Diagnosis and treatment of heart failure with preserved left ventricular ejection fraction
Robert J Henning,College of Public Health,University of South Florida,Tampa,FL33612,United States
Abstract
Key words:Diastolic heart failure;Myocardial stiffness;Incomplete left ventricular relaxation;Echocardiographic heart failure criteria;Pulmonary artery pressure monitoring;Drug treatment
INTRODUCTION
Heart failure is a clinical syndrome,characterized by symptoms of breathlessness and easy fatigability,and signs of fluid retention,such as elevated jugular venous pressure,pulmonary crackles,and ankle edema,that result from the impaired ability of the heart to maintain a cardiac output that meets the metabolic needs of the body.Currently,nearly six million people in the United States have heart failure and require 30.7 billion dollars per year for health care[1].Moreover,the healthcare costs for treating patients with heart failure in the United States are projected to increase to $70 billion in 2030[2].
Approximately 50% of the patients with heart failure have normal,or near-normal left ventricular (LV) systolic heart function with a LV ejection fraction ≥ 50% and a LV end-diastolic volume index < 97 mL/m2.These patients are described as having heart failure with preserved ejection fraction (HFpEF).However,these patients have abnormal LV diastolic function with incomplete LV relaxation due to increased myocardial stiffness.This form of heart failure is becoming the dominant form of heart failure among older adults in the United States and in Europe due,in part,to the increasing longevity of the population.By the year 2020,8% of the United States and European population older than 65 years of age will have HFpEF[3].
Although most physicians have only recently become aware of HFpEF,the first description of HFpEF was in 1966 when “presbycardia” with decreased elasticity of the heart,also known as “senile heart disease”,was first described in elderly individuals[4].Currently,the most common patients with HFpEF are elderly women with comorbid conditions,such as hypertension with LV hypertrophy (60%-80%),ischemic heart disease (35%-70%) that may be occult,atrial fibrillation (15%-65%),obesity (32%-46%),diabetes mellitus (20%-45%),renal disease (51%-58%),and obstructive lung disease (24%-30%)[5,6].In addition,women as they age develop greater systemic arterial and LV stiffness than men which results in concentric LV myocardial remodeling and HFpEF.
The systemic arterial and ventricular stiffness in HFpEF is amplified by the coexistence of hypertension,chronic renal disease,and diabetes mellitus.With an increase in arterial stiffness,the pressure wave ejected from the LV is reflected back to the heart,thereby increasing LV afterload,decreasing diastolic ventricular function,and increasing the hydraulic work and myocardial oxygen requirements of the heart.These hemodynamic effects lead to decreased ventricular diastolic function,increased ventricular diastolic filling pressures,decreased coronary flow reserve,and pulmonary vascular congestion.As a result,patients experience shortness of breath.
Heart disease with preserved ejection fraction is a heterogenous syndrome with multiple different conditions that can contribute to the syndrome.Understanding and targeting the pathological conditions that contribute to this syndrome may have greater patient benefit than targeting the final pathway of cardiac dysfunction alone.The specific conditions that can contribute to HFpEF are listed in Table1 which is adapted in part from[7,8].
Patients who are hospitalized because of HFpEF have complication rates that are similar to those patients with heart failure and reduced ejection fraction (HFrEF),including similar rates of cardiac arrest,acute coronary syndromes,renal insufficiency and failure,and admission to critical care units[9].Despite similar rates of in-hospital complications in the two groups,patients with HFpEF are less likely to receive cardiology consultation while in the hospital than patients with HFrEF.
Table2 compares the clinical characteristics of patients with HFpEF with patient with HFrEF and is adapted in part from[10,11].
The annual mortality rate of patients with HFpEF is approximately 8% per year but increases to approximately 10%-12% per year among patients older than 70 years of age[11-13].The prognosis of patients after the first hospitalization for HFpEF is poor with one-year mortality rates as high as 25% among older patients and five-year mortality rates of 24% to 54%[14].Thirty percent of the patients with HFpEF die of noncardiac causes,compared with 17% of patients with HFrEF,due to the presence of one or more comorbid conditions.In this regard,the predictors of death amongpatients with HFpEF include advanced age,increased systolic blood pressure,atrial fibrillation,a prior cerebral vascular event,renal insufficiency or other major organ dysfunction with sepsis[14,15].

Table1 Conditions that contribute to heart failure with preserved ejection fraction
PATHOPHYSIOLOGICAL MECHANISMS IN HFpEF
LV diastolic dysfunction in individuals with HFpEF is characterized by increased diastolic ventricular stiffness,which slows LV relaxation,increases LV diastolic filling pressures and limits cardiac output[16,17].Different pathophysiologic mechanisms are hypothesized to contribute individually or in combination to LV diastolic dysfunction in patients with HFpEF.The current hypotheses include:(1) Cardiomyocyte titin hypophosphorylation;(2) Vascular endothelial cell inflammation and dysfunction;(3)Abnormal calcium homeostasis;(4) Increased ventricular matrix formation;and (5)Obesity.
CARDIOMYOCYTE TITIN HYPOPHOSPHORYLATION
Resting tension in cardiomyocytes is highly dependent on titin,which is a large sarcomeric protein that functions as a “molecular spring” which stores energy during ventricular contraction and releases energy during ventricular relaxation.Consequently,titin contributes to early myocardial diastolic recoil and late myocardial diastolic distensability[18].A shift in titin isoform expression from the complaint N2BA to the stiff N2B isoform,hypophosphorylation of the N2B isoform,and lower overall phosphorylation of titin increases the resting tension of cardiomyocytes and contributes to increased LV myocardial stiffness and diastolic dysfunction[18-21].Consequently,manipulation of the phosphorylation state of titin and the titin isoforms represents a therapeutic target for the treatment of patients with HFpEF.
INFLAMMATION
Obesity,hypertension,diabetes mellitus,chronic obstructive pulmonary disease,chronic kidney disease,and anemia in patients can induce an inflammatory state with increased circulating C reactive protein,interleukin 1 (IL-1) receptor-like 1 protein,growth differentiation factor 15,IL-6,tumor necrosis factor-a,and pentraxin 3[22-24].See Figure1.Inflammation of the coronary microvascular endothelium produces free oxygen radicals and vascular cell adhesion molecules that attract circulating leukocytes to the microvascular which secrete transforming growth factor-β (TGF-β).TGF-β converts myocardial fibroblasts to myofibroblasts,which increase myocardial collagen deposition and cause interstitial fibrosis.Inflammatory free oxygen radicals also reduce nitric oxide (NO) bioavailability,and increase peroxynitrite (ONOO-),which reduces protein kinase G (PKG),which can increase cardiomyocyte stiffness,promote hypophosphorylation of titin,accelerate pro-hypertrophic signaling in cardiac myocytes and enhance diastolic dysfunction[17,21,25].
In addition,systemic inflammation decreases the vasodilator response of the coronary microvascular to acetylcholine and reduces renal blood flow and the ability of the kidneys to excrete sodium and water with resultant progressive expansion of intravascular volume[3].Systemic inflammation also affects the lungs and skeletal muscle by contributing to pulmonary hypertension,muscle weakness and sarcopenia[3].However,therapies to date that reduce systemic inflammation and/orimprove or increase vascular endothelial function,such as statins,angiotensin receptor blockers,and phosphodiesterase-5 inhibitors have proven ineffective in improving the morbidity or the mortality of patients with HFpEF.
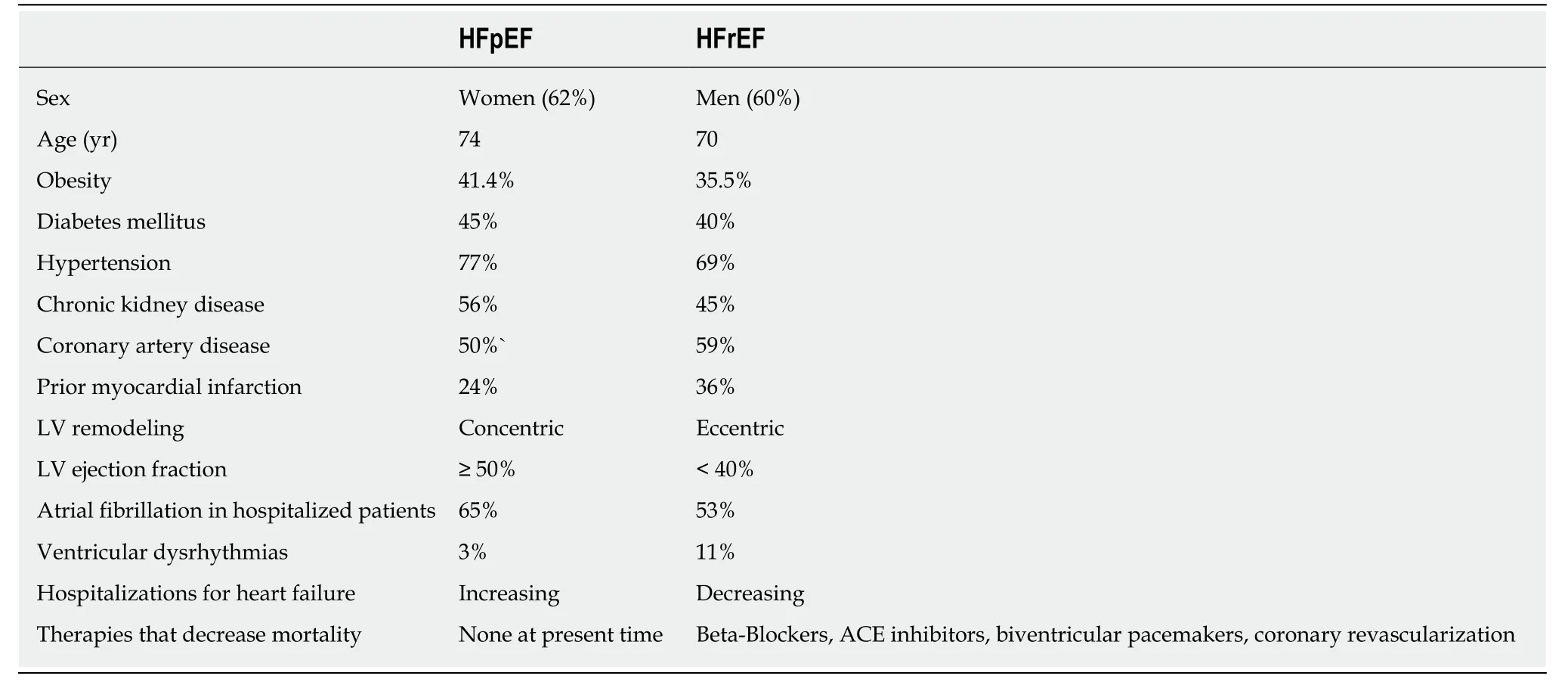
Table2 Clinical characteristics of patients with heart failure with preserved ejection fraction and heart failure and reduced ejection fraction
CARDIOMYOCYTE CALCIUM-HANDLING ALTERATIONS
High circulating concentrations of the free oxygen radical peroxynitrite in patients with HFpEF increase cardiomyocyte protein phosphatase 2A activity,which decreases cardiomyocyte phospholambam phosphorylation,reduces sarcoplasmic reticulum Ca2+uptake,and increases cardiomyocyte diastolic cytosolic Ca2+.In addition,abnormal cardiomyocyte sodium-calcium exchange and also calcium leak from the sarcoplasmic reticulum increase cardiomyocyte diastolic cytosolic calcium concentrations,which increases cardiomyocyte resting tension in HFPEF patients due to a delayed inactivation of actin-myosin crossbridges[26,27].However,reductions in cardiomyocyte calcium overload with ranolazine to date have not significantly improved myocardial relaxation and diastolic dysfunction[28].Nevertheless,abnormal calcium homeostasis is a potential therapeutic target for the treatment of patients with HFpEF.
INCREASED EXTRACELLULAR MATRIX FORMATION
Myocardial biopsies from patients with HFpEF,especially patients with hypertension and HFpEF,show an increase in the collagen volume fraction and an increase in myocardial fibrosis in comparison with patients without heart failure[29,30].In this regard,Inflammation can cause the release of TGF-β from fibroblasts and monocytes/macrophages,which induce the differentiation of fibroblasts into collagen-producing myofibroblasts,while simultaneously decreasing matrix metalloproteinase (MMP)-1 and the tissue inhibitor of (MMP)-1[17,31,32].In addition,endothelin-1,angiotensin II,platelet-derived growth factor,and connective tissue growth factor govern fibroblast activation/differentiation and represent potential therapeutic targets in the treatment of patients with HFpEF[33].
Myocardial collagen synthesis can also be increased during the female menopause where decreased circulating estrogen is associated with activation of the reninangiotensin-aldosterone system[34].Collagen expansion of the myocardial extracellular matrix,and especially an increase in the collagen type 1 fibers and the amount of crosslinked collagen,has an adverse effect on myocardial mechanical,electrical,and microvascular function and contributes to decreased diastolic function in HFpEF.Myocardial fibrosis also decreases myocardial capillary density,coronary perfusion reserve,and myocardial energy production[33].Studies of anti-fibrotic agents,such as Pirfenidone and also valsartan/sacubitril,in the PIROUETTE trial,the Parallax and the PARAGON-HF trials will help to determine in patients with HFpEF whether significant regression of fibrosis in the myocardium can occur and can be associated with improvement in diastolic ventricular function.
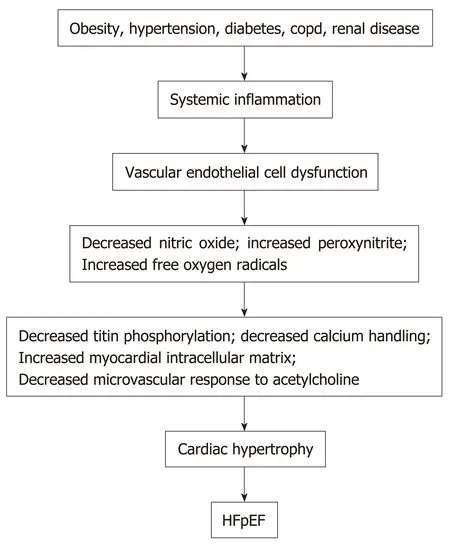
Figure1 Factors that contribute to heart failure with preserved ejection fraction.COPD:Chronic obstructive pulmonary disease;HFpEF:Heart failure with preserved ejection fraction.
MICROVASCULAR DYSFUNCTION
Exercise studies of patients with HFpEF implicate LV stiffness and impaired exercise vasodilation and raise the possibility that the impaired diastolic reserve in these patients may be related to coronary microvascular dysfunction.In an autopsy study of 124 patients with HFpEF compared with 104 age-matched control patients,HFpEF patients had a median of 961 coronary microvessels/mm2vs1316 vessels/mm2in controls[31,35].In addition,patients with HFpEF had more severe coronary artery disease with 65% of patients with ≥ 1 coronary vessel with > 50% diameter stenosis versus 13% in controls[34].The vascular changes in the HFpEF patients were associated with heavier hearts with a median weight of 538 g with 9.6% myocardial fibrosis versus 335 g with 7.1% fibrosis in controls[35].In addition,the myocardial fibrosis increased with decreasing microvascular density.However,cardiomyocyte size and LV hypertrophy are important determinants of the coronary microvascular density in adults and the role of cardiomyocyte size and LV hypertrophy in the determination of microvascular density in HFpEF requires further investigation.
OBESITY
Adipocytes synthesize cell-signaling molecules which include leptin,nerilysin and aldosterone which play a role in systemic inflammatory processes,cardiac fibrosis,and sodium and water retention[36].In this regard,obesity can lead to cardiac fibrosis and increased ventricular diastolic stiffness through:(1) Leptin secretion from adipose tissue which induces cardiac fibrosis through galectin-3,TGF-β and connective tissue growth factors[37];(2) Activation of beta2-adrenergic receptors which can enhance the synthesis of proinflammatory cytokines such as IL-6[38];(3) Increased activity of neprilysin from adipocytes,which by degrading endogenous natriuretic peptides promotes plasma volume expansion,loss of the anti-aldosterone action of endogenous natriuretic peptides,and cardiac fibrosis[39];and (4) Aldosterone secretion,in direct proportion to body mass,which increases collagen synthesis by cardiac fibroblasts[40].
Together these mechanisms can cause myocardial fibrosis and also cause sodium retention with plasma volume expansion.In addition,increased intramuscular fat decreases the supply of oxygen to working muscles and can impair oxidative metabolism in the skeletal musculature and,in this manner,contributes to the decreased functional capacity experienced by patients with HFpEF[5,41].Figure1 summarizes the different factors that can contribute to HFpEF.
DIAGNOSIS
The diagnosis of HFpEF in patients is based on the history,the physical examination,the laboratory data,the echocardiogram,and,when necessary,by cardiac catheterization.
The chest radiograph in patients with HFpEF often shows the presence of cardiomegaly and also evidence of pulmonary congestion.The electrocardiogram shows nonspecific ST-T wave changes although ECG evidence of myocardial ischemia or prior myocardial infarction may be present.Atrial fibrillation is frequently present on the ECG[42].Plasma brain natriuretic peptide (BNP) or N-terminal pro-BNP (NTproBNP) are often mildly increased with BNP > 100 pg/mL or NT-proBNP > 300 pg/mL.The concentrations of these peptides are less than the concentrations in patients with HFrEF[43].Nevertheless,a BNP measurement > 100 pg/mL or a NTproBNP > 300 pg/mL are independent predictors of adverse cardiovascular events in patients with HFpEF[44].Currently,the measurements of natriuretic peptides and troponins are recommended for the diagnosis and the prognosis of patients with heart failure,and the measurements of suppression of tumorgenicity 2,a marker of systemic inflammation,and galectin-3,a marker of cardiac fibrosis,are recommended for additional patient risk stratification and prognosis[45,46].
Echocardiography is a very useful noninvasive technique in the diagnosis of patients with HFpEF and often demonstrates the presence of LV hypertrophy or concentric LV remodeling with a LVEF that is ≥ 50% and a LV volume index that is <97 mL/m2.Left atrial enlargement is oftentimes present with a left atrial volume index> 34 mL/m2in patients who are not in atrial fibrillation.Common echocardiographic indices of significant diastolic dysfunction are listed in Table3 which is adapted in part from[47-49].
However,echocardiographic determined E/A and E/e’ ratios should not be used in isolation in assessing patients,but rather the ratios should be used in conjunction with the patient’s clinical characteristics and laboratory data to accurately diagnose LV diastolic dysfunction and HFpEF[50].In this regard,echocardiographic studies of 745 patients with HFpEF in the I-PRESERVE study reported that the LV end-diastolic volume was within normal limits in > 95 percent of the patients,LV hypertrophy or concentric remodeling was present in 50 to 60 percent,LV diastolic dysfunction (mild to severe) was present in > 70 percent,and left atrial area enlargement was present in> 65 percent of patients[51].
Recently a scoring system has been developed to facilitate the diagnosis of HFpEF in patients with dyspnea and distinguish these patients from patients with noncardiac causes of dyspnea[52].The scoring system uses 6 clinical and echocardiographic characteristics (H2FPEF:Heavy,Hypertension,Atrial Fibrillation,Pulmonary Hypertension,Elderly,LV filling Pressure) that are obtained in the evaluation of patients with unexplained exertional dyspnea to determine the probability of HFpEF.Patients with score high s of 6 to 9 have a probability of HFpEF > 90%.Conversely,patients with low scores of 0 to 1 have a probability of HFpEF ≤ 23%.Patients at intermediate probability with H2FPEF scores of 2 to 5 require additional testing to determine the cause of dyspnea.Table4 lists the H2FPEF scoring system and is adapted from[52].
CARDIAC MAGNETIC RESONANCE (CMR)
In patients with HFpEF,the myocardial extracellular matrix can be determined with CMR T1 mapping and is a predictor of LV myocardial stiffness and LV diastolic dysfunction.Excessive extracellular matrix deposition is a major contributor to the impaired cardiac relaxation and cardiac stiffness that are the hallmarks of HFpEF.Moreover,the CMR detection of myocardial extracellular matrix can precede the clinical diagnosis of HFpEF and correlates with patient myocardial biopsy specimens and hospitalizations for heart failure or death from cardiovascular causes[48,53-56].
In 410 patients with HFpEF or at risk for HFpEF,CMR determinations of increased myocardial extracellular volume (ECV) as an estimate of myocardial fibrosis were strongly associated with hospitalizations for heart failure or death during thesubsequent four years[56].In this investigation,myocardial ECV was more strongly associated with outcome than age,LV mass,atrial fibrillation,or previous myocardial infarction.See Table5 which is adapted from[56]and information kindly provided by Erik Schelbert,MD.
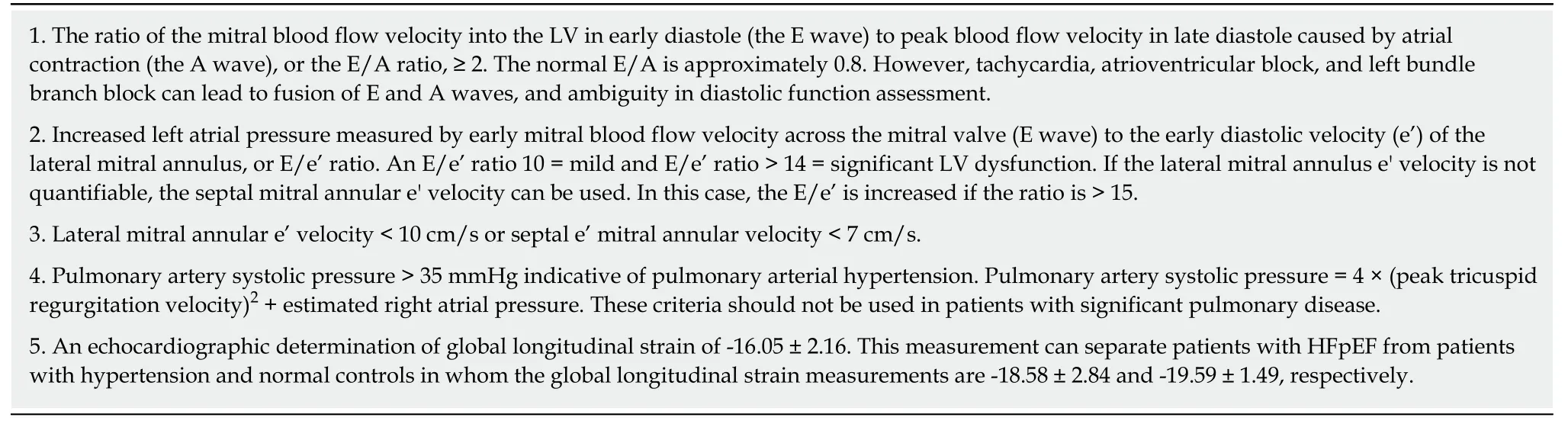
Table3 Echocardiographic indices of diastolic dysfunction
CMR determined ECV measurements provide risk stratification for HFpEF during the ensuing four years.Patients with ECV > 30% had decreased event-free survival during the subsequent four years.
However,the ECV fraction can be normal in as many as one-third of patients with HFpEF,which demonstrates the pathophysiological variation in this syndrome and the necessity to utilize the patient’s history,the physical examination,laboratory data,echocardiography,and,if necessary,cardiac catheterization in order to establish the diagnosis.
HEART CATHETERIZATION
For patients for whom the probability of HFpEF remains intermediate after history,physical examination,natriuretic peptide determinations,and echocardiography have been performed,invasive hemodynamic assessment of cardiac filling pressures,with provocative stress maneuvers such as exercise,is useful to make or exclude the diagnosis of HFpEF.
Right heart catheterization
Many patients with HFPEF have normal right and LV end-diastolic pressures at rest,when measured either invasively or non-invasively.In these patients,right heart catheterization measurements of pulmonary artery wedge pressure,cardiac output,and oxygen extraction during exercise are valuable tools in the diagnosis of patients with dyspnea due to HFpEF.During submaximal exercise,patients with HFpEF display pulmonary artery wedge pressures > 25 mmHg or an increase of 7 ± 3 mm Hg above the resting measurement,and pulmonary artery systolic pressures ≥ 45 mmHg in contrast to patients with noncardiac dyspnea[57,58].With exercise,older patients with HFpEF demonstrate a marked rise in arteriovenous oxygen content difference of 10.8± 1.8,driven by enhanced oxygen extraction,and lower increments in cardiac output in comparison with younger patients with HFpEF[5,59].This is especially true in older women who have small LV chambers and rely on heart rate increases to meet the cardiac output demands of exercise.
Left heart catheterization and coronary artery angiography
Community-based studies show that coronary heart disease (CAD) is common in HFpEF,and is present in 40% to 60% of patients with HFpEF[60-62].Patients with CAD are more likely to be men and to have CAD risk factors,including hypertension,diabetes,hyperlipidemia,and tobacco use[63].However,symptoms of angina and heart failure are similar in patients with and without CAD,as are measures of cardiovascular structure,function,and hemodynamics.Nevertheless,HFpEF patients with CAD experience a fourfold greater decline in EF over time compared with patients without CAD[63].The presence of CAD is associated with worse outcome in HFpEF,which appears to be independent of other predictors.In a study of elderly patients with a mean age of 72 years,patients with HFpEF and CAD have a 1-2-year mortality rate of 20%[63].In these patients,coronary revascularization is associated with a decrease in mortality and with outcomes that are not different from patientswith HFpEF without CAD[62,63].Consequently,patients with HFpEF should be subcategorized according to the presence or absence of CAD.If significant coronary artery disease is present,the patients should be evaluated for coronary revascularization[62,63].

Table4 Heart failure preserved ejection fraction scoring system:Heart failure with preserved ejection fraction
TREATMENT
Patients with HFpEF and major obstructive coronary artery disease should be treated with coronary artery revascularization.In addition,since many hospitalizations and deaths in patients with HFpEF are due to noncardiovascular causes such as chronic obstructive lung,chronic kidney disease,and diabetes,these disorders must be identified early in the clinical course and aggressively treated.In patients with NYHA class II and III heart failure and iron deficiency (ferritin < 100 ng/mL or 100 to 300 ng/mL if transferrin saturation is < 20%),intravenous iron replacement is reasonable to improve functional status and quality of life[46].
GENERAL TREATMENT OF PATIENTS WITH HFpEF WITH OBESITY,HYPERTENSION,AND ATRIAL FIBRILLATION
Medical treatment in HFpEF patients with non-obstructive coronary artery disease includes weight reduction and control of blood pressure,heart rate,and fluid status.More than 80% of patients with heart failure with HFPEF,are overweight or obese and deconditioned.A caloric restriction diet is feasible and safe in older,obese patients with HFpEFs,and significantly improves patient dyspnea,peak oxygen consumption,and quality of life[64].Caloric restriction combined with endurance exercise,such as walking exercise for one hour three or more times per week,is additive and the combination can produce a 2.5 mL/kg/min increase in peak oxygen consumption and an increase in exercise capacity[64].Exercise training,such as can occur with a cardiac rehabilitation program,can also improve the maximum heart rate and the diastolic function of the LV as measured by the echocardiographic ratio of early to late mitral valve filling (E/A ratio) and the LV filling pressure E/e′ ratio[65].Moreover,a decrease in body weight and an increase in peak oxygen consumption is strongly correlated with a decrease in the systemic biomarkers of inflammation in the body and is primarily attributable to increased peripheral microvascular and skeletal muscle function[66].
Fifty-one percent of patients with HFpEF have hypertension,whereas among patients with HFrEF,hypertension is present in 41%[67].In a meta-analysis of 123 studies with 613815 hypertensive patients,a 10 mmHg decrease in systolic BP reduced the risk of heart failure complications by 28%,independently of the baseline BP or co-morbidity status[68].In this regard,diuretic drugs are effective for blood pressure control and for the prevention of volume overload.Analysis from the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack (Allhat)Trial found that the diuretic chlorthalidone reduced the risk of HFpEF and the incidence of HFpEF hospitalizations among high-risk hypertensive patients when compared with lisinopril,amlodipine,and doxazosine[69].For patients whom are persistently hypertensive despite diuretic therapy,renin-angiotensin-aldosterone inhibition with an angiotensin converting enzyme inhibitor or an angiotensin receptor blocker are recommended for reduction in the blood pressure to ≤ 130/80 mmHg[46].In addition,mineralocorticoid antagonists,such as spironolactone or eplerenone,can play an important role in treatment of patients with HFpEF by limiting aldosterone,which is a volume retention and profibrotic hormone,and blood pressure.In theTreatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist (TOPCAT) Trial,spironolactone reduced patient hospitalizations for heart failure,reduced the composite end-point of cardiovascular death,heart failure hospitalization,and aborted cardiac arrest in patients from the Americas but not patients from Russia and Georgia[70].Furthermore,two recent retrospective studies report that the long-term treatment with a mineraloreceptor antagonist,such as spironolactone or eplerenone,and a beta-adrenergic receptor blocker drugs is associated with a reduction in the incidence of new-onset HFpEF in patients with hypertension[9,71,72].Consequently,the effective control of vascular volume and blood pressure in hypertensive patients can prevent patient progression to symptomatic HFpEF.However,while diuretics and antihpertensive medications can be effective in bringing about symptomatic relief,care must be taken to avoid hypotension and azotemia in patients with HFpEF.
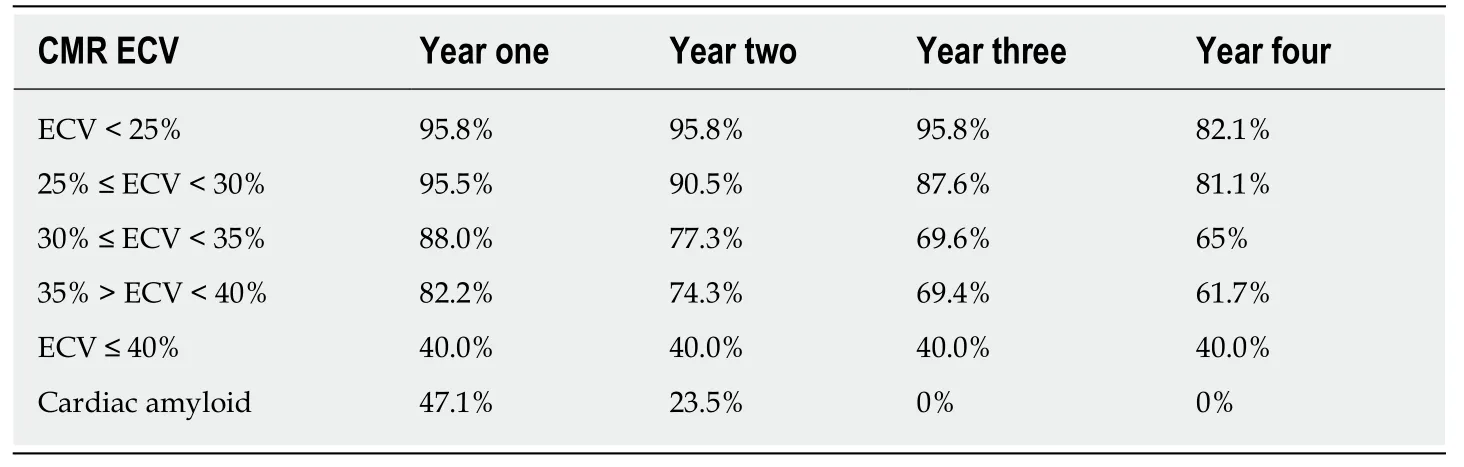
Table5 Survival after cardiac magnetic resonance determined extracellular volumedetermination
Atrial fibrillation is present in 15% to as many as 41% of patients with HFPEF[73].The arrhythmia may be associated with increased fatigue and exertional intolerance,natriuretic peptide elevation,left atrial remodeling and an increase in the risk of death[74,75].The treatment includes identification and treatment of the causes of atrial fibrillation,such as poorly controlled hypertension,obstructive sleep apnea,diabetes mellitus,or thyrotoxicosis.Patients with atrial fibrillation,HFpEF,and sleep apnea shoud undergo a sleep study in order to determine whether the sleep apnea is predominantly obstructive or central in nature and patients with obstructive sleep apnea treated with continuous positive airway pressure[46,76].Anticoagulation is recommended for patients with a CHA2DS2 VAS score ≥ 2.Beta-adrenergic receptor blocking drugs or non-dihydropyridine calcium channel blocking drugs,such as verapamil or diltiazem,are suggested for rate control.Experience with atrial catheter ablation of atrial fibrillation in patients with HFpEF is limited.In a small,single center study,catheter ablation of atrial fibrillation improved diastolic function in patients who maintained sinus rhythm[77].Additional investigations of catheter ablation of atrial fibrillation in patients with HFpEF are necessary.
Recent clinical trials of sodium glucose cotransporter-2 inhibitors have shown promising effects on heart failure outcomes in patients with heart failure and diabetes mellitus.In the EMPA-REG Cardiovascular Outcome Event Trial in patients with type 2 diabetes mellitus,the sodium-glucose cotransporter-2 (SGLT2) inhibitor empagliflozin was associated with a reduction in major adverse cardiovascular endpoints and a significant reduction in heart failure hospitalizations[78].SGLT2 inhibitors,such as empagliflozin,promote gylcosuria and diuresis by reducing glucose and sodium absorption in the proximal renal tubule,without activating the sympathetic nervous system.SGLT2 inhibitors also appear to decrease markers of inflammation[79].Additional studies of SGLT2 inhibitors in patients with HFpEF and diabetes mellitus are in progress.
In summary,inhibitors of the sympathetic nervous and renin-angiotensinaldosterone systems should be considered in patients with HFpEF who have coronary artery disease,hypertension,and atrial fibrillation[46].Sodium glucose cotransporter-2 inhibitors should be considered in patients with HFpEF and type 2 diabetes mellitus[78].Patients with major obstructive coronary artery disease that contributes to heart failure should be evaluated for coronary artery revascularization[62,63].
GENERAL PHARMACOLOGY TREATMENT OF PATIENTS WITH HFpEF
The pharmacologic therapy trials for LV dysfunction in patients with HFpEF with beta-adrenergic receptor blockers,calcium channel blocking agents,angiotensin converting enzyme inhibitors,angiotensin receptor blockers,and nitrates are not consistent and,in general,have been neutral in decreasing patient mortality.This is,in part,due to differences in trial design and patient population heterogeneity with differences in heart failure etiologies or stages of disease.Recently,patients with LV ejection fractions between 41% and 49% have been categorized as heart failure with mid-range ejection fraction.Patients in this intermediate category match a phenotype that is closer to the clinical profile of HFrEF and have a higher risk of sudden cardiac death and cardiovascular death than patients with HFpEF.Table6 lists the major pharmacologic studies that have been performed in patients with HFpEF and patients with heart failure with mid-range ejection fraction.Table6 is adapted,in part,from[80].
MEDICAL DEVICES
Patients with HFpEF and chronotropic incompetence are currently being tested with rate-adaptive atrial pacing (government trial NCT02145351).Patients with HFpEF can have normal resting left atrial pressures but develop marked increases in left atrial pressures and pulmonary hypertension with exercise due to a decrease in LV diastolic compliance.This is typically manifested as exertional dyspnea.Innovative medical devices,listed below,inserted into patients with HFpEF in clinical trials have shown some promise in improving patient symptomatology.
INTERATRIAL SEPTOSTOMY
An 8 mm unidirectional interatrial left to right shunt device in patients with HFpEF has been investigated to reduce left atrial pressure by 3 to 11 mmHg and provide symptomatic patient relief from dyspnea that is due to increased pulmonary venous pressure.In a randomized Reduce-Left Atrial Pressure in Heart Failure Trial I in 39 patients,the decrease in pulmonary wedge pressure during exercise and the improvement in workload corrected pulmonary capillary wedge pressure,exercise duration,and peak exercise workload compared to sham controls were numerically better in the treatment group but the differences from controls did not achieve statistical significance[109,110],A larger trial (REDUCE LAP-HF II Governmental Trial NCT03088033) is currently examining the effects of an interatrial septal device on clinical outcomes and quality of life.The long term effects of volume loading with this device and similar devices,such as the V Wave device (Governmental Trial NCT02511912) and the Atrial Flow Regulator (Governmental Trial NCT03030274),on right heart chambers and function,the pulmonary circulation,the cardiac rhythm,and the potential for paradoxical embolism in older patients who typically have HFpEF,require further investigation.
PULMONARY ARTERY PRESSURE MONITORING
The CHAMPION (CardioMEMS Heart Sensor Allows Monitoring of Pressure to Improve Outcomes in NYHA Class III Heart Failure Patients) Trial was a prospective,randomized controlled trial that investigated whether medical treatment based on daily pulmonary artery pressure monitoring would significantly reduce hospitalizations for heart failure treatment[111].A 15 mm electromechanical pressure sensor was permanently implanted by right heart catheterization into a branch of the right pulmonary artery of each patient and transmitted pulmonary artery pressures by radio signals to an internet web-based system that notified the patient’s physician if the daily pulmonary pressure measurements were outside of a defined range.After 17.6 mo of follow-up,the hospitalization rate was 50% lower in patients where medical treatment decisions were made based on the pulmonary artery pressure measurements[111].Hemodynamic-guided management using PA pressure measurements appears to be a successful strategy to improve the outcome of patients with HFpEF.More recently,the HEMODYNAMICALLY GUIDED MANAGEMENT OF HEART FAILURE (Guide-HF Governmental Trial NCT03387813) Trial is examining the effects of pulmonary artery pressure monitoring on patient mortalityfrom heart failure.

Table6 Pharmacologic studies in heart failure with preserved ejection fraction

LVEF:Left ventricular ejection fractions;HF:Heart failure;EF:Ejection fraction.
MECHANICAL CIRCULATORY ASSISTANCE
For patients with severe refractory HFpEF in whom medical therapies have provided no benefit,mechanical circulatory support is being investigated to decrease LV filling pressures and pulmonary congestion and increase cardiac output.In this regard,a partial mechanical circulatory microdevice,which is inserted with a minimally invasive approach,directs blood from the left atrium into the subclavian artery[112].This system is reported to decrease pulmonary and left atrial pressures in patients with HFpEF.However,patients with HFpEF have small LV dimensions and further decreases in LV stroke volume may limit patient coronary and cerebral blood flow.Additional investigations are needed to evaluate whether this invasive therapy decreases patient morbidity and mortality.
FUTURE PERSPECTIVES
The specific etiologies of HFpEF,the mechanisms,the clinical manifestations,and the contributions of comorbidities to the morbidity and mortality of HFpEF must be fully identified in patients.Such identifications will be facilitated by the pursuit of clinical registries and large clinical trials that focus on collecting clinical,imaging,laboratory,and outcome data and treatments.Successful pursuit of these goals will ultimately permit the development of specific drugs and medical devices that will decrease the morbidity and mortality of HFpEF in patients.
SUMMARY
The American College of Cardiology,the American Heart Association and the Heart Failure Society recommend the following treatments for patients with HFpEF and symptoms and signs of heart failure (adapted from[46,113]):(1) Treatment of hypertension in all HFpEF patients with angiotensin-converting enzyme inhibitors,angiotensin receptor blockers,calcium channel blockers or beta-adrenergic receptor blocking drugs;(2) Treatment of patients with HFpEF with volume overload with diuretics;(3) In patients with HFpEF with increased BNP,creatinine < 2.5 mg/dL,glomerular filtration rate > 30 mL/min and potassium < 5 mEq/L,treatment with an aldosterone antagonist;(4) Control of heart rate with medications in patients with atrial fibrillation;(5) In patients with HfpEF and type 2 diabetes mellitus,treatment with a SGLT-2 inhibitor such as Empaglifozin should be considered;and (6)Treatment of patients with symptomatic obstructive coronary artery disease and myocardial ischemia that contributes to heart failure with coronary revascularization.
HFpEF BULLET POINTS
Introduction
Currently,5.7 million people in the United States have heart failure and require 30.7 billion dollars per year for health care and medications.
50% of patients with heart failure have normal,or near-normal LV systolic heart function but abnormal LV diastolic function with incomplete LV relaxation due to increased myocardial stiffness.These patients have HFpEF.
The most common patients are elderly women with hypertension,ischemic heart disease,atrial fibrillation,obesity,diabetes mellitus,renal disease,or obstructive lung disease.
Patients who are hospitalized because of HFpEF have complication rates that are similar to those patients with HFrEF.
The annual mortality rate of patients is approximately 8% per year but increases to approximately 10%-12% per year among patients older than 70 years of age.
Pathophysiological mechanisms in HFpEF
Current hypotheses include:(1) Cardiomyocyte titin hypophosphorylation;(2)Vascular endothelial cell inflammation and dysfunction;(3) Abnormal calcium homeostasis;(4) Increased ventricular matrix formation;and (5) Obesity.
Clinical manifestations/diagnosis
The diagnosis is based on the patient’s history and physical examinat ion with the assistance of laboratory data,echocardiography,and,when necessary,by cardiac catheterization.
A scoring system facilitates the diagnosis of HFpEF in patient with dyspnea and distinguish these patients from patients with non-cardiac causes of dyspnea.
In patients with HFpEF,the myocardial extracellular matrix can be determined with CMR T1 mapping and is a predictor of LV myocardial stiffness,LV diastolic dysfunction,and patient outcome.
HFpEF treatment
Since many hospitalizations and deaths in patients with HFpEF are due to noncardiovascular causes such as chronic obstructive lung,chronic kidney disease,and diabetes,these disorders must be identified early and aggressively treated.
Patients with obesity,hypertension,and atrial fibrillation require weight reduction,an exercise program,and aggressive control of blood pressure and heart rate.
Patients with volume overload should be treated with diuretics.
Pharmacologic therapy trials with beta-adrenergic receptor blockers,calcium channel blocking agents,angiotensin converting enzyme inhibitors,angiotensin receptor blockers,and nitrates have,in general,been neutral in decreasing patient mortality.
Medical treatment based on pulmonary artery pressure monitoring with a permanently implanted right pulmonary artery microsensor significantly reduces hospitalizations for treatment of heart failure.A clinical trial is currently examining the effects on patient mortality from heart failure.
If significant coronary artery disease is present and contributes to heart failure,the patient with HFpEF should be evaluated for coronary revascularization.
 World Journal of Cardiology2020年1期
World Journal of Cardiology2020年1期
- World Journal of Cardiology的其它文章
- ls there a role for ischemia detection after an acute myocardial infarction?
- Radial artery access site complications during cardiac procedures,clinical implications and potential solutions:The role of nitric oxide
- lmpact of training specificity on exercise-induced cardiac troponin elevation in professional athletes:A pilot study
- Prognostic impact of body mass index on in-hospital bleeding complications after ST-segment elevation myocardial infarction
- Phrenic nerve displacement by intrapericardial balloon inflation during epicardial ablation of ventricular tachycardia:Four case reports
- Salmonella typhimurium myopericarditis:A case report and review of literature
