Strontium Isotope Compositions of Hydrothermal Barite from the Yonaguni IV: Insight into Fluid/Sediment Interaction and Barite Crystallization Condition
ZHANG Xia, ZHAI Shikui, and YU Zenghui
Key Laboratory of Submarine Geosciences and Prospecting Techniques, Ministry of Education, College of Marine Geosciences, Ocean University of China, Qingdao 266100, China
(Received September 27, 2018; revised May 29, 2019; accepted September 16, 2019)
© Ocean University of China, Science Press and Springer-Verlag GmbH Germany 2020
Abstract Hydrothermal barite is a typical low-temperature mineral formed during the mixing of hydrothermal fluid and seawater.Because of its extremely low solubility, barite behaves as a close system after crystallization and preserves the geochemical fingerprint of hydrothermal fluid. In this study, the elemental contents and Sr isotope compositions of hydrothermal barites from the Yonaguni IV were determined using electron microprobe and LA-MC-ICP-MS respectively. On these bases, the fluid/sediment interaction during the hydrothermal circulation and physicochemical condition of barite crystallization were discussed. Results show that the 87Sr/86Sr values of hydrothermal barites from the Yonaguni IV are apparently higher than those of the seawater and associated volcanic rocks, indicating the sufficient interaction between the hydrothermal fluid and overlying sediment. Monomineral Sr abundance shows large variations, reflecting the changes in barite growth rate during the fluid mixing. The mineralization condition in the Yonaguni IV was unstable. During the crystallization of barite, hydrothermal fluid and seawater mixed in varying degrees, with the proportions of hydrothermal fluid varied from 36% to 72%. The calculated crystallization temperatures range from 109 to 220℃. Sediment plays a critical role during the mineralization process in the Yonaguni IV and incorporation of sediment component into hydrothermal system was prior to barite crystallization and sulfide mineralization.
Key words strontium isotope; hydrothermal barite; fluid/sediment interaction; crystallization condition; Okinawa Trough
1 Introduction
Barite, a common hydrothermal mineral in the back-arc basin hydrothermal fields, is generally formed in the lowtemperature stage of mineralization (Tivey et al., 1990).As a key structural component to hydrothermal edifices,its occurrence and abundance can affect hydrothermal deposit morphology, chimney growth and the retention of metals within a deposit (Tivey and Delaney, 1986; Hannington et al., 1995). Hydrothermal barite was formed during the mixing of barium-rich fluid and sulfate-rich seawater(Damn, 1990; Hannington et al., 2005; Humphris and Bach,2005). Because of its extremely low solubility in seawater,barite behaves as a close system and is not prone to diagenetic alteration after crystallization (Averyt and Paytan,2003; Widanagamage et al., 2014), preserving the geochemical fingerprint of hydrothermal fluid. Thus, geochemical compositions of barite can indicate the regional mineralization characteristics and physicochemical conditions of barite crystallization (Jamieson et al., 2016).
Due to the common substitution of Sr to Ba during barite crystallization under hydrothermal condition, the contents of Sr are generally high (reach up to 4.6%; Hanor, 2000)in natural barite, which makes it possible to determine the Sr isotope compositions in barite. Strontium isotope compositions of barite in hydrothermal fields have been studied sufficiently, which revealed the fluid mixing process,source of mineralization materials and fluid/sediment interaction (Marchev et al., 2002; Paytan et al., 2002; Cardellach et al., 2003; Noguchi et al., 2011; Staude et al., 2011;Jamieson et al., 2016). While the Sr isotope research about hydrothermal barite in the Okinawa Trough is extremely poor, only Noguchi et al. (2011) reported the Sr isotope compositions of hydrothermal barite from the JADE hydrothermal field. Up to now, no Sr isotope research about hydrothermal barite in the Southern Okinawa Trough has been carried out. Yonaguni IV hydrothermal field is located in the southern Okinawa Trough. Barite-rich Ba-Zn-Pb type hydrothermal deposit was developed in this region, which provides the perfect sample for Sr isotope compositions study of hydrothermal barite. In this study,we report the first data on elemental contents and Sr isotope compositions of barites from the Yonaguni IV. On these bases, the hydrothermal fluid/sediment interaction during the hydrothermal circulation and physicochemical condition of barite crystallization were discussed.
2 Geological Setting
The Okinawa Trough, an important part of the East China Sea, is a tectonically active intracontinental backarc basin generated by the subduction of the Philippine plate under the Eurasian continent (Halbach et al., 1993).The heat-flow values in the Okinawa Tough are extremely high. Since the first discovery of hydrothermal activity in the middle Okinawa Trough in 1984, a large number of hydrothermal fields have been reported gradually.
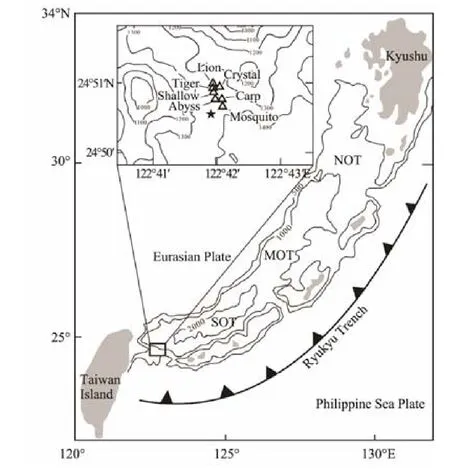
Fig.1 Sampling locations and bathymetric map of the Okinawa Trough (after Gena et al., 2013). The triangles are the major hydrothermal vents in this field and the pentagram is the sample location of this study.
Yonaguni IV hydrothermal field is located in the southernmost part of the Okinawa Trough (Matsumoto et al.,2002; Fig.1). Venting sites in this field are aligned along a 1000 m-long and 500 m-wide elongated valley which was interpreted by Suzuki et al. (2008) to represent a fault that may have controlled the fluid pathways to the seafloor.The valley is surrounded by several seamounts with the maximum water depth up to 1400 m. The local topography within the valley is almost flat and the seafloor is covered by thick brown sediments which are cemented by native sulfur, quartz and barite indicating the influence of hydrothermal fluid. Seven hydrothermal vents (lion, crystal, tiger, swallow, abyss, crap and mosquito) were discovered in this field (Gena et al., 2005), with the highest fluid temperature about 330℃ (Inagaki et al., 2006; Suzuki et al., 2008; Nunoura and Takai, 2009). Between the Tiger and Swallow sites, liquid CO2droplet was observed emerging from the seafloor (Konno et al., 2006). Suzuki et al. (2008) studied the mineralogy of hydrothermal chimney from lion, crystal, tiger and swallow and recognized five types of hydrothermal deposits: i) anhydrite-rich chimneys; ii) massive Zn-Pb-Cu sulfides; iii) Ba-As chimneys;iv) Mn-rich chimneys; v) pavement, silicified sediment.They proposed the diverse series of mineralization was the result of phase separation.
3 Sampling and Analytical Methods
The hydrothermal deposit sample studied in this paper was grabbed from the Yonaguni IV hydrothermal field(122˚41΄55˝E, 24˚50΄26˝N) during the HOBAB3 cruise in 2014 with water depth of 1390 m. Mineralogically, the sample is similar to the Ba-As chimneys recognized by Suzuki et al. (2008) in this region, which mainly consist of barite, sphalerite and galena.
The hydrothermal sample was firstly ultrasonic cleaned in distilled water for 45 min to remove the effect of seawater and sediment contamination. Then the dried sample was cut into thin sections and doubly-polished for electron microprobe and LA-MC-ICP-MS analyses. The elemental abundances of barites were determined in the Key Laboratory of Submarine Geosciences and Prospecting Techniques, Ministry of Education, by JXA-8230 Electron Microprobe. The instrument parameters were acceleration voltage of 15 kV, a beam current of 20 nA and beam diameter of 3 μm. The Sr isotope compositions of hydrothermal barites were analyzed in State Key Laboratory of Isotope Geochemistry, Guangzhou Institute of Geochemistry,Chinese Academy of Sciences using Neptune Plus LAMC-ICP-MS and the analyzing method followed Zhang et al. (2018a). The laser parameters were set as follow:beam diameter, 33 μm; repetition rate, 6 Hz; energy density,4 J cm-2. Helium was chosen as the carrier gas (800 mL min-1). Each analysis consisted of 250 cycles with an integration time of 0.262 s per cycle. The first 30 s was used to detect the gas blank with the laser beam off, followed by 30 s laser ablation for sample signals collection with laser beam on. During the measurement of this study, the gas blank of83Kr and88Sr were less than 2.5 mv and 0.5 mv. The interferences of84Kr and86Kr on84Sr and86Sr were corrected by subtracting gas blank from the raw time-resolved signal intensities. Seven Faraday cups, on a Neptune Plus MC-ICP-MS instrument, were used to receive the signals on m/z 82, 83, 84, 85, 86, 87 and 88 simultaneously for the Sr isotope analysis of hydrothermal barite.85Rb was used to correct the interference of87Rb on87Sr with a natural85Rb/87Rb = 2.593 (Catanzaro et al., 1969). The instrumental mass fraction of87Sr/86Sr was corrected to86Sr/88Sr = 0.1194 with an exponential law. Finally, the residual analytical biases of87Sr/86Sr were corrected with a relationship between the deviations of87Sr/86Sr from the reference values.
4 Results
Barites within hydrothermal deposit sample from the Yonaguni IV occur as two types of crystal habits, wellformed tabular and needle-like crystal, with sizes varying from 50 to 300 μm. Later-formed sulfide minerals fill in the interface of barite (Fig.2). The analysis results of monomineral elemental abundance (Table 1) show that the contents of BaO in the barite are relatively homogeneous(62.59% – 64.81%), while the contents of SrO have a large variation between 0.30% and 2.54%. The contents of CaO are extremely low (lower than 0.4%), indicating that the substitution of Ca to Ba was weak during the barite crystallization.
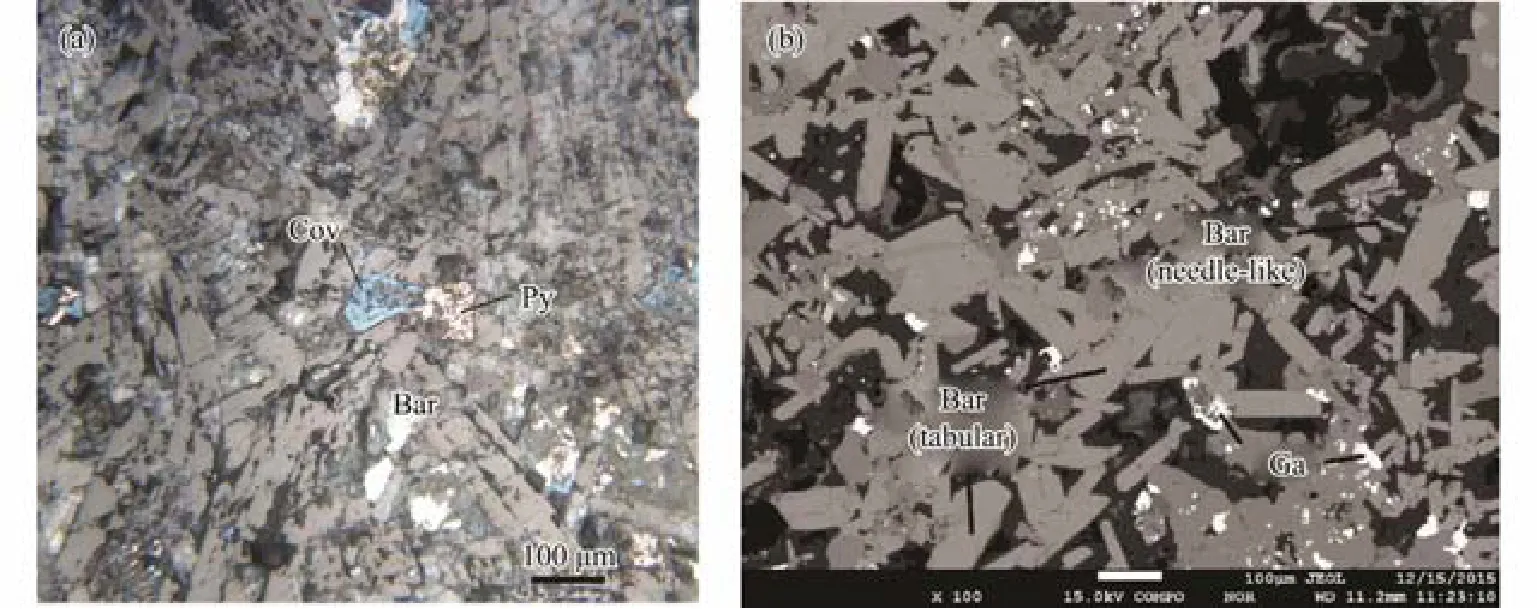
Fig.2 (a) Reflection light and (b) backscatter scanning photomicrographs of hydrothermal deposit from the Yonaguni IV.Bar, barite; Cov, covellite; Ga, galena; Py, pyrite.
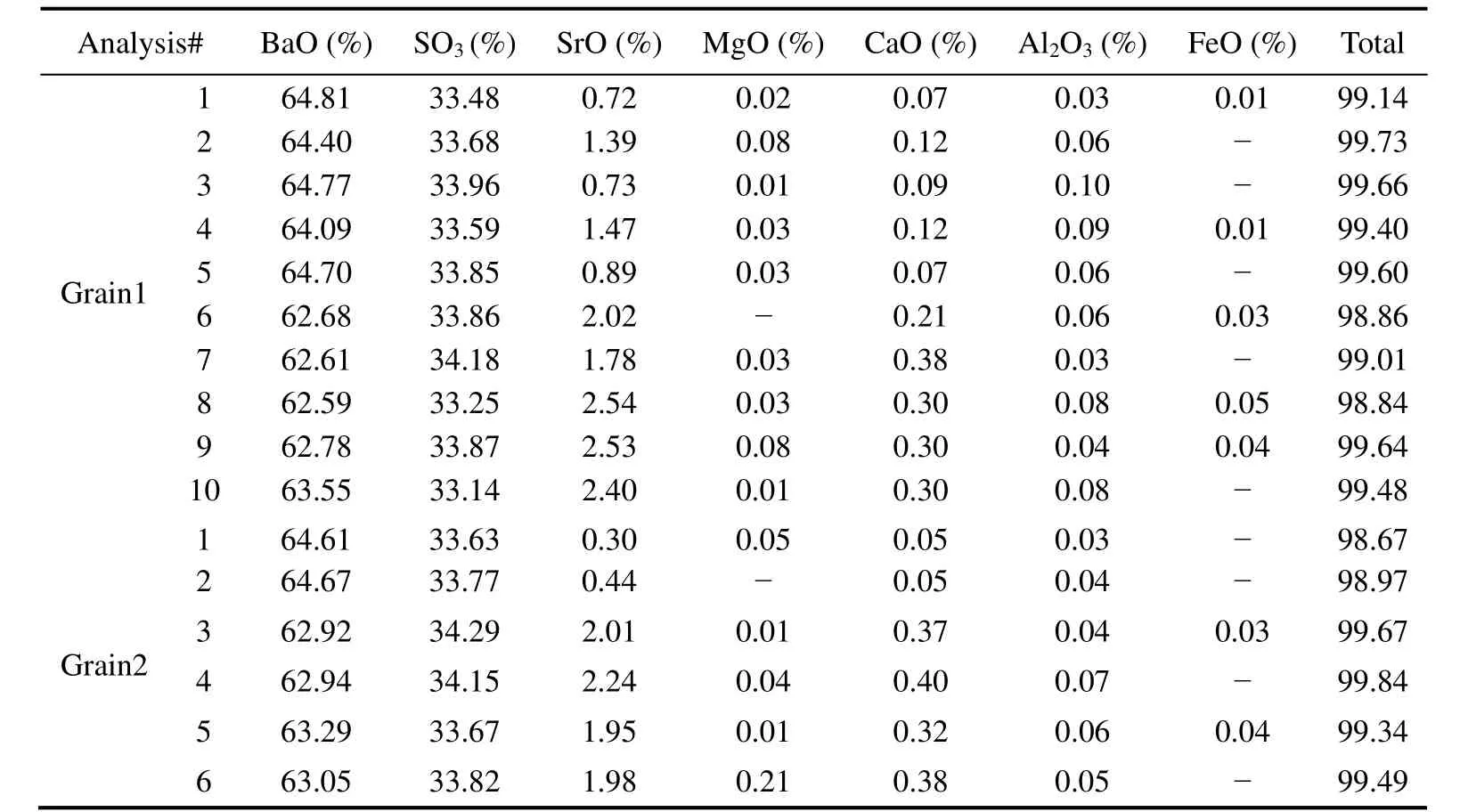
Table 1 Electron microprobe analysis results of elemental contents across single barite
The Sr isotope compositions of barites show large variation, with the87Sr/86Sr values varying from 0.71284 to 0.71749 (Table 2), which are apparently higher than those of seawater (0.7092; Butterfield et al., 2001) and regional volcanic rocks (0.704035–0.708635; Guo et al.,2018), but fall between the values of seawater and Yonaguni IV sediment (0.72212; Dou et al., 2016). Compared with other hydrothermal fields, barites from the Yonaguni IV also have apparently higher87Sr/86Sr which are the highest values reported up till now (Fig.3).
5 Discussion
5.1 Hydrothermal Fluid/Sediment Interaction
Strontium isotope compositions of barites from the sediment-starve hydrothermal fields generally lie between those of basement rocks and seawater (Fouquet et al., 1993),reflecting the mixing of magmatic and seawater-derived Sr. While the87Sr/86Sr values of hydrothermal barites from the Yonaguni IV are apparently higher than those of seawater and associated volcanic rocks, but fall between the values of seawater and Yonaguni IV sediment. These values suggest the interaction between hydrothermal fluid and local sediment during the hydrothermal circulation.Chiba et al. (1993) reported that the87Sr/86Sr value in the Minami-Ensei Knoll hydrothermal fluid was higher than those in regional volcanic rocks and seawater and proposed that the composition of hydrothermal fluid in this region was affected by the interaction with sediment during the late stage of hydrothermal circulation. Noguchi et al.(2011) found the87Sr/86Sr values of hydrothermal barites from the Hakurei field were higher than that of seawater and attributed it to the interaction between hydrothermal fluid and sediment as well. Yonaguni IV hydrothermal field is covered by thick terrigenous sediment (Dou et al.,2016), which may interact with hydrothermal fluid during the hydrothermal circulation. Suzuki et al. (2008) detected large amounts of NH4+in the Yonaguni IV hydrothermal fluid and found the hydrothermal-altered sediment, providing the incontrovertible evidences for fluid/sediment interaction in this field. Thus, we propose that the abnormally high87Sr/86Sr values of hydrothermal barites from the Yonaguni IV field are the result of fluid/sediment interaction. Fluid/sediment interaction will elevate the Sr isotope ratio in the hydrothermal fluid and lead to the high87Sr/86Sr values of barites. The high Sr isotope ratios of the Yonaguni IV barites imply that the fluid/sediment interaction in this region is very sufficient and sediment might play a critical role during the hydrothermal mineralization.
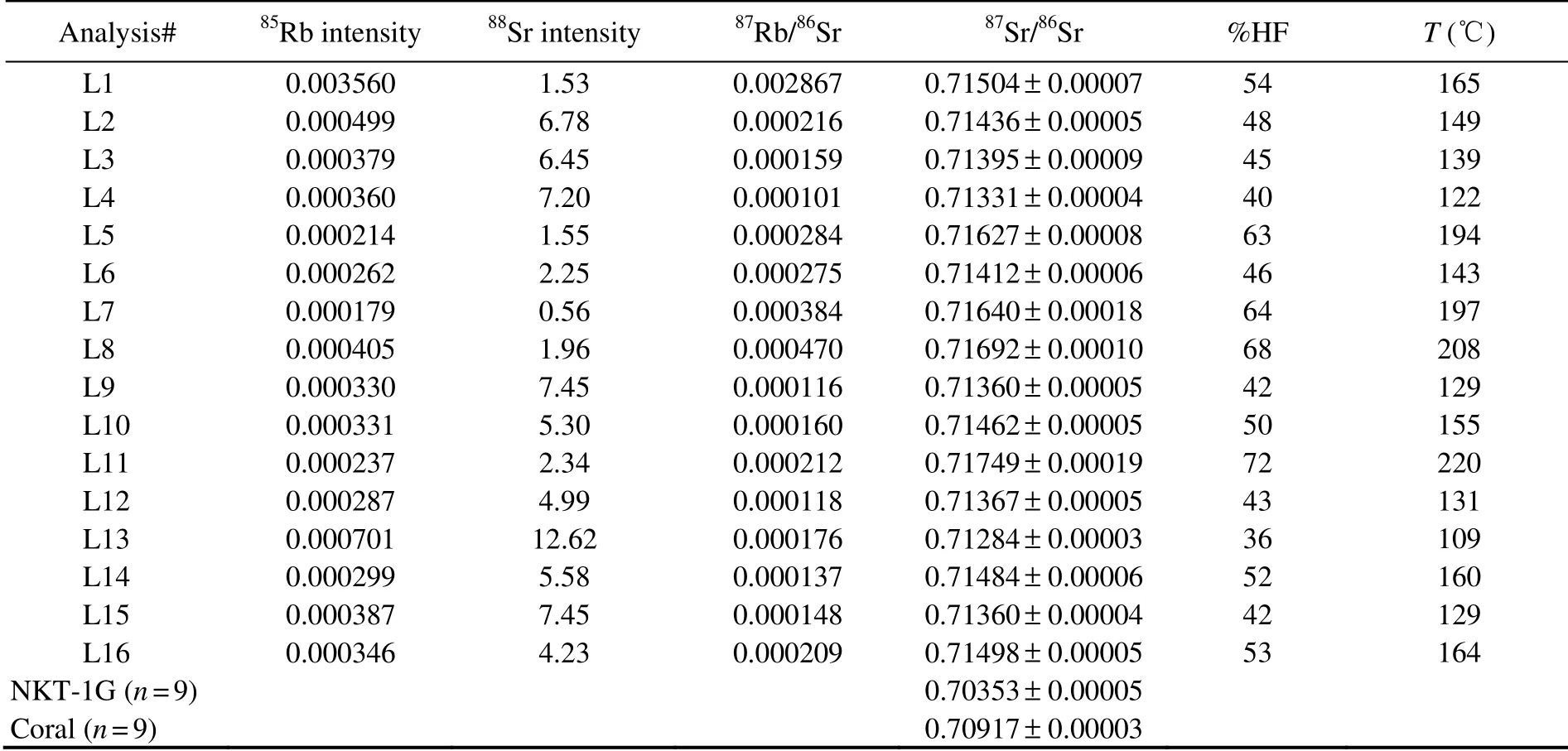
Table 2 LA-MC-ICP-MS analysis results of Sr isotope compositions within the hydrothermal barites and standard references
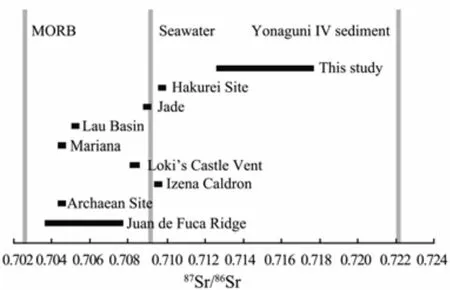
Fig.3 Strontium isotope compositions of hydrothermal barites from the modern hydrothermal fields. Juan de Fuca Ridge (Jamieson et al., 2016); Archaean Site, Izena Caldron, Hakurei Site (Noguchi et al., 2011); Loki’s Castle Vent (Eickmann et al., 2014); Mariana (Paytan et al.,1993); Lau Basin (Fouquet et al., 1993); Jade (Marumo and Hattori, 1999).
5.2 Crystallization Condition of Barite in the Yonaguni IV
5.2.1 Factors controlling Sr abundances in the barite
It’s very common that Sr substitutes Ba into crystal lattice of barite in natural systems, thus, the contents of Sr in barite are generally high (Hanor, 2000). Under hydrothermal environment, trace elements substitution within crystals is controlled by the composition of hydrothermal fluid,pressure, temperature and crystal growth rate (Sasaki and Minato, 1983; Hannington and Scott, 1988; Averyt and Paytan, 2003). Laboratory researches demonstrated that substitution of Sr into barite crystal increases with fluid temperature (Hanor, 2000; Averyt and Paytan, 2003). Alternatively, Shikazono et al. (2012) and Jamieson et al.(2016) linked increase Sr partitioning to higher degrees of saturation and fast crystal growth. Meanwhile, all of these researches have excluded the influence of hydrothermal fluid compositions on Sr contents in hydrothermal barite.Thus, the fluid source is not the controlling factors of Sr contents in barite. The elemental contents in monocrystal of barite show that the contents of Sr in barite have a large variation from the core to rim (Fig.4), demonstrating that the mineralization condition (fluid temperature or crystal growth rate) changed greatly during the barite crystallization, which is consistent with the conclusion obtained from the mineralogy study by Zhang et al. (2018b).
In the plots of88Sr intensity versus87Sr/86Sr values(Fig.5), hydrothermal barites from the Yonaguni IV are aligned along a hyperbola, which is the typical characteristic of two end-members mixing (Faure, 1986), i.e., seawater (with high Sr content and low87Sr/86Sr values)mixed with hydrothermal fluid (with low Sr content and high87Sr/86Sr). This demonstrates that the fluid mixing is the major cause of the variation in Sr contents and87Sr/86Sr values of barites from the Yonaguni IV. Previous researches (Hanor, 2000; Averyt and Paytan, 2003) demonstrated that mineralization fluid temperature decreased with the enhancing of fluid mixing, which will restrain the substitution of Sr to Ba and lower the Sr contents in barite. While the Sr contents (i.e.,88Sr intensity) in the barites from the Yonaguni IV increase with the fluid mixing degrees (Fig.5),which is contradictory to fluid temperature-controlled substitution of Sr to Ba. In this way, the change in barite crystal growth rate during the fluid mixing is the cause of Sr contents variation. Thus, although the mixing between hydrothermal fluid and seawater will lead to the changes both in fluid temperature and barite crystal growth rate,the fluid temperature change is apparently not the cause of Sr contents variation in the Yonaguni IV barites. Besides, it’s noteworthy that the contents of Sr in barites show a decrease trend from the core to rim (Fig.4), which indicates that the fluid mixing degrees decreased during the barite crystallization and crystal growth rate reduced from the early to late stage. This is consistent with the growth stages of hydrothermal chimney: in the early stage of hydrothermal activity, the fluid mixing was intensive,causing the fast crystallization of barite and large amounts of Sr substituted Ba into crystals; with the development of hydrothermal activity, the chimney sealed gradually, restraining the fluid mixing and decreasing the growth rate of barite, Sr contents reduced as a result.
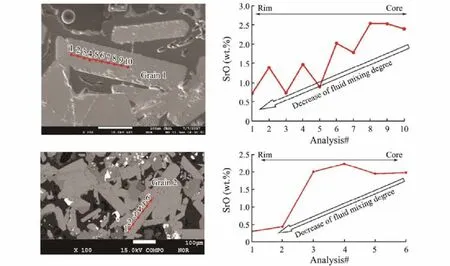
Fig.4 Electron microprobe analysis results of SrO contents across single barite.
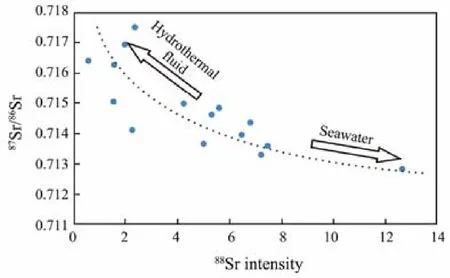
Fig.5 The 87Sr/86Sr versus 88Sr intensity diagram of hydrothermal barites from the Yonaguni IV. The dotted line is simulated hyperbola which indicates the mixing of two end-member fluids (Faure, 1986). We propose that the two end-members are seawater (SrSW = 87 μmol kg-1; 87Sr/86Sr= 0.7092; Seyfried et al., 2003) and sediment-influenced hydrothermal fluid (SrHF = 61 μmol kg-1; 87Sr/86Sr = 0.72212;Kishida et al., 2004; Dou et al., 2016). Here SrSW and SrHF are Sr concentrations of seawater and hydrothermal fluid respectively.
5.2.2 Sources of Ba
The source of Ba2+is a key factor for barite crystallization. Barite contents are generally low in hydrothermal fields developed in the mid-ocean ridge due to the substrates being usually Ba-poor mid-ocean ridge basalts (Noguchi et al., 2011). In immature back-arc basin hydrothermal fields, the contents of barite are generally high for the substrates are Ba-rich felsic volcanic rocks which provide a large amount of Ba2+to the hydrothermal systems (Noguchi et al., 2011; Jamieson et al., 2016). In addition, the dehydration of subducted slab can add soluble Ba ion into the magma during plate subduction, which further elevated the Ba2+abundances in the substrates(McCulloch and Gamble, 1991; You et al., 1996). Yonaguni IV hydrothermal field is hosted by subduction component-influenced felsic volcanic rocks (Guo et al., 2017,2018; Zhang et al., 2019a), which can provide large amounts of Ba2+to the hydrothermal system and facilitate the crystallization of barite. Besides, the Yonaguni IV is covered by thick sediment which can also contribute abundant Ba2+to the hydrothermal fluid during the fluid/sediment interaction (Noguchi et al., 2011).
5.2.3 Fluid mixing
Sr isotopes cannot fractionate below 400℃ or during barite crystallization (Matter et al., 1987), therefore, strontium isotope compositions of barite record the isotope ratio of the mineralization fluid (Staude et al., 2011). The hydrothermal fluid/sediment interaction occurred during the hydrothermal circulation in the Yonaguni IV. Kuhn et al. (2003) proposed that if hydrothermal fluid/sediment interaction reached equilibration, the87Sr/86Sr value of fluid should be equivalent to those of the sediments. We assumed that fluid/sediment interaction reached Sr isotope fractionation equilibration in the Yonaguni IV and took the average87Sr/86Sr value of Southern Okinawa sediment (0.72212, n = 22; Dou et al., 2016) as the Sr isotope composition of hydrothermal fluid. Then the relative contribution of hydrothermal fluid and seawater in the mixing fluid can be quantified by applying a two component mixing model:
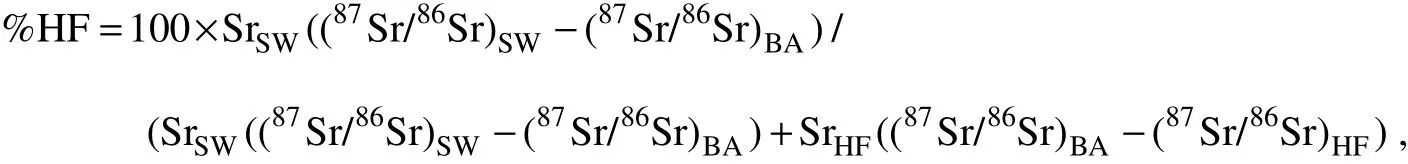
where %HF is the proportion of hydrothermal fluid (with the remaining proportion being seawater), SrSWand SrHFare the Sr concentrations of seawater (87 μmol kg-1; Seyfried et al., 2003) and hydrothermal fluid (61 μmol kg-1;Kishida et al., 2004), respectively, and (87Sr/86Sr)SW, (87Sr/86Sr)BAand (87Sr/86Sr)HFare Sr isotope ratios for seawater(87Sr/86Sr = 0.7092; Seyfriedet et al., 2003), barite and hydrothermal fluid (87Sr/86Sr = 0.72212).
Calculations show that hydrothermal fluid and seawater mixed in different proportions during the barite crystallization. The contribution of hydrothermal fluid component in the mixing fluid varied from 36% to 72% (Table 2),which further demonstrates that the mineralization condition in the Yonaguni IV is unstable and physiochemical condition of mixing fluid changes greatly during the mineralization process.
5.2.4 Crystallization temperature of hydrothermal barite
Hydrothermal barite is formed during the mixing of hydrothermal fluid and seawater (Damm, 1990; Hannington et al., 2005; Humphris and Bach, 2005). Thus, its crystallization temperature can be inferred using a linear thermal mixing relationship:
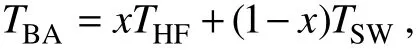
where TBAis the crystallization temperature of barite in degrees Celsius, THFand TSWare the temperature of endmember hydrothermal fluid (310℃; Seyfried et al., 2003)and seawater (2℃; Glasby and Notsu, 2003; Suzuki et al.,2008), and x is the fraction of hydrothermal fluid in the mixture.
Results show that the crystallization temperatures of hydrothermal barite in the Yonaguni IV vary from 109 to 220℃, which indicates that the temperature of mixing fluid changed greatly during the barite precipitation and the mineralization condition is unstable. Due to the fluid temperature within a chimney is controlled not only by the mixing between hydrothermal fluid and seawater, but also by conductive cooling of hydrothermal fluid (Jamieson et al., 2016); the temperature calculated by the formula should be a little higher than the true crystallization temperature of barite.
5.2.5 Fluid saturation
Crystallographic features of barite reflect the physiochemical condition of hydrothermal fluid (Judat and Kind,2004; Li et al., 2007; Feng and Roberts, 2011). Dendritic crystals grow quickly from the highly saturated fluid (Turcotte and Brown, 1997), while well-formed crystals grow slowly at lower saturation (Shikazono, 1994). The barites from the Yonaguni IV occur as two types of crystal, wellformed tabular and needle-like crystal (Fig.2), which were formed in different stages of mineralization. In the early stage, the fluid mixing was intensive and high saturation of BaSO4resulted in fast crystallization of the dendritic barite. With the development of hydrothermal activity, the chimney sealed gradually restraining the fluid mixing and lowering the BaSO4saturation in the fluid, barite crystalized as well-formed tabular as a result (Paytan et al., 2002;Ray et al., 2014).
In conclusion, the mineralization environment is unstable in the Yonaguni IV. During the barite crystallization,hydrothermal fluid mixed with seawater in different proportions, which led to the large variation in barite crystallization temperature, crystallographic features, Sr contents and87Sr/86Sr values.
5.3 New Insight into Mineralization Material Source
According to the traditional hydrothermal leaching model, ore forming metals are mainly derived from the reaction between seawater and basement rocks at high temperature (Bischoff and Dickson, 1975; Humphris and Thompson, 1978; Mottl and Holland, 1978). Sediment may contribute a part of mineralization material in sediment covered hydrothermal fields (Lehuray et al., 1988; Zierenberg et al., 1993; Cousens et al., 2002; Zeng et al., 2017;Zhang et al., 2019b). With the deepening of research,importance of contribution from magmatic fluid to the hydrothermal systems has been realized recently (Yang and Scott, 1996; Kamenetsky et al., 2001). Wang et al.(2018) reviewed the results of previous researches and put forward the ‘double diffusive convection model’which emphasized the contribution of both water/rock reaction and magmatic fluid. In the above models, sediment contribution to the hydrothermal systems is put on the back burner. The hydrothermal barite from the Yonaguni IV has extremely high87Sr/86Sr, implying that sediment plays a critical role during the mineralization process. Sr isotope compositions of barite from other hydrothermal fields are generally fall between those of basement rocks and seawater (e.g., Fouquet et al., 1993), reflecting the mixing of magmatic and seawater-derived strontium. The measured87Sr/86Sr values of barites in this study are apparently higher than the values of regional volcanic rocks and seawater but lie between those of seawater and Yonaguni IV sediment, which indicates that the hydrothermal fluid in the Yonaguni IV sufficiently interacted with the overlying sediment during the hydrothermal circulation. In this process, sediment may contribute a large amount of mineralization material to the hydrothermal system.
Previous researches have demonstrated that the mineralization characteristics in the Yonaguni IV are apparently different from those in the middle and northern Okinawa(Gena et al., 2013). For instance, Pt-rich bismuthinite is found in the Yonaguni IV and the Platinum group elements mineralization is generally associated with sediment (Hodge et al., 1986). Yonaguni IV hydrothermal field is covered by thick terrigenous sediment which may be the major source of Pt for Pt-rich bismuthinite. The hydrothermal deposit sample in this paper is Ba-Zn-Pb type and the barite contents are high up to 50%, such large amounts of barite require adequate supply of Ba2+, while mere water/rock reaction can hardly satisfy the requirement. Sediment is rich in Ba2+, which can provide large amounts of Ba2+to the hydrothermal system during the fluid/sediment interaction. All the evidences above indicate that the hydrothermal deposit from the Yonaguni IV is seemingly different from the VHMS (volcanic-hosted massive sulfides) ores. Sediment plays a critical role during the mineralization process and probably is the major source of mineralization material. Moreover, fluid/sediment interaction changed the compositions of Yonaguni IV hydrothermal fluid greatly, especially in the Sr contents and87Sr/86Sr values. This sediment-influenced fluid (with abnormally high87Sr/86Sr values) mixing with seawater caused the crystallization of the hydrothermal barites.Thus, incorporation of the sediment component into the hydrothermal system was prior to the barite crystallization and sulfide mineralization.
6 Conclusions
1) The hydrothermal fluid sufficiently interacted with the sediment during mineralization process in the Yonaguni IV, which led to the apparently higher87Sr/86Sr values of barites.
2) The mineralization environment is unstable in the Yonaguni IV. During the barite crystallization, hydrothermal fluid mixed with seawater in different proportions causing the large variation in barite crystallization temperature, crystallographic features, Sr contents and87Sr/86Sr values.
3) The hydrothermal deposit from the Yonaguni IV is largely different from the VHMS ores. Sediment plays a critical role during the mineralization process.
Acknowledgements
This study is financially supported by the National Basic Research Program of China (No. 2013CB429702). We thank the two anonymous reviewers for their helpful comments in the early version of the paper.
 Journal of Ocean University of China2020年2期
Journal of Ocean University of China2020年2期
- Journal of Ocean University of China的其它文章
- Abyssal Circulation in the Philippine Sea
- Numerical Study of Storm Surge Inundation in the Southwestern Hangzhou Bay Region During Typhoon Chan-Hom in 2015
- Interannual Variability and Scenarios Projection of Sea Ice in Bohai Sea Part I: Variation Characteristics and Interannual Hindcast
- Probability Distribution of the Hull Motion and Mooring Line Tension of Two Floating Systems
- Suppression of Vortex-Induced Vibration by Fairings on Marine Risers
- The Characteristics of Storm Wave Behavior and Its Effect on Cage Culture Using the ADCIRC+SWAN Model in Houshui Bay, China
