate colony counts (APCs) for the gutted and whole Pacific saury reached 7.0 log10 CFUg-1 on days 6 and 10, respectively. Organoleptic,biochemical and microbiological analyses revealed that shelf-lives should be 6–8 days for gutted fish and 10 days for whole fish.Microbiota analysis results showed that gutting partly changed the initial microbiota, but didn’t alter the dominant bacteria during storage. When the fish were spoiled, high proportion of Pseudomonadaceae was detected in both groups.
Key words Cololabis saira; gutting; shelf life; microbiota
1 Introduction
Pacific saury (Cololabis saira) is widely distributed in the North Pacific Ocean and is the only member of the family Scomberesocidae that has been intensively harvested from the 17th century to present (Baitaliuk et al.,2013). Pacific saury is one of the most popular fish in Asia due to its special taste and flavor, high-quality protein, and high content of polyunsaturated fatty acids such as eicosapentaenoic acid (EPA) and docosahexaenoic acid(DHA).
Aquatic products such as fish and shellfish are perishable. Freshness is one of the most important contributors to the overall quality of fish as food. Various food preservation techniques are being studied to extend the shelf life of seafood, such as freezing, modified atmosphere packaging, chemical or biological preservatives, and some novel techniques (Mañas and Pagán, 2005). However, the mechanism of fish spoilage is not entirely clear.Among the factors that influence the spoilage of fish,microorganism has been confirmed as a crucial one.Gram and Dalgaard (2002) proposed that the ‘spoilage’microbiota is composed of microorganisms that contribute to spoilage as well as those that have grown but cause no unpleasant changes. Accurate microbiota analyses will help to furtherly reveal the process of fish spoilage. Most previous studies on fish microbiota were based on traditional culturing methods. However, most microorganisms are nonculturable, therefore experimental data based on culture methods cannot clarify the microbiota accurately(Caporaso et al., 2012). Molecular biological techniques,such as real-time polymerase chain reaction (real-time PCR), denaturing gradient gel electrophoresis (DGGE),and terminal restriction fragment length polymorphism(TRFLP), present advanced capabilities, but still cannot fully examine the microbial species and their proportions in specific samples. Nowadays, emerging high-throughput sequencing (HTS) technology is attracting much attention for its efficiency and accuracy in microbiota analyses (Parlapani et al., 2018).
Fish quality during storage has been studied in many species over the past decade, including freshwater and marine fish (Cao et al., 2009; Peter, 2010; Sampels, 2015).In China, Pacific saury is usually sold as whole fish in wholesale markets, or in its gutted form which is easy for consumers to cook in retail markets. To date, only limited information is available in the literature on quality changes and shelf life assessments of Pacific saury in different forms during storage, not to mention the microbiota aspect. The present paper reports a quality and shelf life assessment of (I) whole untreated and (II) gutted Pacific saury during refrigerated storage by evaluating sensory, chemical and microbiological changes. The two groups’ microbiotas are also reported.
2 Materials and Methods
2.1 Fish Samples
The Pacific saury (Cololabis saira) used in this study were obtained from Penglai Zhongbai Ship Industry Co.,Ltd. (a leading fishery enterprise located in Shandong Province). The fish were captured in the north Pacific region from October to November 2016 and were frozen immediately using quick-freeze equipment (chilling temperature -35℃) on the fishing vessel, then preserved and transported below -28℃. It took less than two months for the fish to be transported to the dock after capture. When the transport-vessels reached the dock, the fish used for experiment were transported to the laboratory immediately.
2.2 Sample Preparation
After arriving at the laboratory, a whole plate of frozen fish totaling more than 200 specimens were vacuumpacked and thawed with running water. After thawing, all fish were cleaned under running water, then divided into two lots and one lot was gutted.
The gutting was performed by skilled personnel, simulating a procedure currently used in most fisheries. Fish were immediately sampled (day 0), while the others were stored in a refrigerator (2 ± 1℃) for 14 d. Fish were randomly chosen for each sampling and examined at 48-hour intervals for changes in sensory attributes, biochemical indicators and bacterial counts.
2.3 Sensory Evaluation
The sensory properties of Pacific saury were measured by a panel of 6 trained assessors from the Food Engineering and Nutrition Department by the methods proposed by Sallam et al. (2007). Fish samples were assessed based on appearance, odor and texture characteristics.Each characteristic was evaluated using a three-point scale (0, dislike extremely to 3, like extremely). The overall score was between 0 and 9, and acceptability was determined as having a score over 6. The data from 6 independent panelists were pooled, and points represented mean values of six measurements ± standard deviation.
2.4 Biochemical Tests
Total volatile basic nitrogen (TVB-N) was measured by a microdiffusion method using a Conway’s unit.
Lipid oxidation was assessed by measuring thiobarbituric acid-reactive substances (TBARS) and was expressed as milligrams of malonaldehyde (MDA) per kilogram of fish meat (Ke et al., 2010).
2.5 Bacterial Counts
Fish were sampled aseptically in a vertical laminarflow cabinet, 10.0 g of fish meat was transferred to a stomacher bag containing peptone water. Samples were homogenized for 60 s. Ten-fold serial dilutions were made.Then, 0.1 mL of solution was spread on marine agar plates. After incubation at 30℃ for 48 h, aerobic plate counts (APC) were determined by counting the number of colony-forming units.
2.6 Microbiota Analysis
The total microorganismal DNA from fish meat was extracted via a PureLink Microbiome DNA Purification Kit (Thermo Fisher Scientific Co., Ltd., Shanghai, China).Target DNA was amplified by polymerase chain reaction(PCR) following the protocol described previously(Caporaso et al., 2011). A specific primer set was used to amplify the V3+ V4region of the 16S rDNA gene (Bergmann et al., 2011). The tag-encoded high-throughput sequencing was conducted using an Illumina sequencing platform (Guangzhou Genedenovo Biotechnology Co.,Ltd., Guangzhou, China).
The raw sequencing data were analyzed and refined through the quality clinic process chart provided by QIIME 1.6.0 software (Caporaso et al., 2010) to guarantee a higher level of accuracy in detecting the operational taxonomic units (OTUs). The final effective tags were obtained after detecting and removing chimeric sequences(Bokulich et al., 2013). OTUs defined by a 97% similarity were picked using UPARSE software (Edgar, 2013).The representative sequences were submitted to the Ribosomal Database Project (RDP) classifier (Wang et al.,2007) to obtain the taxonomic assignments for phylum,class, order, family and genus.
Based on the species annotation results and richness information, the top 42 families were selected for the cluster analysis on both microbial species and specimen levels. Fast-tree software (Price et al., 2009) and R-languages were used to draw the heat map.
2.7 Statistical Analysis
Results are presented as mean values ± standard deviation (n = 2×3). The program used for statistical evaluation was SYSTAT, version 11 (StatSoft Inc., Beijing, China).P value less than 0.05 was considered as significant different between experimental groups and storage periods.
3 Results and Discussion
3.1 Shelf Life Determination
3.1.1 Sensory properties
The acceptability of fish depends largely and directly on their sensory attributes. Changes in sensory scores for the Pacific saury during storage are shown in Fig.1. No significant differences in sensory scores were detected between the group of (I) whole fish and (II) gutted fish before storage (P > 0.05), indicating that no organoleptic disadvantage resulted from the gutting treatment. During storage, sensory scores gradually decreased for both groups. No significant differences between the two groups were noticed during the first 4 d; however, after day 4, scores sharply decreased for the gutted fish and became unacceptable on day 8, while the whole fish scores decreased more slowly, remaining acceptable for up to 10 days.
The primary cause of the decreased sensory scores was the odor deterioration, and the secondary cause was the altered appearance. This may be because Pacific saury are rich in unsaturated fatty acids, and gutting may accelerate the oxidation rate of fish lipids by increasing the surface area exposed to air (Cao et al., 2009).
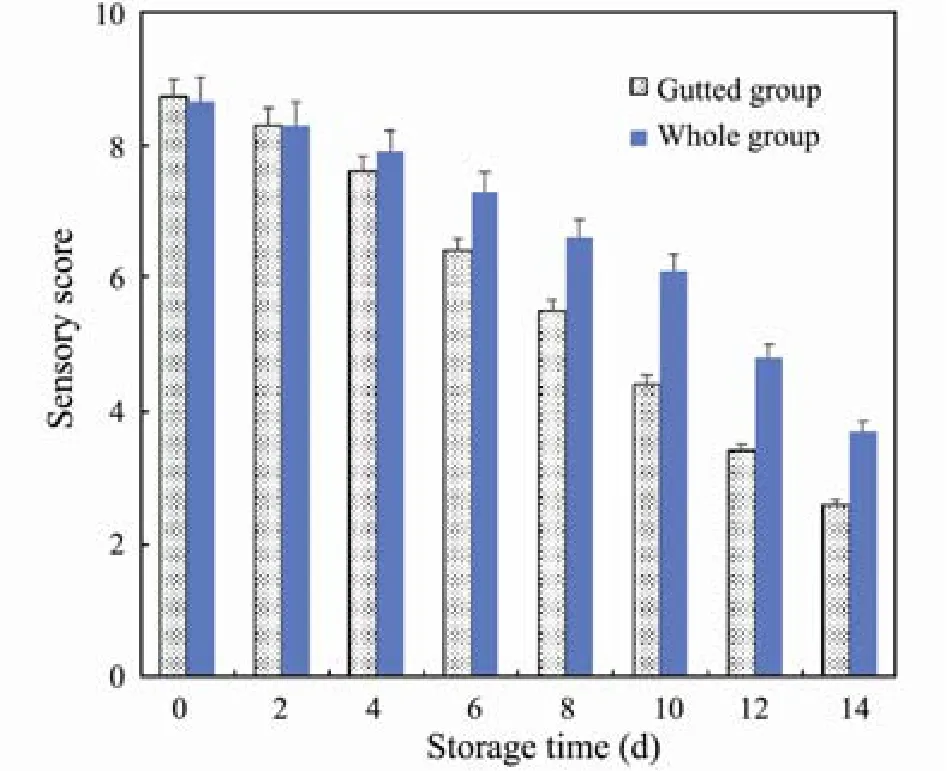
Fig.1 Changes in sensory scores for (I) whole and (II) gutted Pacific saury during refrigerated storage.
Sensory methods are simple, fast, and provide immediate quality information (Paulo et al., 2008); however, they have some disadvantages in that human sensory responses can vary, particularly with fatigue or outside distractions.Thus, non-sensory methods based on biochemical, physical and/or microbial analyses are necessary to comprehensively evaluate fish quality.
3.1.2 Biochemical analysis
TVB-N has been widely reported as a fish spoilage indicator, which is mainly composed of trimethylamine,dimethylamine, ammonia and other volatile basic nitrogen compounds, resulting from the degradation of proteins and nonprotein nitrogenous compounds that mainly result from microbial activity (Ruiz-Capillas, 2001).Changes in TVB-N values of Pacific saury during storage are shown in Fig.2a. No significant differences (P > 0.05)were detected between the two lots on days 0 and 2. From day 4, TVB-N values of the gutted fish increased rapidly,while TVB-N values of the whole samples increased at a relatively slower rate. TVB-N value of 30 mgN (100g)-1is considered the spoilage level, above which, fish products are considered unfit for human consumption (Campos et al., 2005). TVB-N values of the gutted fish got close to 30 mgN (100g)-1on day 6, while those of the whole samples surpassed 30 mgN (100g)-1on day 10.
The TBARS value represents the degree of lipid oxidation. The presence of TBA-reactive substances arises from a series of secondary reactions, which follow the primary auto-oxidation process and lead to the decomposition of lipid hydroperoxides to aldehydes and ketones(Ke et al., 2010). Changes in TBARS values for the Pacific saury during storage are shown in Fig.2(b). TBARS values from both lots increased over the 14-day storage period. The present results indicate that the lipid oxidative rancidity remained relatively low in the whole Pacific saury samples. Similarly, lower TBARS values have been reported for other fish species in their whole form, such as tilapia (Oreochromis niloticus) (Cao et al., 2009) and sea bass (Dicentrarchus labrax) (Papadopoulos et al.,2003). Higher TBARS values of gutted fish samples are likely due to the gutting process, which exposes fish lipids to atmospheric oxygen, thus accelerating oxidative rancidity. A TBARS value of 5.0 mgkg-1is normally regarded as the spoilage level of fatty fish (Beyza et al.,2008), above which, fishery materials and products will develop offensive odors and tastes for human consumption. The TBARS value of the gutted fish got close to 5.0 mgkg-1on day 10, while that of the whole samples surpassed 5.0 mgkg-1on day 14. TBARS alone should be considered as a rather poor freshness indicator for Pacific saury, because of its lag response compared with the sensory scores and TVB-N values.
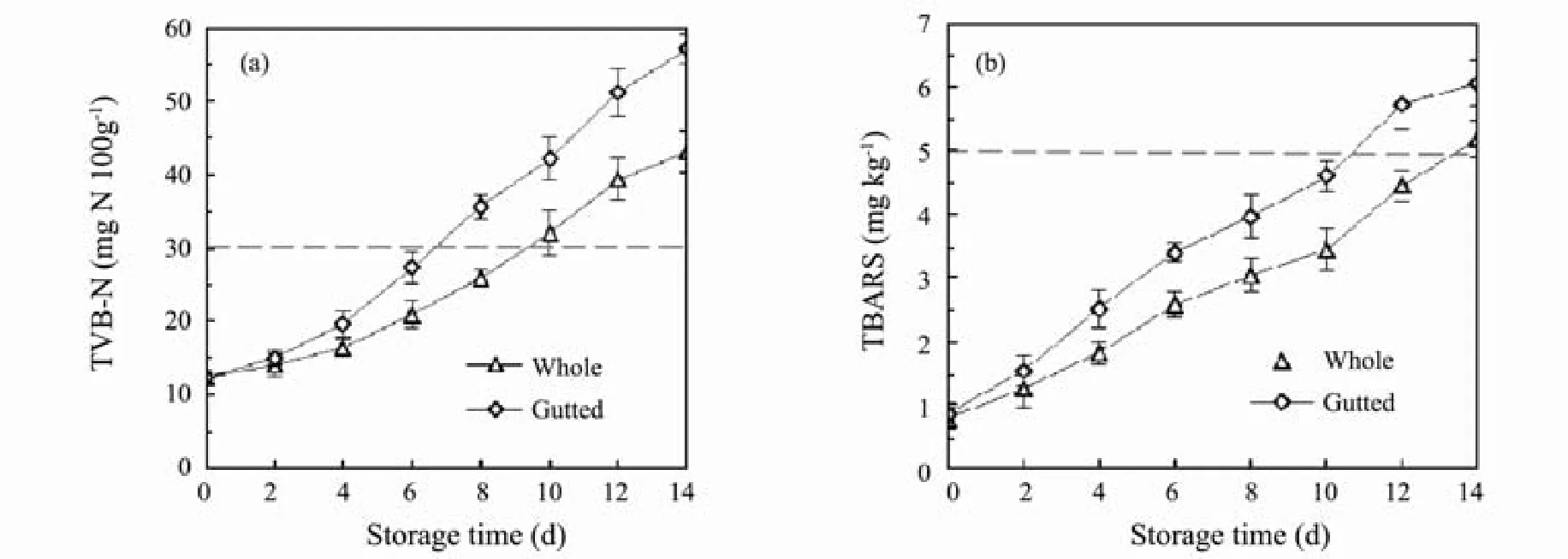
Fig.2 Changes in (a) TVB-N values and (b) TBARS values of (I) whole and (II) gutted Pacific saury during refrigerated storage.
3.1.3 Bacterial counts
Changes in APC of the Pacific saury during refrigerated storage are shown in Fig.3. Many microorganisms exist in the internal organs of fish. Theoretically, gutting could reduce the initial bacterial load and delay the quality deterioration caused by microorganisms during storage. However, experiment results showed that the initial APC of the gutted fish was slightly higher than that of the whole samples, which were approximately 3.75 log10CFUg-1and 3.29 log10CFUg-1, respectively (day 0). This difference may be attributed to the gutting procedures(processing tables and knives) and the bigger fish flesh surface area exposed to environmental microbial contamination (Cao et al., 2009).
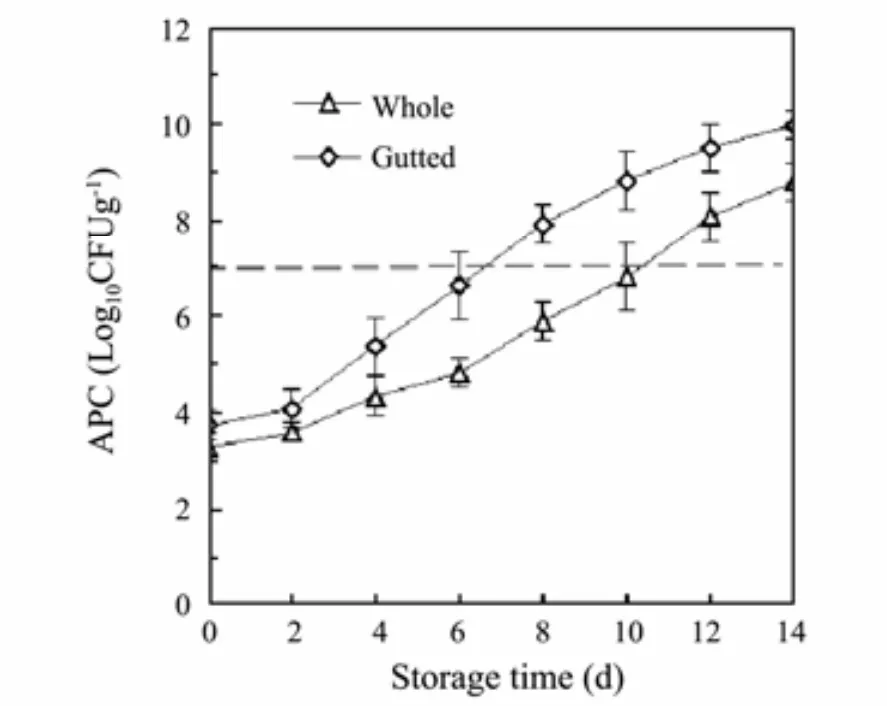
Fig.3 Changes in APC of (I) whole and (II) gutted Pacific saury during refrigerated storage.
During the 14-day storage period, continuous increases in APC were detected in both lots. No significant differences in APC were observed between the two lots during the first 4 d of storage. However, over the next 8 d, bacterial counts in the gutted fish were approximately 1-2 log cycles higher (P < 0.05) than those of the whole samples.This may be attributed to the relatively higher initial APCs of the gutted samples. More importantly, the gutting treatment can affect the microorganismal composition, which could influence the APC greatly, as different bacteria have different growth rates under specific conditions. For marine fish, 7.0 log10CFUg-1is considered as the normal acceptability level. The APC of (I) whole and(II) gutted Pacific saury got close to this level on days 10 and 6, respectively.
Comprehensively considering the sensory, biochemical and microbiological indicators, the shelf life was determined to be 6–8 d for gutted Pacific saury and 10 d for the whole fish under refrigerated storage.
3.2 Microbiota Characteristic
3.2.1 PCR amplification results
Bacterial 16S rDNA is the ideal molecular label for species identification due to its appropriate molecular size,species specificity and low mutation rate. Fig.4 shows the electrophoretogram of amplification of the V3+ V4zone of the 16S rDNA. The average lengths of the PCR products’ valid target bands were within the sequence length scope of the 16S rDNA V3+ V4zone.

Fig.4 Electrophoretogram of PCR amplification products.M represents the 100-bp DNA ladder, loading amount 2 μL; 1-6 represent the whole fish sample number, responding to storage times of 0, 2, 4, 6, 8 and 10 d, respectively, loading amount 5 μL for each; 7-11 represent the gutted fish sample numbers, responding to storage times of 0, 2, 4, 6 and 8 d, respectively, loading amount 5 μL for each.
3.2.2 Species annotation
Valid tags were obtained after filtering low-quality reads and trimming adapters, barcodes, and primers. For each sample, the tags number was more than 60000, and the OTU number was in the range of 617 to 958. Fig.5 shows the species annotation results at the family level.The dominant bacteria of the whole Pacific saury on day 0 belonged to Pseudomonadaceae (43.4%). Enterobacteriaceae (6.8%) and Rhodospirillaceae (6.0%) played subordinate roles. The numbers and taxonomic composition of the bacterial populations often relate to those of the surrounding environment (Austin, 2018) and can be influenced by many factors such as harvest season and capture method (Gram and Huss, 1996). Gram-negative bacteria have been reported as the dominant microorganism species in fresh aquatic products. Gennari et al. (1998)found that the microbiota of fresh sardine was composed by non-fermenting Gram-negative bacteria, such as
Pseudomonas, Flavobacterium, Psychrobacter, Acineobacter and Shewanella. During the first 6 d, the proportion of Pseudomonadaceae fluctuated and ranged from 35.8% to 53.4%. This fluctuation indicated that the growth of Pseudomonadaceae might be influenced by other kinds of bacteria. From day 8, the proportion of Pseudomonadaceae increased to more than 95%. This rapid increase of proportion was largely related to Pseudomonadaceae’s strong tolerance to low temperature.Moreover, Pseudomonadaceae can cause offensive odors by producing ammonia, H2S and TMAO (Dalgaard,1995). Pseudomonadaceae played important roles in deteriorating the fish quality during refrigerated storage.Therefore, treatment that can inhibit or delay the growth and metabolism of Pseudomonadaceae will help to improve the storage quality and extend the shelf life of fish.
For the gutted fish, the dominant bacteria were also Pseudomonadaceae. Although the proportion of Pseudomonadaceae on day 0 was slightly lower than that of the whole fish, the proportion increased rapidly and continually during storage. On day 8, the proportion of Pseudomonadaceae surpassed 90%. This unconstrained Pseudomonadaceae growth suggests that gutting did not inhibit or delay Pseudomonadaceae growth. On the contrary,gutting partly accelerated Pseudomonadaceae growth.The cause might be that gutting destroyed the Pacific saury’s initial microbiota by removing some bacteria with antagonistic effects against Pseudomonadaceae.
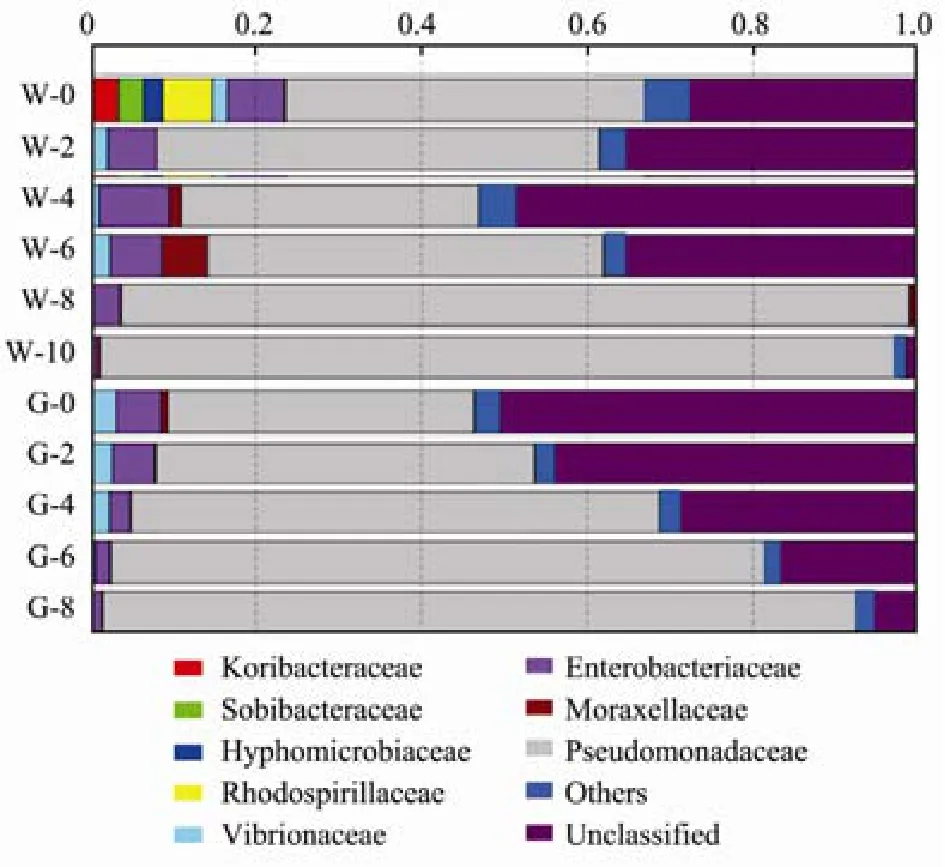
Fig.5 Bacterial species annotation of Pacific saury at the family level. ‘Others’ represent species with abundances less than 2%; tags that could not be annotated to the ‘family’ level were categorized as ‘unclassified’; ‘W’ on the vertical ordinate represents the whole fish samples; the numbers indicate storage times; ‘G’ on the vertical ordinate represents the gutted fish samples; the numbers indicate storage times.
3.2.3 OTU clustering heat map
OTU clustering heat maps can be applied to identify differences and similarities among bacterial communities in different fish samples. As shown in Fig.6, the top 42 bacterial families were selected to construct the heat map.For the whole fish, the proportions of Mycoplasmataceae,Caulobacteraceae, Gemmataceae, Leuconostocaceae,Rhodospirillaceae, Koribacteraceae, Solibacteraceae, Ktedonobacteraceae, Hyphomicrobiaceae and Alcaligenaceae decreased dramatically in the spoiled fish samples, potentially due to their poor tolerance of low temperatures.Pseudomonadaceae could grow at low temperatures and became dominant. Gutting clearly changed the microbiota,as the proportions of Isosphaeraceae, Lactobacillaceae,Methylobacteriaceae, Vibrionaceae and Xanthomonadaceae increased in the gutted samples. However, these bacteria did not affect Pseudomonadaceae proliferation.The dominant bacteria in the gutted fish were also Pseudomonadaceae.
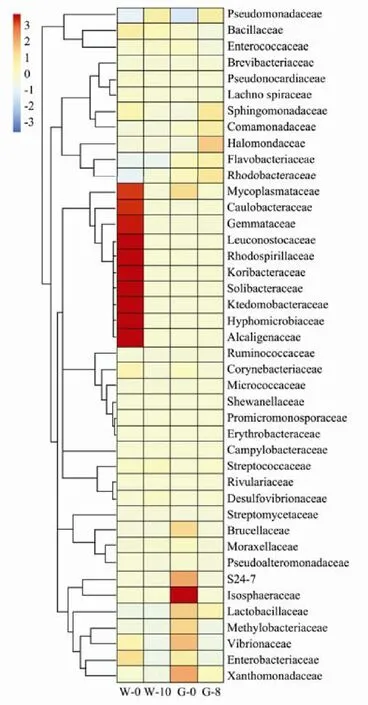
Fig.6 OTU clustering heat map on family level. The color intensity in the same line represents the proportional discrepancies in the bacteria in the fish samples; ‘W’ on the vertical ordinate represents the whole fish samples; the numbers indicate storage times; ‘G’ on vertical ordinate represents the gutted fish samples; the numbers indicate storage times.
4 Conclusions
Based on sensory, biochemical and microbial analyses,the shelf life of Pacific saury stored at 2 ± 1℃ was determined to be 10 d for whole fish and 6–8 d for gutted fish.Gutting slightly increased the initial APC of the fish,which was mainly caused by the gutting procedures and the bigger fish flesh surface area exposed to environmental microbial contamination. Pseudomonadaceae was dominant in the initial microbiota of whole Pacific saury and played a leading role in the spoilage progress of fish.Gutting partly changed the microbiota, but did not inhibit or delay the growth of Pseudomonadaceae. Pseudomonadaceae was the dominant family in both whole and gutted fish during storage.
Acknowledgements
Financial support from the National Key R&D Program of China (No. 2018YFD0901004) and Central Public-interest Scientific Institution Basal Research Fund,YSFRI, CAFS (No. 20603022018028) are greatly acknow- ledged.
 Journal of Ocean University of China2020年2期
Journal of Ocean University of China2020年2期
- Journal of Ocean University of China的其它文章
- Genome Sequence of a Marine Carotenoid Producing Yeast Rhodotorula mucilaginosa CYJ03
- Researches on the Internal Molecular Weight Uniformity of Chitosan Biomaterials
- Transcriptome Responses of Hygromycin B Resistance Gene-Transformed, Hygromycin B-Adaptive and Wild Nannochloropsis oceanica Strains to Hygromycin B
- Stock Structure Analysis of the Japanese Spanish Mackerel Scomberomorus niphonius (Cuvier, 1832) Along the China Coast Based on Truss Network
- Comparative Analysis of Nutrient Composition of Caulerpa lentillifera from Different Regions
- Triterpene-Enriched Olive Extract as an Immunopotentiator in Black Sea Bream (Acanthopagrus schlegelii)
