Curcumin alleviates LPS-induced retinal inflammation by inhibiting PI3k/Akt signaling pathway
Yan Li1, Hua Li2, Xue Li3, Shan-Bi Zhou
1Department of Ophthalmology, the Affiliated Hospital of North Sichuan Medical College, Nanchong 637000, Sichuan Province, China 2Department of Ophthalmology, the Guizhou Provincial People’s Hospital, Guiyang 550002, Guizhou Province, China 3Department of Ophthalmology, the First Affiliated Hospital of Chongqing Medical University, Chongqing 400000, China 4Department of Ophthalmology, University-Town Hospital of Chongqing Medical University, Chongqing 401331, China
Abstract
INTRODUCTION
In many ophthalmologic disorders, inflammation is an important pathogenic component, finally resulting in visual impairment, even blindness[1]. For instance, retinal inflammation, a common inflammatory ocular condition, is one of the world’s main sight-threatening diseases.
Endotoxin-induced uveitis (EIU) is a commonly used animal model of human uveitis which is induced by injection of LPS, a ingredient of the gram-negative bacteria[2-3]. Among the prominent characteristics of EIU is infiltration of leukocytes into ocular blood vessels and leakage of protein into the anterior chamber of the eye, which reach a maximum at 24h after LPS injection[4]. LPS administration can activate Toll-like receptor 4 (TLR4) to trigger the cellular inflammatory pathways, including the NF-κB, and lead to the release of inflammatory mediators, such as IL-6, TNF-α, which are believed to be important contributors to the development of EIU[5-7]. In addition, the retina plays a crucial role in the formation of vision in the central nervous system. Among the retina, RPE cells which are located in a monolayer of pigmented cells between the photoreceptors of the neural retina and choroid possess a variety of complex biochemical functions, including modulating the transport of nutrients and debris and phagocytizing rod outer segment discs undergoing circadian shedding[8]. Thus, RPE cells play essential functions in the maintenance of homeostasis in the neural retina. However, inflammatory responses are the main contributing factor to the dysfunction of RPE cells[9]. Thus,invitrowe explored the role of curcumin in LPS-induced inflammation in ARPE-19 cells.
Curcumin, a phenolic natural product derived from Curcuma longa, is widely reported to have some potent anti-inflammatory, anti-oxidative, anti-carcinogenic and neuroprotective function[10-13]. Accumulating evidence has indicated that curcumin regulates various signaling molecules to produce different effects[14-16]. In recent years, extensive studies have demonstrated that reduction of inflammatory response of curcumin seem to be associated with inhibition of NF-κB activation by preventing IκB[17-19]. By affecting methylation pattern of TNF-α promoter curcumin was reported to regulate the expression of TNF-α[20]. Curcumin also alleviates inflammatory responses related to asthma by activating Nrf2/HO-1sig as well as down-regulating the inflammatory cytokines of expression of IL-6, TNF-α and IL-1β[21]. However, the benefits of curcumin have not been completely elucidated in ophthalmic inflammatory disorders. Due to its potent effects, in this study, we explored the anti-inflammatory effects of the administration of curcumin in EIU mice and explored the underlying mechanism in ARPE-19 cells.
MATERIALS AND METHODS
AnimalsC57BL/6 mice were purchased from the Laboratory Animal Center of Chongqing Medical University (Chongqing, China). The protocol was approved by the Ethics Committee of the Chongqing Medical University. EIU was induced by intraperitoneal injection of LPS (Sigma-Aldrich) diluted in PBS at the dose of 10 mg/kg body weight (BW). Mice were pretreated with curcumin by peritoneal injection at the dose of 40, 80, 120 mg/kg BW for 3d until the application of LPS. The level of inflammatory cytokines was measured by RT-PCR to evaluate the inhibitory effect of curcumin in EIU (data not shown). Base on this result, we chose 80 mg/kg curcumin for following animal experiments. The study was approved by the Medical Ethics Committee and was conducted according to the Declaration of Helsinki Principles.
CellCultureThe ARPE-19 cell line was purchased from the American Type Culture Collection (ATCC, USA). Cells were cultured in complete DMEM/F12 medium containing 10% fetal bovine serum (FBS; Invitrogen). The cells were incubated in humidified 5% CO2condition at 37℃. The cell was collected by centrifugation to plate for subculture after reaching confluence.
Real-timeQuantitativePCRAnalysisTotal RNA was extracted from cells and the mice freshly detached eyeballs by using Trizol Reagent (Invitrogen, USA).Then tested RNA concentrations and synthesized cDNA with PrimeScript RT reagent kit (Takara, China). RT-PCR was performed with a sequence detection system (ABI Prism 7500, CA) with the PCR kit (Bio-Rad). The cycling protocol and calculation were used as previously reported. All primers sequences were shown in Table 1.
LectinLabelingofRetinalVasculatureandAdherentLeukocytesA perfusion labeling technique was used to analyze the effect of curcumin on adherent retinal leukocytes in EIU as previously reported[22-23]. The adherent leukocytes on retinal vasculature were imaged with fluorescein-isothiocyanate (FITC)-coupled concanavalin A lectin (FITC-ConA; Vector Laboratories, CA). 24h after LPS administration, the mice were deeply anesthetized with by intraperitoneal pentobarbital sodium injection. Chest cavity was opened then introduced perfusion cannula (24 gauge) into the left ventricle. The mice were perfused 5 mL PBS to remove non-adherent leukocytes and erythrocytes after drainage was achieved from the right atrium. FITC-ConA (40 μg/mL in PBS; 5 mg/kg BW) was perfused to label adherent leukocytes, followed by removal of residual unbound lectin with 2 mL PBS perfusion. The eyes of mice were enucleated and fixed in 4% paraformaldehyde for 5min at room temperature. The retinas were carefully removed and mounted on microscope slides. The flat mounts were imaged with fluorescence microscope (Model DM6000, Leica, Germany).
AqueousHumorMeasureThe mice were anesthetized. Pupils were dilated with tropicamide eye drops 5min prior to collect aqueous humor (AH). AH sample was collected from the anterior chamber. Protein concentration was determined with a protein quantification kit (Beyotime) and absorbance was measured with a multifunction microplate reader (Molecular Devices, Sunnyvale, USA).

Figure1CurcumininhibitedthemRNAexpressionsofpro-inflammatorycytokinesinEIUThe expressions of IL-6 (A), IL-1β (B)and TNF-α (C) were increased in the LPS group when compared with untreated group. Curcumin pre-treatment suppressed the mRNA expression of IL-6, IL-1β and TNF-α in EIU.
Table1Sequencesoftheprimersforreal-timePCR

GenesForwardprimer(5 -3 )Reverseprimer(5 -3 )Mus-IL-6CCCCAATTTCCAATGCTCTCCCGCACTAGGTTTGCCGAGTAMus-IL-1βGGGCCTCAAAGGAAAGAATCTACCAGTTGGGGAACTCTGCMus-TNF-αCCCTCACACTCACAAACCACCCTTTGAGATCCATGCCGTTGMus-GAPDHGTATGACTCCACTCACGGCAAAGGTCTCGCTCCTGGAAGATGHum-IL-6AGTGAGGAACAAGCCAGAGCCAGGGGTGGTTATTGCATCTHum-IL-8GACATACTCCAAACCTTTCCACCCCCAGACAGAGCTCTCTTCCATCAGHum-MCP-1CTCATAGCAGCCACCTTCATTCTCACAGCTTCTTTGGGACACTTHum-βactinGGATGCAGAAGGAGATCACTGCGATCCACACGGAGTACTTG
CellViabilityAssayThe viability of ARPE-19 cells was detected by Cell Counting Kit-8 (CCK8, SigmaAldrich) assay. The cells were seeded on culture plate with 100 μL medium and incubated for 2d. Washed with PBS and starved with 100 μL of serum-free medium for 24h. The ARPE-19 cells were treated with various concentrations (5, 10, 20, 30 and 40 μmol/L) of curcumin for 24h. In addition, the ARPE-19 cells were pretreated with above-mentioned concentrations of curcumin for 1h and then stimulated with or without LPS (5 μg/mL) for 24h. After stimulation, WST-8 incubated the cells for 4h subsequently. Optical densities of the supernatant were read at 450 nm with a multifunction microplate reader (Molecular Devices, CA). Cells cultured without curcumin were used as a control.
Enzyme-linkedImmunosorbentAssayThe ARPE-19 cells were plated and incubated with curcumin for 1h with LPS in the presence or absence for 24h. The cell-free supernatant was collected after 24h application with LPS. ELISA kits (R&D Systems, CA) detected the concentrations of IL-6, IL-8 and MCP-1. The absorbance at 450 nm was determined using a microplate reader.
WesternBlotAnalysisWashed cells with PBS. Extracted protein and detected protein concentration by relevant reagents (Beyotime, China).All protein samples were denatured with SDS loading buffer (Beyotime) at 100°C for 8min. Equal amounts of protein (30 μg) were separated by SDS-PAGE electrophoresis with 8% polyacrylamide gels. The proteins were electroblotted onto polyvinylidene difluoride membranes (Millipore, MA), Membranes were blocked with 5% nonfat skim milk for 1h and washed three times containing 0.1% PBST. Incubated membranes with primary antibodies against Akt, p-Akt, IκB-α, p-IκB-α (1∶1000 dilutions; CST) and β-actin (1∶5000; zoonbio biotechnology, Nanjing, China) overnight at 4℃. Afterwards, washed membranes three times and incubated with the secondary antibody (1∶5000; Zoonbio biotechnology, Nanjing, China) for 1h at 37℃. After washing three times with PBST, bands were visualized by ECL kit (Advansta, CA). The β-actin protein level was normalized in western blotting analysis.
StatisticalAnalysisAll data were shown as mean±SEM. Statistical analysis was used with the GraphPad Prism software (GraphPad Software, USA). Comparisons of multiple groups were analyzed by one-way ANOVA followed by Dunnett test.P<0.05 was considered statistically significant.
RESULTS
CurcuminDecreasedthemRNAExpressionsofPro-inflammatoryCytokinesinEIUThe mRNA expressions of IL-6, IL-1β and TNF-α increased significantly in the LPS group when compared to the untreated group. In the curcumin pretreated mice, the mRNA expressions of inflammatory mediators were significantly decreased (Figure. 1).
CurcuminInhibitedtheRetinalLeukocyteAdhesionTo evaluate the changes of inflammatory cell responses in EIU,by perfusion labeling with FITC-coupled ConA We imaged leukocytes adherent on the retinal vasculature. We chose three part of a retina including the posterior retina (Figures 2A-2C), the midperipheral retina (Figures 2D-2F) and the anterior retina (Figures 2G-2I). The results showed that there was nearly no leukocyte adhesion in the untreated group (Figures 2A, 2D, 2G). After LPS injection, Retina-adherent leukocytes were numerous in LPS group mice (Figures 2B, 2E, 2H). However, compared with LPS group mice, curcumin administration significantly inhibited leukocyte adhesion on the retina vasculature (Figures 2C, 2F, 2I).

Figure2CurcuminsuppressedtheretinalleukocyteadhesionFlat-mounted retinas from untreated group (A, D, G), LPS-treated group (B, E, H) and LPS+curcumin group (80 mg/kg BW) (C, F, I). LPS-treated mice showing significantly increased leukocytes (white arrows) adhering to the retinal vasculature compared with untreated mice. Pre-treatment with curcumin resulted in the suppression of leukocyte adhesion. Scale bar, 20 μm; magnification ×200 (n=3).
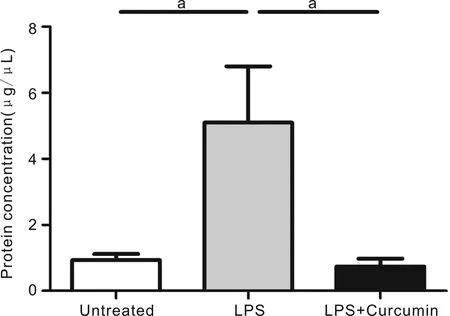
Figure3CurcumindecreasedtheanteriorchamberproteinleakageProtein concentration in the aqueous humor in LPS-treated mice, significantly increased compared with those in age-matched untreated mice, was significantly suppressed by pre-treatment with curcumin.

Figure4TheoptimumconcentrationofcurcumininculturedARPE-19celllineARPE-19 cells were treated with various concentrations (5, 10, 20, 30 and 40 μmol/L) of curcumin for 24h. In addition, above-mentioned concentrations of curcumin pretreat ARPE-19 cells for 1h and then stimulated with LPS for 24h.
CurcuminReducedtheAnteriorChamberProteinLeakageWe examined protein leakage into the aqueous humor (AH) by protein quantification kit. The results indicated that protein concentration in the AH was significantly higher in the LPS group mice than in the normal age-matched controls. However, pretreated curcumin to EIU mice led to a significant suppression in protein leakage compared with LPS group mice (Figure 3).
DeterminetheOptimumConcentrationofCurcumininARPE-19CellsTreatment with different concentration of curcumin (5, 10, 20, 30 and 40 μmol/L) for 24h had no noticeable effect in cells (Figure 4). Stimulation with LPS alone for 24h, the viability of cells was influenced a little. Interestingly, we found that curcumin was indicated to be capable of protecting ARPE-19 cells from LPS cytotoxic effects. Before LPS application, pretreatment with curcumin for 1h increased the viability of APRE-19 cells. The effect of curcumin was reached maximum at 20 μmol/L (Figure 4). Therefore, the concentration of 20 μmol/L curcumin was used for in subsequent experiments.

Figure5ThemRNAandproteinexpressionsofpro-inflammatorycytokinesincurcuminpre-treatedARPE-19cellsfollowedbyLPSstimulationA: Effects of curcumin on mRNA expression of IL-6, IL-8, and MCP-1 were determined by real-time RT-PCR. Cells were pretreated with curcumin (20 μmol/L) for 1h then administered with or without LPS (5 μg/mL) for 6h; B: Effects of curcumin on IL-6, IL-8, and MCP-1 production were measured by ELISA. Cells were pre-incubated in medium containing above-mentioned concentration of curcumin for 1h and then stimulated with LPS for 24h. Supernatants were then and the amounts of IL-6, IL-8 and MCP-1 were detected.
CurcuminDownregulatedtheExpressionsofPro-inflammatoryCytokinesinLPS-stimulatedARPE-19CellsWe detected the change of IL-6, IL-8, and MCP-1 both mRNA and protein expression level. The results indicated that curcumin had no effect on the production of IL-6, IL-8 and MCP-1 both mRNA and protein expression level (Figures 5A, 5B). The expression of pro-inflammatory cytokines was significantly increased in the LPS group compared to the control group at both mRNA and protein levels (Figures 5A, 5B). Meanwhile, pretreatment curcumin significantly reduced pro-inflammatory cytokine production.
EffectofCurcuminonPI3K/AktActivityinLPS-stimulatedARPE-19CellsPrevious study demonstrated that curcumin ameliorated inflammation through the PI3K/Akt signaling pathway[19,24]. We detected the expressions of Akt, p-Akt, IκB-α and p-IκB-α in APRE-19 cells pre-treated for 1h with or without curcumin and following stimulated with LPS for 24h by western blotting. The results were that the expressions of p-Akt and p-IκB-α were significantly increased in the LPS-stimulated cell group when compared with the untreated cell group and the curcumin pre-treatment cell group. In addition, curcumin pre-treatment significantly decreased the expression both p-Akt and p-IκB-α (Figures 6A-6D).
DISCUSSION
In this study, we demonstrated the effect of curcumin on LPS-induced inflammatory responseinvivoandinvitro. Curcumin in EIU mice ameliorated leukocyte adhesion and decreased the expression of pro-inflammatory cytokines in the retina as well as protein leakage in the AH. Leukocyte adhesion, played an important role in the inflamed retina of EIU, was significantly suppressed by pre-treatment of curcumin (Figures 2C, 2F, 2I). Meanwhile, the pathogenesis of EIU is involved activation inflammatory cells to releases TNF-α, IL-1β, and IL-6[4,25]. Here, we found that curcumin pre-treatment decreased the mRNA expressions of IL-1β, TNF-α and IL-6 in EIU mice. At both mRNA and protein levels curcumin pre-treatment reduced the expression of LPS-induced inflammatory cytokines including IL-6, IL-8, and MCP-1 in ARPE-19 cells. We also found that curcumin pre-treatment decreased the expression of p-Akt and p-IκB-α levels compared to LPS-stimulated cells. Thus, these findings suggested that curcumin attenuated the production of the pro-inflammatory mediators in LPS-stimulated by suppressing the PI3K/Akt-mediated NF-κB pathway.
Curcumin has been widely used in many countries for centuries. Accumulating evidence indicated that the effects of curcumin involved numerous aspects of pharmacological properties, which consists of antioxidant activities, hypoglycemic, anti-inflammatory activities and anti-cancer activities. Besides these activities, curcumin also plays its beneficial effects by regulating various signaling molecules, such as transcription factors, cell survival proteins, cytokines,adhesion molecules, growth factors andetc[26-27]. Related studies have shown that curcumin pretreatment protects against PM2.5 induced oxidative stress and inhibits LPS-induced endotoxemia and airway inflammation in mice[28-29]. In addition, curcumin also alleviates DSS-induced colitis, reduces MPO activity, apoptotic cell death and myeloperoxidase activity by modulating JNK, p38, MAPK pathways[30]. Meanwhile, there has been reported curcumin ameliorates LPS-induced liver failure and sepsis by related signaling[24]. Using different types of disease models explored the mechanisms of curcumin in anti-inflammatory activity. In this study, we administered LPS to mice to build retina inflammationinvivoand successfully found pre-treatment of curcumin decreased retinal inflammation. In line with previous studies, our results also demonstrated the anti-inflammatory effect of curcumininvivo.

Figure6SuppressioneffectofcurcuminonLPS-inducedPI3K/AktactivityinARPE-19cellsAkt phosphorylation was detected after pre-treatment with curcumin by Western blot analysis in LPS-activated cells (A, B). Activation of NF-κB was indicated by phosphorylation IκB-α (C, D). Curcumin treatment resulted in significantly reduction of phosphorylation of AKT and IκB-α compared with the LPS-stimulated group.
It is well known that the endotoxin initiates the vascular and cellular inflammatory responses after the exposure to LPS. Generally, a broad spectrum of chemokines and pro-inflammatory cytokines trigger the retinal inflammation process[31]. It is reported that TNF-α, IL-1β and IL-6 play a pivotal role in the EIU[4,25,32]. Apart from the chemical mediators of inflammation, during the early inflammation cell adhesion molecules play a crucial role in leukocyte adherence to vascular endothelial cells. Then damaged vascular lead to the leakage of protein in the AH. Meanwhile, LPS can stimulate RPE cells to secret IL-6, IL-8 and MCP-1[9]. Here, our data indicated that curcumin significantly inhibited these pro-inflammatory cytokines. However, further study of curcumin on animal model is warranted to explore the mechanisms of anti-inflammatory activity in retinal inflammation.
To the best of our knowledge, there are many signaling pathways about inflammation. PI3K/Akt-mediated NF-κB is a classical inflammatory signaling pathway. PIP3 is generated by PI3K in response to far-ranging of signals. Then the PDK1 is activated. PDK1 is translocated to the plasma membrane. Then PKB/Akt is activated by phosphorylating its kinase domain at Thr308 and Ser473 residues.Phosphorylation AKT regulates the activity of NF-κB, which plays a vital role in regulating several pro-inflammatory genes at the transcriptional level[33]. Under ordinary circumstances, NF-κB as an inactive form complex resides in the cytoplasm with the IκB-NF-κBp50/p65 trimetric complex. Once external stimuli lead to the IκB is phosphorylated, the p65 is freed and can exert its effect[34]. LPS induced inflammatory responses are reported to be mediated by the activation of NF-κB[35-36]. To investigate whether PI3K/Akt dependent NF-κB activation is involved in the regulation of inflammation by curcumin, we assessed the expression level of p-IκB-α and p-AKT in RPE cells. The results showed that curcumin treatment decreased the level of p-AKT. Moreover, p-IκB-α expression was increased in the LPS-treated cells but increase was reversed by curcumin-treated cell. Curcumin exhibited anti-inflammatory effects are related to suppression PI3K/Akt dependent NF-κB activation. In summary, this study demonstrated that curcumin has a preventive effect on LPS-induced retinal inflammationinvivoandinvitro.
The anti-inflammatory action of curcumin is concerned with inhibition of inflammatory cytokines production and suppression of PI3K/Akt dependent NF-κB activation. These results indicate the potential use of curcumin as a therapeutic strategy for suppressing retinal inflammation.

