Potential new metabolic approach for treatment of diabetes mellitus: converting glucose to ascorbic acid (vitamin C)
Payam Hashemi, Shaghayegh Pezeshki
1.MD (Doctor of Medicine), Faculty of medicine, Tehran University of Medical Science, Tehran, Iran
2.Department of Immunology, School of Medicine, Iran University of Medical Science, Tehran, Iran
Abstract
Key words: Diabetes mellitus, Vitamin C, Glucose conversion, Glycemic control, Hypotheses
Background
Diabetes is a metabolic disorder characterized by high levels of blood glucose over a prolonged period.There was about 451 million individuals with diagnosis of diabetes mellitus in 2017 and it has been estimated that the prevalence will increase up to 693 million in 2045 [1].The economic burden of diabetes will increase from $1.3 in 2015 to $2.1 in 2030 in the united states [2].This metabolic disease is categorized into two main groups type 1 and type 2 .Its worldwide health burden has been increased in the recent decades [3].Type 1 diabetes mellitus is defined as loss of beta cells of pancreatic islets and thereby insulin deficiency.Its prevalence is about 5% to 10% of whole diabetes mellitus cases [4].Type 2 diabetes mellitus is more complicated metabolic disorder than type 1 and is associated with insulin resistance and defective response of body tissues to insulin [5].Both types of diabetes mellitus is associated with many metabolic complications including inflammation and oxidative stress and subsequent clinical side effects including diabetic nephropathy, neuropathy and retinopathy [6,7].Also, atherosclerosis and cardiovascular disease is more prevalent in patients with diabetes mellitus [8].
During the evolution of vertebras, the ability of synthetizing of vitamin C (ascorbic acid) from glucose has been loosed, thus one of the metabolic pathways for controlling of blood glucose level is disappeared [9].This biochemical pathway is conducted with gulonolactone oxidase enzyme, which converts glucose to ascorbic acid in older vertebras [10].But the gene responsible for expressing this enzyme, GULOP (L-gulono-gamma-lactone oxidase), has been turned off about 60 to 63 million years ago [11].
The pathogenesis of type 2 diabetes mellitus is characterized by insulin resistance in peripheral tissues including muscles, liver and adipose tissue beside with insulin deficiency due to impairment of beta cells of pancreas [12].There has been discovered several drug groups for management of diabetes in terms of glycemic control and reducing the complications of this disease.Metformin, the only Biguanide available in market, is usually prescribed as the first choice for treatment of type 2 diabetes.There are many anti-diabetic agents including Sulfonylureas [13], Thiazolidinediones [14], Alpha-glucosidase inhibitors [15], Dipeptidyl peptidase-4 inhibitors [16], SGLT-2 inhibitors [17], Meglitinides [18], for controlling of hyperglycemia and management of diabetes complications.The other agent for management of diabetes is insulin which has different analogues and all types of them are prescribed subcutaneously [19].Despite the growing body of researches and drug discoveries for management of diabetes, the outcome of treatment of this disease is not satisfying enough, concerning clinical complications that involve many patients.(Fig.1)
According to the biochemical pattern demonstrated in the pathway, we can design a novel treatment for controlling of blood glucose in diabetes mellitus by converting the excess amount of glucose to ascorbic acid.By using this method, we can also manage the inflammation and oxidative stress with producing excess amount of vitamin C.On the other hand, ascorbic acid can reduce the glucose concentration in accordance with medical literatures.
Given the growing prevalence of diabetes mellitus, we review the common approaches for treatment and controlling of side eあects of this complicated disease.Due to insuきcient control in patients with diabetes mellitus, we propose a new approach for management of this disease.Our hypothesis is converting the excess amount of glucose to vitamin C and its derivatives in patients with diabetes mellitus.With this treatment we can have optimal glycemic control in addition to inhibition of oxidative stress and chronic inflammation in patients with diabetes mellitus.Concerning many diあerent challenges associated with gene-therapy, we propose pharmacologic way for solving this problem through imitating GULO enzyme function and facilitating this biochemical reaction.(Fig.2)
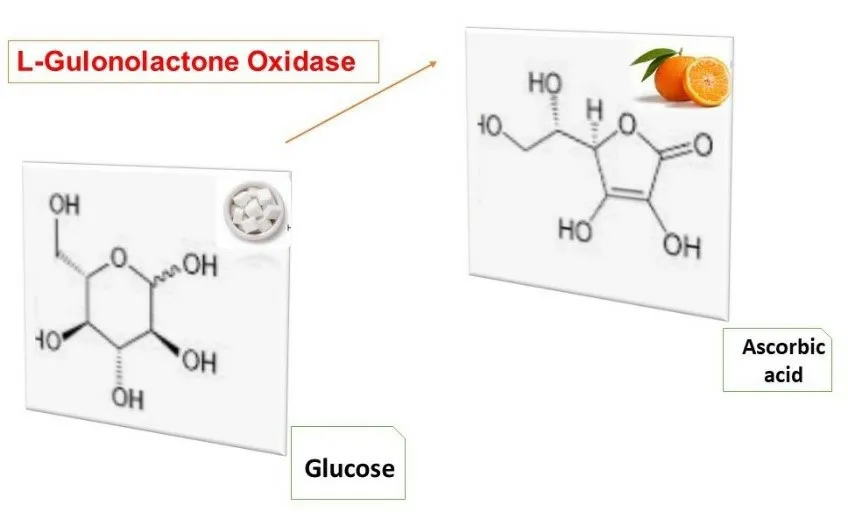
Figure 2 The main role of gulonolactone oxidase
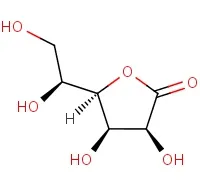
Figure 3 Gulonolactone structure
Evaluation of hypotheses
Regarding to biochemical similarity of vitamin C and glucose and the role of hyperglycemia in pathogenesis of diabetes, for evaluation of available data, the search was done in multiple data base such as Google, Google Scholar, PubMed, Elsevier Science Direct, Web of Science and Cochrane with the key words glucose and vitamin C plus/or diabetes mellitus.The search was limited to english articles about converting glucose to vitamin C as an approach for treatment of diabetes mellitus.Excluding some articles about the role of vitamin C in improvement of hyperglycemia and oxidative stress in diabetes, none of the articles discussed about converting the excess amount of glucose to vitamin C as an approach for treatment of diabetes.
Ganesh N.and colleagues demonstrated that vitamin C can reduce blood glucose level and decrease the rate of protein glycosylation.On the other hand, vitamin C ameliorates oxidative stress and inflammation in patients with type 2 diabetes mellitus.Thus, vitamin C can help for management of clinical complications of diabetes mellitus [20].
Metadichol, an ingredient of olive oil, has been reported to increase the level of vitamin C by activating of glutathione or reduced nicotinamide adenine dinucleotide phosphate (NADPH)-dependent systems.Another probable action of metadichol is activating of GULO gene and converting glucose to vitamin C according to Raghavan PR research [10].(Fig.3)
Discussion
Glucose plays a key role in maintaining of energy hemostasis in human cells.Cells of body provide glucose from three main sources: 1) Absorption of glucose through the gastrointestinal system; 2) Hydrolysis of glycogen in liver and muscle tissues, known as Glycogenolysis; 3) Biological pathways which produce glucose from other substrates such as amino-acids, defined as Gluconeogenesis [21].Insulin secreted into blood from beta cells of pancreas, known as islets of Langerhans, mostly after dietary meals [22].Most cells of human body are dependent on insulin for breaking up glucose and converting it to other biochemical substances like amino acids and glycogen [23].When the blood glucose decrease Glucagon, the other hormone releases from pancreas, stimulates catalysis of glycogen to glucose in liver and muscles tissues [24].
Reducing the consumption and increasing the production of glucose result in disturbance of glucose hemostasis in diabetes mellitus.This metabolic disorder can raise insulin resistance.Then, hyperglycemia and insulin resistance intensify each other during progression of this metabolic disorder.Hence, increasing in glucose and carbohydrate uptake and sedentary lifestyle are risk factors of diabetes mellitus.Insulin hormone has main role in both types of diabetes mellitus that regulates glucose uptake in most tissues of human body.Insulin deficiency, insulin resistance and insulin receptor deficiency are known as pathophysiology of diabetes [25].Other risk factors such as smoking, stress and genetic factors may increase the risk of diabetes through hyperglycemia and insulin resistance [26].Low diagnosis rate of diabetes mellitus and poor medical adherence of some medications for treatment of diabetes mellitus eventuate high level of health burden for this metabolic disorder [27].Clinical diagnosis of diabetes mellitus performed when fasting plasma glucose is more than 126 mg/Dl or/and plasma glucose is more than 200 mg/dl after 2 hours in OGTT test or/and random plasma glucose of 200 mg/dl or higher.Also, HbA1c level 6.5% or higher is another diagnostic criterion for detecting diabetes mellitus [5].
Type 1 diabetes is defined as very little or no release of insulin to blood due to destruction of beta cells of pancreas.Destroying of beta cells may progress in a background of autoimmune disorder [28].Due to childhood appearance, this disease is recognized as juvenile diabetes [29].Insulin is an essential hormone (released from beta cells of pancreatic islets) for consuming of glucose in terms of stimulating of Glycolysis and producing glycogen from glucose in peripheral body tissues.In the absence of insulin in type 1 diabetes mellitus and the subsequent impairment of glucose hemostasis [25], the clinical symptoms of this disorder appear.These symptoms are microvascular injury including diabetic nephropathy, retinopathy and neuropathy and cardiovascular disease known as macrovascular injury [30].Oxidative stress and chronic inflammation which intensify with hyperglycemia play an essential role in progression of micro and macro vascular damages in both types of diabetes mellitus [31].Besides with lifestyle modifications like exercise and lower uptake of carbohydrate, insulin therapy is essential for management of type 1 diabetes.Insulin, the main treatment of type 1 diabetes, may lower blood glucose to the extent of hypoglycemia symptoms.Type 2 diabetes mellitus is usually arise from adulthood and characterized by insulin resistance, high levels of blood glucose and dysfunction of insulin and its receptor [32].Like type 1 diabetes, clinical complications of type 2 diabetes include diabetic nephropathy, retinopathy and neuropathy in addition to cardiovascular disease.In contrast with type 1 diabetes, glycemic and side effects control can be achieved without insulin therapy in type 2 diabetes [33].However, if other treatments would not capable of glycemic and clinical control, insulin can play a key role in this situation.Clinical management of type 2 diabetes is done by administration of metformin one or twice daily unless there are contraindications [34].Metformin exerts its biochemical eあects through several mechanisms including reduction of gluconeogenesis in liver, improvement of insulin resistance, inhibition of mitochondrial respiratory chain I [35], activation of AMP-activated protein kinase [36], inhibition of glucagon-induced elevation of cyclic adenosine monophosphate through reducing activation of protein kinase A, inhibition of mitochondrial glycerophosphate dehydrogenase [37] and beneficial changes in gut microbiota [38].Compared with Sulfonylureas, metformin as a first line therapy has more beneficial eあect in terms of glycemic control and cardiovascular disease [39].There is little systematic data for efficacy of other oral agents as the first line therapy for treatment of type 2 diabetes.The major side effects of metformin are gastrointestinal intolerance such as bloating, abdominal discomfort, nausea and diarrhea.Mentioned side eあects of metformin cause poor medical adherence of this drug in patients with type 2 diabetes mellitus [40].
Oxidative stress can prevent from free radical detoxifying and redox-regulating enzymes in beta cells of pancreas such as Catalase, Glutathione Peroxidase, Superoxide Dismutase, and Thioredoxin.Low levels of antioxidant agents besides with failure to detoxification of H₂O₂ and chronic inflammation result in impairment of beta-cells and thereby insulin deficiency in diabetes mellitus [41].Chronic uncontrolled hyperglycemia leads to significant rate of diabetic retinopathy, nephropathy and neuropathy [42].Some isoforms of NADPH Oxidase (NOX) play a pivotal role in development of diabetic nephropathy.For example, NOX-1 induces renal injury and hypertension in diabetes mellitus.NOX-2 which produces from macrophage may lead to renal cell injury and NOX-5 induces glomerular injury too [43].Diあerent pathologic biochemical processes such as Polyol pathway [44], Hexosamine pathway [45], Protein Kinase C signaling [46], AGE pathway [47], PARP pathway [48], MAP Kinase pathway [49], NFKβ signaling, Hedgehog pathway, TNF-α signaling [50], Cyclooxygenase pathway, WNT pathway, Autophagy and GSK3 signaling play an important role in progression of diabetic neuropathy [51].On other hand, in the setting of minimized activity of antioxidant enzymes, such as Catalase, Glutathione Peroxidase and Lipoperoxidase, Arginase enzyme overactivate in the process of diabetic retinopathy [52].Overactivated Arginase induces lower bioavailability of nitric oxide (NO) which stimulates the progression of diabetic retinopathy.Too many oxidative pathways and chronic inflammatory mechanisms proceed to microvascular injury in diabetes.Also, the altered biochemical setting can lead to cardiovascular complications which defined as macrovascular disease [53].
Regarding to the role of oxidative stress in worsening of inflammatory disease, the role of antioxidants has been investigated [54].In this way, some researchers carried out some observations to illustrate the role of different types of antioxidants for inhibitions of oxidative stress and chronic inflammation.Diabetes, as a chronic disease with high levels of oxidative stress and chronic inflammation has been assessed for controlling of these metabolic complications [55].Rafighi.Z et al showed that vitamin C can alleviate hypertension, hyperglycemia and increase the concentration of Super Oxide Dismutase (SOD) and Glutathione [56].Other clinical observations have shown the role of vitamin C in ameliorating inflammatory status in terms of decreasing interleukin 1,6, TNFα and hs-CRP [29, 30].Furthermore, the inhibitory function of vitamin C on PARP and NFKβ pathway has been investigated.Other clinical studies assessed the antioxidant pattern of vitamin C in improvement of oxidative stress in other disease.For example, John X.Wilson reported the useful role of intravenous vitamin C in reducing NADPH Oxidase and thereby improvement of endothelial function in patients with sepsis [57].Another study explained the reduction of MAP Kinase through activation of Phospholipase D by ascorbic acid in microvascular endothelial cells of lung tissue [58].In animal model ascorbic acid results in increasing the level of Catalase [59].
Despite various medications for management of diabetes, desirable control of its clinical complications has not been achieved [60].Parts of these clinical complications are results of low drug adherence due to different clinical side eあects of current medication [61].In other hand, the current management of this disorder could not appropriately control the chronic inflammation and oxidative stress [62].Also concerning high level of health burden of diabetes mellitus, it is important to find new approaches for management of diabetes in terms of glycemic control and minimizing the clinical complications (3).Vitamin C is an antioxidant and anti-inflammatory molecule that structurally close to glucose [56].Therefore, in order to achievement of more effective management of diabetes, we figure out an idea about converting excess amount of glucose to vitamin C and its derivatives.With consider to high levels of oxidative stress and chronic inflammation in patients with diabetes mellitus, by converting glucose to vitamin C, we can prevent from oxidative stress and chronic inflammation [63].The potential limitation of our idea is increasing concentration of vitamin C up to toxic levels.John X.Wilson reported that high dose of intravenous ascorbic acid up to 1.5 gr/kg is safe in critical patients with cancer [59].However, we propose converting glucose to vitamin C and its derivatives in order to resolve this challenge.The excess amount of vitamin C and its derivatives clear by renal filtration [64].By optimized glycemic control, we can inhibit chronic inflammation and oxidative stress and thereby promoting of lifestyle in these patients.
 Medical Theory and Hypothesis2019年4期
Medical Theory and Hypothesis2019年4期
- Medical Theory and Hypothesis的其它文章
- Analysis of uterine artery and sex hormones in treating diminished ovarian reserve with tiaojing decoction
- Interpretation of data and treatment of Jing-well point temperatures´ test
- Application value of tongue coating microflora in integration of traditional Chinese medicine and modern medicine
- Medical ethics w.s.r to ayurveda
