A Mild Method for the Construction of CL-20/H2O2 Host-guest Energetic Material
YU Zhi-hui,XU Jin-jiang,SUN Shan-hu,WANG Hong-fan,ZHANG Hao-bin,DUAN Xiao-chang,ZHU Chun-hua,WANG Shu-min,SUN Jie
(1.School of Materials Science and Engineering,Southwest University of Science and Technology,Mianyang 621010,China;2.Institute of Chemical Materials,CAEP,Mianyang 621999,China)
Abstract:Hexanitrohexaazaisowurtzitane(CL-20)with a negative oxygen balance,is currently the most powerful commercially available explosive.In this work,the CL-20/H2O2 host-guest energetic material(CL-20/H2O2)was constructed by using urea hydrogen peroxide(UHP)as raw material through the solvent volatilization at low temperature and negative pressure.The structure of the complex was confirmed through X-ray diffraction(XRD)and Raman spectra.Results indicates that CL-20/H2O2 crystallizes in orthorhombic system space group Pbca with a long-range ordered stacked structure.The ratio of CL-20 molecule and H2O2 molecule is 2∶1 stoichiometry according to the thermogravimetry and simultaneous differential scanning calorimetry(TG-DSC)analyses.Furthermore,the polymorph transitions of CL-20/H2O2 with increasing temperature were investigated by in situ high temperature XRD.Results show that CL-20/H2O2 gradually converts to γ-CL-20 with elevated temperature and the rate of transition is faster than that of ε-CL-20.The CL-20 acetonitrile solvate(CL-20/CH3CN)is a key intermediate via a solid state phase transition to form the CL-20/H2O2 host-guest energetic material by tracing the growing process of CL-20/H2O2.
Key words:host-guest energetic materials;hexanitrohexaazaisowurtzitane(CL-20);H2O2;metastable phase;solid state transition phase
1 Introduction
Energetic materials are extensively used for a variety of military purposes,industrial applications and aerospace fields.High energy density materials(HEDMs) with desired properties are already attracting wide attention in recent decades[1-4].The performance of HEDMs is dominated by detonation pressure(p)and velocity(D),which are connected with density and oxygen balance (OB)[5-6].Recent concerns about the level of environmental compatibility of energetic materials have been focused on the green energetic materials based on a good OB[7-8].Many attributions were made to tune these materials through the physical and chemical approaches.In general,load oxidizer such as ammonium nitrate(AN),hydrazine nitroform(HNF),ammonium dinitramide(ADN)and ammonium perchlorate(AP)in energetic materials to enhance the explosive performance.However,this method would decrease the loading of explosives.Therefore,another way is to design and synthesize novel energetic compounds with a good OB,typical nitrogen-rich heterocycles such as tetrazole,triazole,furazan,and tetrazine derivatives[9-10].However,the exploitation of novel energetic materials is a long-term challenge with remarkable obstacle.Current interest has been focused on the development of host guest energetic materials,such as hexanitrohexaazaisowurtzitane(CL-20)/N2O host-guest energetic material and CL-20/H2O2hostguest energetic material(CL-20/H2O2),which can improve the explosive performance significantly[11-12].
As is well known,CL-20 has been widely studied as one of the most powerful commercially available explosives.Given an insight into the lattice packing model of CL-20,the instinct cavities can be used to insert the specific small molecules,such as H2O,CO2and N2O[11-13].As a result,the CL-20/H2O2attracted our attentions.Matzger incorporated H2O2into CL-20 crystal by solvent crystallization,which improved the OB and the crystal density.However,the high concentration H2O2(98%)was used as a solvent,which was a extremely dangerous method.Therefore this synthesis route appeared to be difficult in a large number production of CL-20/H2O2.Therefore,it is urgent to develop a convenient and safe method to prepare CL-20/H2O2.
In this study,a safe and mild method was developed to prepare the CL-20/H2O2.Especially,urea hydrogen peroxide(UHP)is adopted to replace concentrated H2O2.The structure determination,morphology characterization,thermal behaviour,sensitivity and phase transition of this complex were carried out.Furthermore,the mechanism was examined by PXRD and Raman spectra in detail.This study provides an effective method which encapsulating the specific molecules in the lattice cavity to design high-performance energetic materials.
2 Experimental
2.1 Materials
Raw CL - 20 was provided by the Institute of Chemical Materials,Chinese Academy of Engineering Physic(CAEP).Acetonitrile(CH3CN,99.9%,Superdry,dried in the 4Å molecular sieve),was provided by J & K Chemical Reagent Factory.Anhydrous ether(99.5%)was purchased from Chengdu Kelong Chemical Reagent Factory.Urea Hydrogen peroxide(UHP,97%) and molecular sieve were purchased from Aladdin industrial corporation Chemical Reagent Factory.
2.2 Preparation of CL-20/H2O2
According to the properties of H2O2,ether was applied to extract hydrogen peroxide from UHP at low temperature,since it had a high solubility for hydrogen peroxide but not for urea and low boiling point which is easy to volatile.In addition,in this work,it was a good selection to adsorb the solution by molecular sieve.CL-20/H2O2was obtained until the solvent disappeared and there were no by-products.
The preparation of CL-20/H2O2was carried out at 0-5 ℃.As shown in Fig.1a,UHP(5 g)was added into anhydrous ether (20 mL) and exhaustively stirred for 3 h at 0 ℃.Then H2O2was extract by filter operation,as depicted in Fig.1b,10 mL H2O2extract was injected to a glass vial which was loaded with the solution ofε-CL-20(0.2 g)in dry acetonitrile(1 mL).As seen in Fig.1c,the vial was put into a vacuum dryer with molecular sieves at 0-4 ℃and-0.06 - -0.05 MPa.CL-20/H2O2was obtained until the solution evaporated completely.

Fig.1 The preparation sketch of CL-20/H2O2
2.3 Mechanism Experiment of Solid - state Phase Transition
To verify the formation of CL-20/H2O2via a solidstate phase transition,mechanism experiments were carried out.

Fig.2 Mechanism experiments of CL-20/H2O2 solid-state phase transition
Experiment A:As shown in Fig.2a,0.3 gε-CL-20 was dissolved into 600 μL acetonitrile and the solution was added into vial,then 10 mL H2O2was loaded into the vial.The vial was put into a vacuum dryer with molecular sieves to volatilize(the temperature of the vacuum dryer was remained at 0-4 ℃and the pressure of the vacuum was kept at -0.06 --0.05 MPa).
Experiment B:As shown in Fig.2b,0.3 g CL-20/CH3CN was loaded in dense gauze and was suspended in the mouth of vial.And 10 mL H2O2extract was loaded in vial,then the vial was put in a vacuum dryer in the same condition as experiment A.
Experiment C:As shown in Fig.2c,a vial loaded with 10 ml H2O2extract was placed into a vacuum dryer to volatilize at 0-4 ℃and -0.05- -0.06 MPa.When H2O2extract was less than half in vial,the vial was taken out,0.3 g CL-20/CH3CN was placed in a dense gauze and suspended into the mouth,and then put back.Keep evaporating until the disappearance of the solution.
2.4 Characterizations
PXRD were recorded on a Bruker D8 Advance X-ray diffractometer equipped with Cu Kαradiation source(40 kV,40 mA).Raman were conducted on a Renishaw(UK,model InVia)with the 532 nm laser excitation.The thermal behavior was investigated at 25- 300 ℃ through the TG-DSC,the sample was heated with a heating rate of 10 ℃·min-1under the nitrogen flow.Microstructures of CL - 20/H2O2were collected on a scanning electron microscope on an Apollo 300 operating at an acceleration of 3 kV.The crystal morphologies were observed via ZEISS 2000-C optical microscope in reflection mode.The polymorph transitions were analyzed byin situhigh temperature XRD with a temperature programming.The scanning data were collected during temperature from 30- 185 ℃ in an interval of 5 ℃ at a fixed heating rate of 5 ℃ ·min-1.At last,the temperature was reduced to 30 ℃and the final scanning process was carried out.Moreover,the polymorphic con-tents ofγ- CL - 20 were quantified by Topas software[15].The impact sensitivity was according to the GJB772A-1997 standard method 601.2.Conducted by small-scale impact drop testing with a 2 kg drop mass on approximately 30 mg samples,which was determined statistically with the drop height of 50%explosion probability (H50).The friction sensitivity was determined with a WM-1 type friction sensitivity instrument according to GJB -772A-1997 standard method 602.1.Measured with 1.5 kg pendulum mass on 20 mg sample.For comparison,the sensitivity of raw materialε-CL-20 was also tested.
3 Results and Discussion
3.1 Crystallization of CL-20/H2O2
The PXRD pattern of the experimental CL-20/H2O2is shown in Fig.3a,which mathches with the simulated pattern of CL-20/H2O2(CCDC:1495520).Additionally,the experimental data of CL-20/H2O2are in accordance with those ofα-CL-20(CCDC:1495519)which indicate that experimental sample of CL-20/H2O2andα-CL-20 have the same space group of Pbcaand exhibit in a rhombic packing[11].Moreover,Raman spectra of the CL-20/H2O2,α-CL-20 and raw materials(UHP andε-CL-20)are displayed in Fig.3b and Fig.3c.The spectrum of the experimental CL-20/H2O2is similar to that ofα-CL-20 and is different from that ofε-CL-20.The strong peak of experimental CL-20/H2O2at 3557 cm-1could be attributed to the stretching vibration of O—H bond assigned to H2O2.In contrast withα-CL-20,O—H vibration at 3610 cm-1is assigned to H2O.Moreover,the characteristic peak of O—O bond stretching vibration for sample could be seen at 866 cm-1in the experimental CL - 20/H2O2.Compared with that of UHP(located at 871 cm-1),the peak decreases approximately at 866 cm-1,which is caused by the hydrogen bond between H2O2and CL-20.The results of Raman spectra are in good agreement with that of CL-20/H2O2in reference[11].
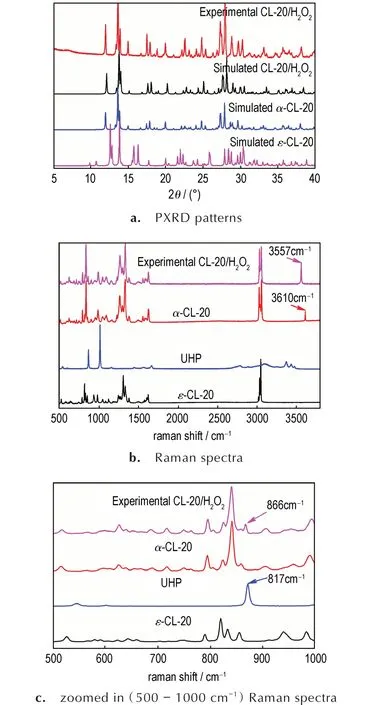
Fig.3 Identification of obtained CL-20/H2O2
The thermal behavior of experimental CL-20/H2O2and raw materialε-CL-20 are shown in Fig.4a and Fig.4b.Compared toε-CL-20,the TG analysis of experimental CL-20/H2O2shows a remarkable mass loss about 3.4% at 160-167.3 ℃.Corresponding to the DSC profile,there are two exothermic peaks(162.5,252.0 ℃)and a sharp endothermic peak at 167.3 ℃ for CL-20/H2O2.In contrast withε-CL-20,there is no mass loss before 200 ℃ in TG curve,and its DSC profile displays an endothermic peak at 166.9 ℃ and one exothermic peak at 238.7 ℃[16].The mass loss is confirmed to be H2O2and the stoichiometric ratio of CL-20 to H2O2is 2∶1(calculated value:3.7%).The H2O2molecules embedded invoids of CL-20 crystal lattice firstly decomposes at 165 ℃,followed by a phase transition to theγ-CL-20 at 167.3 ℃(supported byin situPXRD below).H2O2is unstable and easily broken down in the air.However,the embedding of H2O2into the capsuleshaped voids of CL-20 could delay the decomposition,in other words,the H2O2in the voids is more stable than that in air.Additionally,the structure stability and the decomposition temperature of CL-20/H2O2are higher thanε-CL-20,due to the incorporation of guest molecule and the change of lattice packing.

Fig.4 TG-DSC curves of CL-20/H2O2 and ε-CL-20.
The SEM images of CL-20/H2O2are showed in Fig.5,which revealed the three-dimensional porous distribution on the surface and the inner of experimental CL-20/H2O2randomly.This may result from the embedding of H2O2molecule.In addition,there are some cracks on the surface of the sample.The thermal phenomenon of CL-20/H2O2have a higher decomposition temperature which may be related to its porous microstructure of CL - 20/H2O2.In addition,the appearance of small inhomogeneous holes and cracks may be due to its growth mechanism.

Fig.5 SEM images of CL-20/H2O2
Table 1 shows the impact and friction sensitivity ofε-CL-20 and CL-20/H2O2.The 50% impact height(H50)of CL-20/H2O2is 13.8 cm,close to the raw materialε-CL-20 with 14 cm.The friction sensitivity of CL-20/H2O2is consistent with that ofε-CL-20 which all 100% ignited.The results reveal that CL-20/H2O2andε-CL-20 exhibited similar sensitivity.

Table 1 Sensitivity of ε-CL-20 and CL-20/H2O2
3.2 Phase Transformations of CL - 20/H2O2 with Elevated Temperature
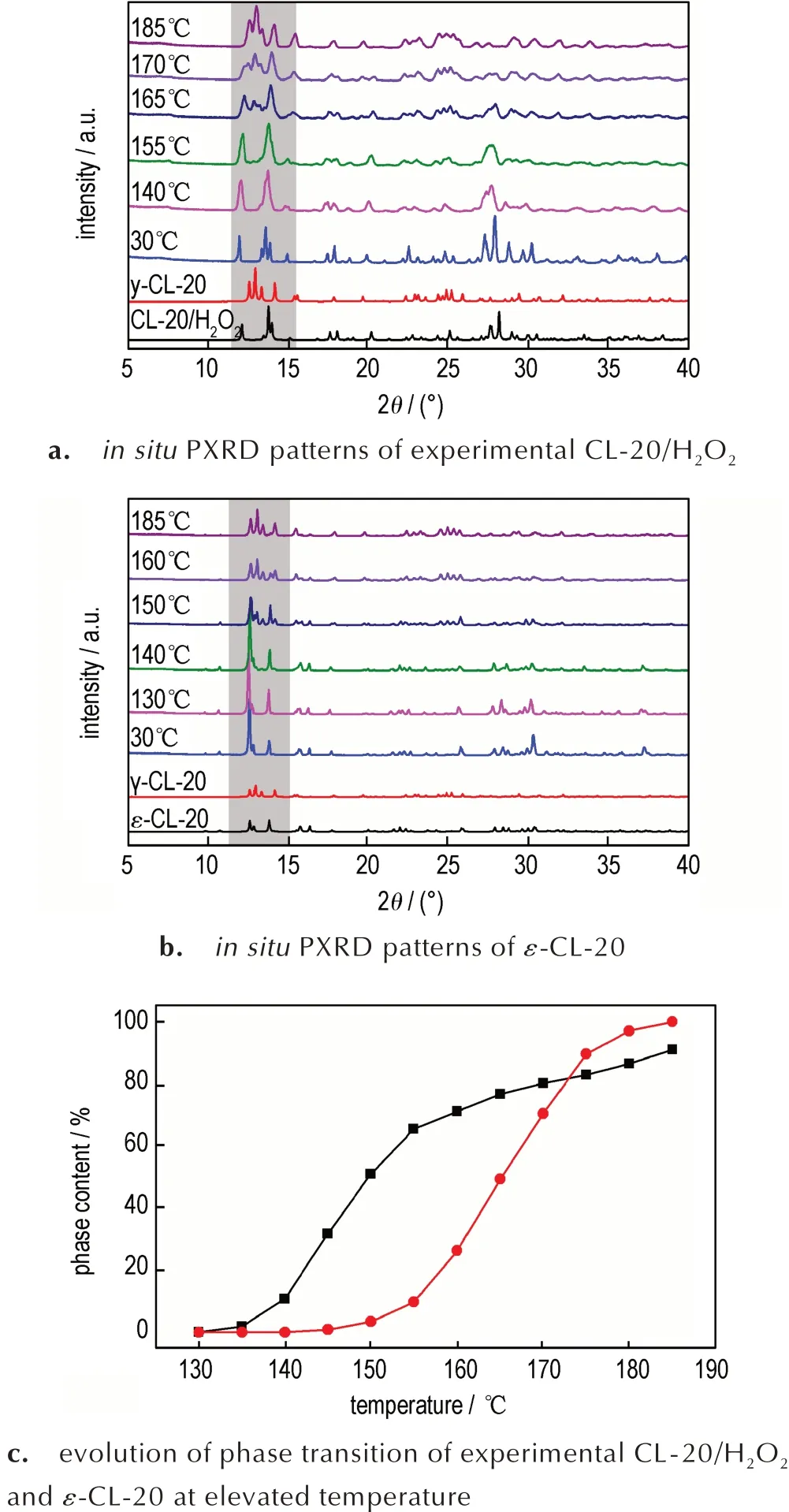
Fig.6 Polymorph transitions of experimental CL-20/H2O2 and ε-CL-20 at elevated temperature.
The phase transitions of CL-20/H2O2with increasing temperature were investigated byin situhigh temperature XRD and the results are presented in Fig.6a.From ambient temperature to 140 ℃,the results of PXRD of CL-20/H2O2agree well with the simulated results of CL-20/H2O2[11].When the temperature is up to 155 ℃,the intensity of the weak peaks at 12.8°(triplet) and 15.5° increases.With the increase of temperature,the intensity of 12.8°(triplet)and 15.5° becomes stronger which is attributed to the emergence of theγ-CL-20 phase gradually.In addition,the characteristic peaks of CL-20/H2O2(12°,15.1°,17.9°and 18.8°)become weak and disappear.And the rate of transition becomes large during 155-165 ℃.Until to 185 ℃,CL-20/H2O2pattern is in accordance with the simulated pattern ofγ-CL-20 completely.Forε-CL-20,the result is identical to the previous work(Fig.6b)[15].The phase contents ofγ-CL-20 of CL-20/H2O2andε-CL-20 with elevated temperature are depicted in Fig.6c.Compared toε-CL-20,CL-20/H2O2has a higher phase transition temperature,indicating that CL - 20/H2O2is more stable thanε-CL-20.Furthermore,it would entirely transform intoγ-CL-20 at 185 ℃,because the H2O2resolves into O2and H2O when temperature is over 140 ℃,and produce interspace in the crystal structure to make the transition easier.[12,17]
3.3 Insight Mechanism of CL-20/H2O2 Formation
In order to make the formation of the CL-20/H2O2energetic material clear,Raman spectra were utilized to analysis the crystallization process of samples.Crystallization process can be divided into 3 stages.When the H2O2extract was added into the CL-20 acetonitrile solution,crystal precipitated from the solvent,and its spectrum is shown in Fig.7a.In comparison with the literature[18],the crystal is found to be CL-20/CH3CN and this process is defined to be stage 1.The crystal was exposed to gaseous environment gradually as solvent volatilize.The spectral analysis indicates that the composition of the solid phases starts to change and the critical state is called state 2.Peak at 2255 cm-1dominated by CL-20/CH3CN became weaker,simultaneously,two characteristic peaks at 866 cm-1and 3557 cm-1appeared which was attributed to the formation of CL-20/H2O2[11].This indicates that the stage 2 is the state in which CL-20/CH3CN gradually transformed to CL-20/H2O2.With the disappearance of solvent,stage 3 emerges,the spectral analysis shows that the characteristic peak at 2245 cm-1of CL-20/CH3CN has disappeared totally,peaks at 866 cm-1and 3557 cm-1ascribedto CL-20/H2O2has developed further.These changes suggest that the complete emergence of CL-20/H2O2.

Fig.7 The formation process of CL-20/H2O2
PXRD was employed to detect the formation process accurately.As seen in Fig.7b,the results show a good agreement between the PXRD pattern of the samples from solvent(stage 1)and the simulated CL-20/CH3CN(CCDC:1569059).Once the crystal exposed to gas environment(stage 2),the PXRD pattern begins to change.Diffraction peak at 8.94° of CL-20/CH3CN becomes increasingly weaker as the solvent volatilizes,and the diffraction peaks referred to CL-20/H2O2(a triplet around 13.7° and a peak of 17.62°)can be observed clearly.As the disappearance of the solvent (stage 3),diffraction peaks of CL - 20/H2O2can be observed merely[18].The changes of PXRD are in accordance with Raman results,revealing that the CL-20/CH3CN is a key intermediate for the formation of CL-20/H2O2.
Along with morphological change of the sample in preparation process,CL-20/CH3CN as an intermediate and preparation process can be concluded as a solid-state phase transition.The image of sample at the end of stage 1 is shown in Fig.7c.It is a transparent sample with a colourless cubic crystal.By contrast,the crystal from stage 3(Fig.7d)retained original shape,however,it becomes an opaque crystal[19].It can be implied that the CL-20/H2O2is obtained based on CL-20/CH3CN without the dissolution-recrystallization process.
To explore mechanism further,the crystal structure of CL-20/CH3CN and CL-20/H2O2were investigated(Fig.8).The formation of CL-20/CH3CN depends on hydrogen bonds between CL-20 molecules and acetonitrile molecules,with intermolecular distances of 2.362 Å,2.573 Å and 2.411 Å,respectively.For CL-20/H2O2,the H2O2molecule interaction with two CL-20 molecules are via hydrogen bonds,with bond lengths of 2.224,2.294,2.224 Å and 2.259 Å,respectively.Manifestly,each hydrogen bond in CL-20/H2O2is stronger than that of CL-20/CH3CN.Therefore,the transformation from CL-20/CH3CN(metastable phase)to CL-20/H2O2(stable phase)is a spontaneous process thermodynamically[20].It was a convincing evidence that the formation of CL-20/H2O2via an intermediate of CL-20/CH3CN.

Fig.8 Hydrogen bonding and crystal packing diagrams of CL-20/H2O2 and CL-20/CH3CN
The morphology change has implied that there is a solid-state phase transition between the CL-20/H2O2and CL-20/CH3CN.To make the transformation process clearer,the mechanism experiments were carried out.The samples from experiment A,B and C were tested by PXRD and Raman spectra.As shown in Fig.9a,the PXRD patterns of the samples of experiment A and C were assigned to CL-20/H2O2.However,the peaks of sample in experiment B can be well identified toβ-CL-20.The results of mechanism experiments were further confirmed by Raman spectra shown in Fig.9b.In experiment A,CL-20/CH3CN was soaked in solution,as the ether evaporated,phase transition occurred until it is exposed to the concentrated H2O2gas molecules.In experiment B,however,when the CL-20/CH3CN was suspended in vial,the gas molecules were almost ether vapor,few H2O2molecules exist.And in experiment C,CL-20/CH3CN was surrounded by the gas molecules H2O2and ether.Therefore,it can be concluded that the preparation of CL-20/H2O2is a solid-state phase transition process which is induced by H2O2gas molecules.

Fig.9 PXRD patterns and Raman spectra of mechanism experiments A,B and C
4 Conclusions
A mild method is utilized to prepare CL - 20/H2O2by evaporation of the solution contained H2O2and CL-20 without by-product.It is found that CL-20/H2O2would have cause a mass loss about 3.4%around 162.5 ℃ caused by the deformation of H2O2,and the phase transitions was detected within situhigh temperature XRD.It is found that the CL-20/H2O2was stable up to 140 ℃,then converted toγ-CL-20 quickly during the temperature range of 140-170 ℃.And the SEM show that CL-20/H2O2by this method would have a three-dimensional porous structure,and it may lead to a similar impact sensitivity and friction sensitivity toε-CL-20.Moreover,a convincing evidence given by PXRD patterns,Raman spectra and optical images pointed out that the formation of CL-20/H2O2via a metastable phase CL-20/CH3CN solvate.The conditions of the phase transition was studied by the mechanism experiments,which reveal that CL - 20/CH3CN transformed into CL-20/H2O2is a process induced by the H2O2molecules.The simple preparation strategy of CL-20/H2O2hostguest energetic material makes it possible for a largescale production.Furthermore, the preparation method of stable phase via a metastable phase can be a promising approach to construct a novel energetic materials.

