ZIF-derived Co-N-C ORR catalyst with high performance in proton exchange membrane fuel cells
Ruixing Wng ,Pengyng Zhng ,Yucheng Wng,∗∗ ,Yuesheng Wng ,Krim Zghi ,Zhiyou Zhou,∗
a State Key Laboratory of Physical Chemistry of Solid Surfaces,Collaborative Innovation Center of Chemistry for Energy Materials,College of Chemistry and Chemical Engineering,Xiamen University,Xiamen,361005,China
b Center of Excellence in Transportation Electrificationand Energy Storage Hydro Québec,Québec,J3 × 1S1,Canada
ABSTRACT Metal and nitrogen-doped carbon (M-N-C) materials have been considered as the most promising non-precious metal oxygen reduction(ORR)catalysts to replace expensive Pt catalysts.Due to high Fenton catalytic activity of Fe element and the resulting instability,Co-based N-C (Co-N-C) catalysts without Fenton catalytic activity should be a worthier ORR catalyst being explored.Although the high ORR activity of Co-N-C catalyst has been demonstrated in aqueous half-cell tests,their performance under PEMFC working condition is still far away from that of state-of-the-art Fe-N-C catalysts.In this study,a high-performance Co-N-C catalyst was synthesized by one-step pyrolyzing Co-doped ZIF-8 (zeolitic imidazolate framework-8) particles in-situ grown on the highsurface-area KJ600 carbon black with high electronic conductivity.The resulting Co-N-C catalyst exhibited high intrinsic ORR activity,fast mass transfer rate and high electronic conductivity,and thus yielded a remarkable peak power density of 0.92 W cm-2 in H2-O2 PEMFC,which is comparable to state-of-the-art Fe-N-C catalyst.This strategy is helpful to synthesize highly active M-N-C ORR catalysts with improved mass transfer and electric conductivity.
Keywords:Non-precious metal electrocatalysts Fuel cell Oxygen reduction Co-based catalyst ZIF-8
1.Introduction
Proton exchange membrane fuel cells(PEMFCs)have been regarded as one of the most promising energy-conversion devices due to high energy efficiency,quick refueling and zero pollutant emission [1].Despite their advantages,PEMFCs currently require the use of expensive Pt catalysts for the oxygen reduction reaction (ORR),which makes such devices cost-prohibitive for many applications [2].In this regard,the exploration of non-precious metal ORR electrocatalysts to replace expensive Pt catalysts is indispensable for the commercialization of PEMFCs [3].Pyrolyzed metal and nitrogen-doped carbon(M-NC) materials have been considered as the most promising non-precious metal ORR catalysts to replace expensive Pt catalysts [4,5].Recently,the ORR activity of Fe-based nitrogen carbon (Fe-N-C) materials has been demonstrated to be comparable to that of Pt catalysts with a halfwave potential (E1/2) of higher than 0.8 V (vs.RHE,Reversible Hydrogen Electrode) in aqueous solution and a peak power density exceeding 1 W cm-2under membrane electrode assembly (MEA) working condition [6-10].However,these highly-active Fe-N-C catalysts suffered from poor stability.One possible reason for their instability is because Fe ion will react with some H2O2released by incomplete ORR,i.e.,Fenton’s reagent,which generates highly oxidizing ⋅OH radical.The ⋅OH can chemically oxidize the catalytic active sites,carbon matrix,and Nafion membrane [11].Due to a higher equilibrium potential of Co3+/2+,Co element is not able to undergo the Fenton’s reaction[12].As such,Co-N-C materials should be better ORR catalysts as compared to Fe-N-C materials.Very recently,the high ORR activity of Co-N-C catalysts has been demonstrated under aqueous solution testing condition with anE1/2of 0.84 V [13].However,the performance of Co-N-C catalysts under fuel cell working condition is still far away from that of state-of-the-art Fe-N-C catalysts.
The pyrolysis of metal-organic framework (MOFs) has been regarded as an efficient route to synthesize highly active M-N-C ORR electrocatalysts[14,15].The pristine structure of MOFs can be partially retained,providing an avenue to design ORR catalysts with controlled structure[16].However,some problems still exist for MOFs-based ORR electrocatalysts,such as poor electronic conductivity due to their polyhedral shape and a relatively low pyrolysis temperature(<1100 °C),slow mass transfer rate due to relatively large particle sizes (>100 nm) and low mesoporosity [17].To improve the mass transfer rate and electronic conductivity,an efficient strategy was the incoporatation of an inactive phase with high mesopore content and excellent electronic conductivity into the final ORR catalysts [18-20].However,the introduction of inactive phase may also dilute the density of ORR active phase [21].So far,the influence of adding such an inactive phase on the ORR activity of MOFs-based catalysts in aqueus solution testing condition has been extensively investigated [18-20],but the relevant research under MEA working condition is still lacking.
In this work,a high-performance MOFs-based Co-N-C catalyst(denoted as CoNC@KJ600)was synthesized by one-step pyrolyzing Codoped ZIF-8 particles in-situ grown on the high-surface-area KJ600 carbon black with high electronic conductivity.The high specific surface area and porous structure can separate ZIF-8 particles spatially and prevent the aggregation of ZIF-8 particles during pyrolysis process,which contributes to the formation of highly-active Co-N coordination structure.Such a strategy can increase the density of ORR-related active species and thus counteracting the dilution effect of inactive additive on ORR active phase.Moreover,the mesopores structure and high electronic conductivity of KJ600 carbon black can be retained in the final CoNC@KJ600 catalyst.Owing to its improved mass transfer rate and electronic conductivity,the concentration overpotential and ohmic drop of CoNC@KJ600 cathode was significantly lowered,thus resulting in a remarkable peak power density of 0.92 W cm-2in H2-O2PEMFC,which is comparable to the state-of-the-art Fe-N-C catalysts.
2.Materials and experimental
2.1.Materials
2-Methylimidazole (98%,Alfa Aesar),sulfuric acid (suprapur 96.0%,Merck),ketjenblack EC600J (KJ600,Akzo Nobel),Nafion(D520,EW 1100,5%,Dupont) were used as received.Zinc nitrate hexahydrate [Zn(NO3)2,99.0%],Cobalt (Ⅱ) nitrate hexahydrate [Co(NO3)2·6H2O,99.0%],and methanol (CH3OH,99.7%),Nitric acid(HNO3,65%-68%) were purchased from China Medicine Shanghai Chemical Reagent Corp.High purity Ar (99.999%),O2(99.998%) and N2(99.99%) were purchased from Linde.
2.2.Catalysts synthesis
KJ600 carbon black was pre-treated with 8 M HNO3solution at 80 °C for 4 h to increase the carboxyl and hydroxyl groups on the surface.The oxygen-containing groups could coordinate with metal ions,and consequently making ZIF nanocrystals uniformly dispersed on the surface of KJ600 carbon black.To synthesize CoNC@KJ600 catalyst,acid-pretreated KJ600 carbon black (200 mg),Co(NO3)2(50 mg),and Zn(NO3)2(50 mg) were ultrasonically dispersed in 50 mL of methanol (Solution A).Solution B was prepared by dissolving 50 mg of 2-methylimidazole in 50 mL methanol.Then,solution B was added dropwise to solution A,followed by stirring for 24 h.Afterward,the precursor (labeled as Co-ZIF-8@KJ600) was collected by centrifugation,washed with methanol and dried at 60°C for 12 h under vacuum.The as-prepared Co-ZIF-8@KJ600 precursor was then pyrolyzed at 800 °C for 2 h under the atmosphere of 10% NH3and 90% N2.The heating rate was 5°C min-1.To illustrate the dispersion effect of KJ600 carbon black on the final CoNC@KJ600 catalyst,a control sample without the addition of KJ600 carbon black,labeled as CoNC,was also prepared using the same procedure.
2.3.Physical characterization
X-ray photoelectron spectroscopy (XPS) was conducted on a Qtac-100 LEISS-XPS instrument.Brunauer-Emmett-Teller(BET)specific area and pore size distribution was measured on a Micromeritics ASAP 2020 sorption analyzer.X-ray diffraction (XRD) was performed on Rigaku Ultima IV with Cu Kα radiation.The morphologies were characterized by a FEI Tecnai G2 20 S-TWIN(F20)electron microscope at 200 kV and a Hitachi S4800 field emission scanning electron microscope at 6 kV.
2.4.Electrochemical measurements
All the electrochemical data were collected on a CHI-760D bipotentiostat equipped with a rotating ring-disk electrode (RRDE,Pine Inc.).A standard three-electrode system was selected for ORR performance test.A glassy carbon (GC,5.61 mm diameter) disk−Pt ring RRDE,a thin graphite plate (5 × 2 cm) and a RHE were used as the working electrode,counter and reference electrode,respectively.The electrochemical cell was placed in 30 °C water baths.To prepare the working electrode,6 mg of the Co-based catalyst was ultrasonically dispersed in the mixture of 0.5 mL deionized water and 0.5 mL ethanol,followed by the addition of 50 μL Nafion(5 wt%,Dupont).The 25 μL of the catalyst ink was dropped onto the freshly polished GC disk of RRDE.The catalyst loading was 600 μg cm-2.Before each test,electrolyte solution was bubbled with high-purity N2or O2for at least 15 min.ORR polarization curves were recorded with potential scanning from 0.2 to 1.0 V at 10 mV s-1in O2-saturated 0.1 M H2SO4solution.Double-layer charging current was corrected by the voltammogram recorded in Arsaturated solution.The solution ohmic resistance was compensated for ORR polarization curves.
2.5.Membrane electrode assembly (MEA) preparation and evaluation
To prepare the cathode catalyst ink,10 mg of Co-N-C catalyst was added into ethanol (1 mL),followed by the addition of 217 μL Nafion(5 wt%).After dispersing ultrasonically for 1 h,the resulting ink was coated on a gas diffusion layer to obtain Co-N-C cathode.The loading of Co-N-C was controlled at 4 mg cm-2.The MEA was fabricated by hotpressing a Co-N-C cathode (4 mg cm-2),an commercial Pt/C anode(0.4 mg cm-2),a NRE 211 Nafion membrane (Dupont),and a PTFEpretreated Toray 060 carbon paper with a microporous layer at 135 °C and 3 M Pa for 2 min.The active area of MEA was 1.21 cm2.The weight ratio of the Co-N-C catalyst to Nafion was controlled at 1:1.Polarization curves were measured at 80°C on Model 850e fuel cell test system (Scribner Associates Inc.).The ohmic resistance of the cell was represented by high-frequency resistance(HFR)at 1000 Hz.The ohmic resistance of the cell was also calculated by analyzing polarization curves [22] and compared with the values obtained by HFR method(Fig.S1).H2and O2with 100% RH were fed as fuel and oxidant at a flow rate of 0.2 slpm.The back pressure was controlled at 1 or 2 bar.
3.Results and discussion
3.1.Materials synthesis and characterizations
The CoNC@KJ600 catalyst was synthesized via a one-step pyrolyzing Co-doped ZIF-8 (labeled as Co-ZIF-8) particles grown on highsurface-area KJ600 carbon black (Fig.1).The BET surface area of KJ600 carbon black was 1400 m2g-1(Table S1).After treated by concentrated HNO3solution,the carbonaceous surface of KJ600 was rich in carboxyl and hydroxyl groups [23].The high surface area,combined with strong interactions between the oxygen-containing groups and ZIF particles,enabled the uniform growth of Co-ZIF-8 particles on the carbonaceous surface.The successful growth of Co-ZIF-8 particles on the KJ600 was confirmed by the observation of typical XRD pattern of ZIF-8 structure (Fig.S2).Notably,compared with pure KJ600,the morphology of Co-ZIF-8@KJ600 changed little,and no considerable ZIF-8 crystals could be observed in TEM images of the Co-ZIF-8@KJ600(Fig.S3).The above two points indicated that a thin layer of ZIF-8 particles was uniformly coated on the carbonaceous surface of KJ600 carbon black or confined in the pores.After pyrolysis at 950°C for 3 h,the BET surface area of KJ600 was still high (1200 m2g-1) (Table S1),implying that the porous structure of KJ600 carbon black was retained.It is therefore expected that the porous structure of KJ600 would isolate ZIF-8 particles spatially and consequently prevent the aggregation of ZIF-8 particles during the high-temperature treatment process.To illustrate the dispersion effect of KJ600 carbon black,a control sample without the addition of KJ600 carbon black,labeled as CoNC,was also prepared using the same procedure.In this case,ZIF-8 crystals were found to be aggregated.
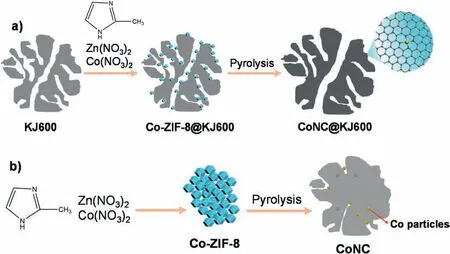
Fig.1.Schematic of the synthesis process of CoNC@KJ600 and CoNC catalysts.
Fig.2a-c revealed the SEM and TEM images of the final CoNC@KJ600 catalyst.The SEM images showed that CoNC@KJ600 catalyst consisted of highly dispersed porous carbon sphere(Fig.2a),with little Co nanoparticles observed in the TEM images at different magnifications(Fig.2b and c).This implied that Co element is highly dispersed in the CoNC@KJ600 catalyst and consequently more likely to be existed in the form of Co-Nxcoordination structure[24].The highly-dispersed Co element in the CoNC@KJ600 catalyst was further confirmed by energy dispersive X-ray spectroscopy (EDS) mapping analysis (Fig.3).In addition,the TEM images also revealed that the mesopore structure of KJ600 black carbon was retained in the final CoNC@KJ600 catalyst.In contrast,without the separation effect of KJ600 carbon black,ZIF-8 particles aggregated under high-temperature condition as shown in the SEM image (Fig.2d).TEM analysis revealed a large number of Co nanoparticles in the CoNC catalyst (Fig.2e and f),implying that the aggregation of Co-ZIF-8 particles during the pyrolysis process promoted the formation of Co nanoparticles.The formation of Co nanoparticles will not only decrease the density of highly-active Co-Nxstructure for ORR but also block the pores for mass transport [25].
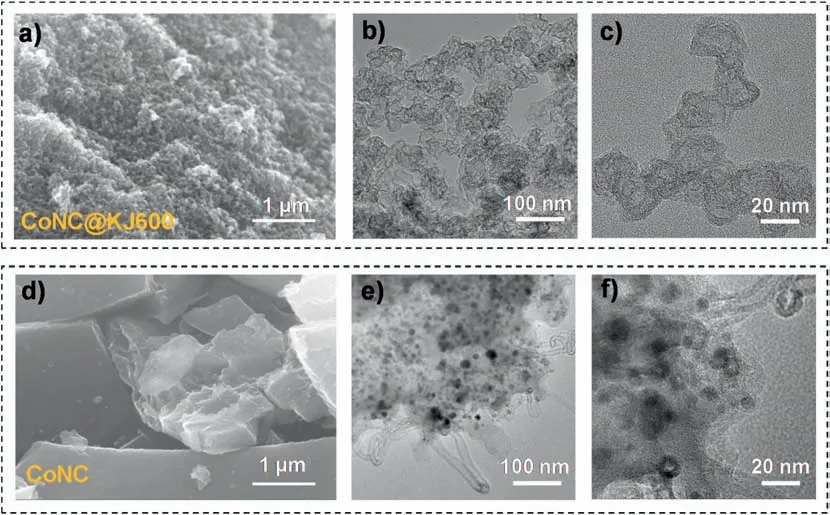
Fig.2.(a) SEM and (b,c) TEM image of CoNC@KJ600 catalyst.(d) SEM and (e,f) TEM image of CoNC catalyst.
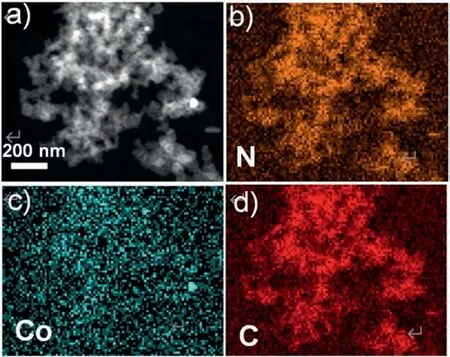
Fig.3.(a)STEM of CoNC@KJ600 and the responding element mapping images of (b) N,(c) Co and (d) C.
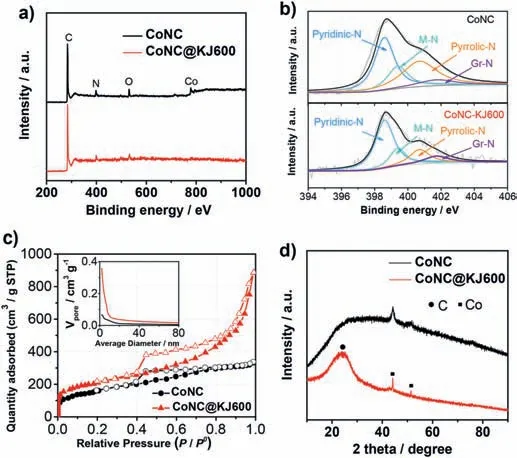
Fig.4.(a) XPS survey spectra;(b) High-resolution N 1s XPS and the corresponding peak deconvolution;(c) N2 adsorption/desorption isotherm (Insert shows the BJH mesopore distribution);(d) XRD pattern of CoNC and CoNC@KJ600 catalysts.
The surface chemical components of the two catalysts were analyzed by XPS.Fig.4a exhibited the XPS survey spectra of the two Co-N-C catalysts.Both the two catalysts contain C,N,O,and Co element.The atomic percentage of C,N,O,and Co were 92.8%,4.68%,1.94%,and 0.55%,respectively,for CoNC@KJ600 catalyst,while 81.0%,7.36%,10.2% and 1.39% for CoNC catalyst.A relatively lower Co (0.55 at%vs.1.39 at%) and N (4.68 at%vs.7.36 at%) content of CoNC@KJ600 than CoNC catalyst was attributed to the addition of inactive phase (KJ600 carbon black) and thus,diluting the content of Co and N on the catalyst surface.The high-resolution of Co 2p XPS shows that Co is mainly in the form of Co2+species for the both two catalysts (Fig.S4) [13].The high-resolution N 1s XPS and the corresponding peak deconvolution of the two catalysts were shown in Fig.4b and Table S2.Among all N species,metallic-N and pyridinic-N were determined to contribute to the ORR activity of M-N-C catalyst [26].Due to the addition of inactive component (KJ600 carbon black support),the content of metallic-N of CoNC@KJ600 (0.81 at%) was 0.5-fold lower than that of CoNC catalyst (1.31 at%),but their pyridinic-N content was found to be very similar(2.56 at%vs.2.96 wt%).The XPS signal of metallic-N was generally assigned to be M-Nxspecies,thus a lower content of metallic-N implied a lower content of M-Nxspecies in CoNC@KJ600.However,among all M-Nxspecies,only a part of such structures were active or accessible for the ORR[27,28].CoNC@KJ600 catalyst exhibited much smaller particles sizes,which implied a higher utilization of active sites and compensated the possible activity loss caused by a lower content of M-Nxspecies.This might be attributed to the dispersion effect of KJ600 on ZIF particles during pyrolysis process,thus forming more active or accessible Co-N coordination structures[29].
The specific surface area and pore size distribution of the two Co-N-C catalysts were analyzed by N2adsorption/desorption isotherm.As shown in Fig.4c,CoNC@KJ600 shows a type-IV isotherm with a large adsorption capacity in the low-pressure region and a hysteretic loop in the range of 0.4-1.0P/P0,indicating the coexistence of micropores and mesopores in the CoNC@KJ600 catalyst.CoNC catalyst exhibited a similar pore structure,but the BET surface area(494 m2g-1)and mesopore content(Fig.4c,insert)were lower than those of CoNC@KJ600(653 m2g-1),which agreed with different TEM images observed for the two catalysts (Fig.2c and f).It had been determined that micropores were the host of ORR active sites while mesopores could allow sufficient mass transfer in M-N-C ORR catalyst[30].The CoNC@KJ600 catalyst,having abundant micropores and mesopores,should therefore be expected to have excellent ORR activity.
The structures of the two catalysts were further analyzed by XRD.Fig.4d shows the XRD patterns of CoNC@KJ600 and CoNC catalysts.The diffraction pattern attributed to metallic Co crystal was observed in the CoNC catalyst.The absence of diffraction signal of graphitic structure reveals a low graphite degree and consequently low electronic conductivity of CoNC catalyst.In contrast,the diffraction peak located at 25° of CoNC@KJ600 catalyst corresponded to the(002) plane of the graphitic structure.The graphitic structure was also observed by the HRTEM image of CoNC@KJ600 catalyst (Fig.S5).Considering that KJ600 carbon black was partially retained in the final CoNC@KJ600 catalyst,the graphitic signal should be attributed to that of the KJ600 carbon black.The graphitic carbon matrix of CoNC@KJ600 can ensure high electronic conductivity and consequently accelerate ORR,especially in fuel cell operating conditions [21].Unexpectedly,metallic cubic-phase Co corresponding to the diffraction peaks at around 44.2°and 51.5° was also observed in the CoNC@KJ600 catalyst.The XRD result is obviously contradictory to the above TEM images (Fig.2b and c)with little Co particles.This discrepancy may be because the density of Co particles is too low to be observed by the local TEM images easily.
3.2.Electrochemical ORR performance
The ORR activity of the two Co-N-C catalysts was initially evaluated by a rotating disk electrode (RDE) test in O2-saturated 0.1 M H2SO4solution.Under RDE test condition,where mass-transport effects in catalyst coating layer on the electrode could be negelected,the intrinsic ORR activity of the target catalyst could be represented by the kenetic current at 0.8 V (RHE) [31].A higher kenetic current implies a superior ORR kinetic activity[32].As shown in Fig.5a,CoNC@KJ600 catalyst exhibited a slightly higher kenetic current density than CoNC catalyst after mass-transfer correction through Koutecky-Levich equation(1.58vs.1.28 A g-1@0.8V).The much lower diffusion-limiting current on the CoNC than on the CoNC@KJ600 could be attributed to large catalyst particles of the CoNC,resulting in poor dispersion on the electrode surface and slow O2diffusion to the active sites located at the interior of catalyst nanoparticles.Similar kenetic current at 0.8 V(RHE)implied that the intrinsic ORR activities of two catalysts were similar.However,the CoNC@KJ600 catalyst has open pore structure and highly graphitizated carbon (high conductivity),which are very important for the fuel cells.We then carried out a H2-O2PEMFC test,as shown in Fig.5b.Under fuel cell conditions,the current density of at high cell voltage is mainly controlled by electrochemical kinetics,i.e.,catalyst intrinsic activity.At the cell voltage higher than 0.65 V,the fuel cell performance of CoNC@KJ600 cathode is just slightly higher than that of CoNC catalyst (Fig.5b and Fig.S6),consistent with the RDE test result(Fig.5a).However,at the lower cell voltage,where mass transfer and ohmic resistance become limiting[33],the current density at 0.6 V(practical working voltage of fuel cell) and the peak power density of CoNC@KJ600 cathode(0.34 A cm-2&0.72 W cm-2)is 1.5 and 1.6 times larger than those of CoNC cathode(0.23 A cm-2&0.45 W cm-2)at 1 bar back pressure,respectively(Fig.5b).At a higher back pressure of 2 bar,the peak power density of H2-O2PEMFC based on CoNC@KJ600 cathode could reach 0.92 W cm-2.To the best of our knowledge,this is the highest peak power density reported so far for H2-O2PEMFCs with Co-N-C catalysts as cathodes (Table 1) and comparable to most of highly active Fe/N/C cathodes (Table S3).However,the polarization performance of CoNC@KJ600 cathode was far inferior to that of Pt/C cathode,which should be improved further (Fig.S7).
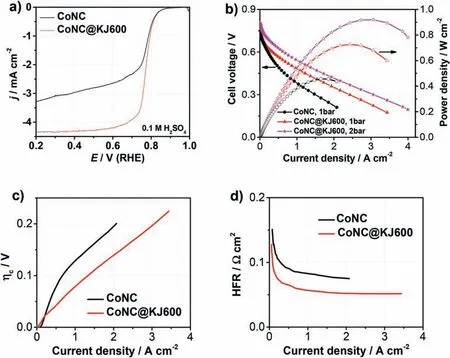
Fig.5.(a)ORR polarization curves of CoNC and CoNC@KJ600 catalysts in O2-saturated 0.1 M H2SO4 solution.Scan rate:10 mV s-1;rotation rate:900 rpm,solution temperature:30 °C;catalyst loading:0.6 mg cm-2.(b)Polarization and power density curves of H2-O2 PEMFCs employing CoNC and CoNC@KJ600 as the cathode catalyst,respectively.(c) Concentration overpotential and (d) High-frequency resistance (HFR) of H2-O2 PEMFCs employing CoNC and CoNC@KJ600 as the cathode catalyst,respectively.Flow rate:200 sccm for both H2 and O2;NRE 211 membrane;cathode catalyst loading:4.0 mg cm-2;anode catalyst:Pt/C (40 wt%,Johnson Matthey) with 0.4 mgptcm-2.
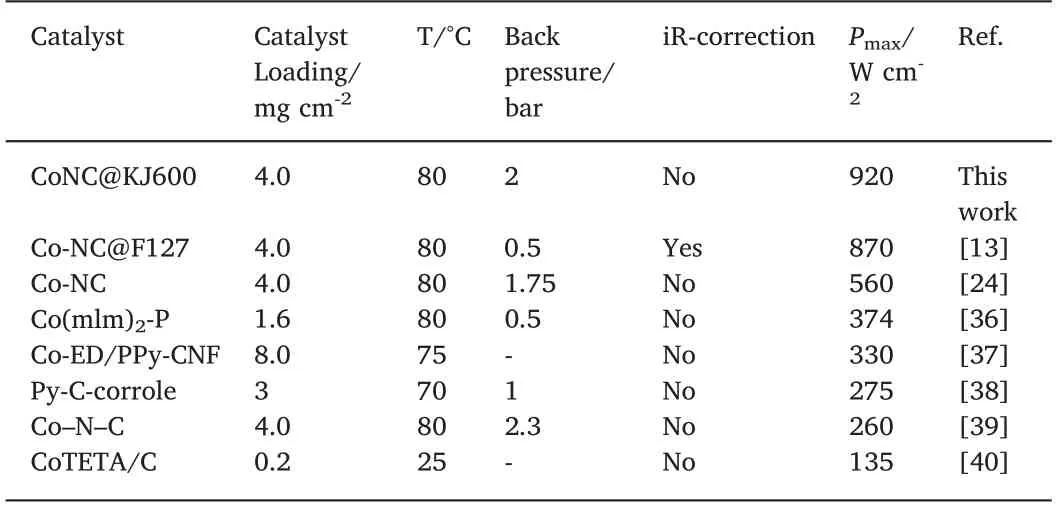
Table 1 Peak power density of H2-O2 PEMFC employing Co-N-C cathode.
Due to the similar intrinsic ORR activity of the two catalysts,the superior power density of CoNC@KJ600 over CoNC cathode should be attributed to the faster mass transfer and higher electronic conductivity.The mass transfer could be evaluated by comparing the concentration overpotential(ηC).The lower ηCindicates the higher mass transfer rate.The ηCcan be determined by the voltage gap between the polarization curve extrapolated by Tafel’s equation and the measured curve after the iR correction[34,35].As shown in Fig.5c and Fig.S8,the ηCof CoNC@KJ600 is considerably lower (0.08 V at 1 A cm-2) than that of CoNC(0.13 V at 1 A cm-2),which confirmed the faster mass transfer of the CoNC@KJ600 cathode.The electronic conductivity could be readily evaluated by high-frequency resistance.As shown in Fig.5d,the highfrequency resistance of PEMFC with CoNC@KJ600 cathode is close to 50 mΩ cm2,which was about 25 mΩ cm2lower than that of PEMFC with CoNC cathode.
Apart from ORR activity,the stability is another critical parameter to evaluate the ORR catalysts.The stability of CoNC@KJ600 and CoNC was evaluated by a chronoamperometric measurement at a half-wave potential (0.77 Vvs.RHE) in an O2-saturated 0.1 M H2SO4solution.After 20-h test,the CoNC@KJ600 and CoNC exhibited 27%and 46%of ORR activity loss,respectively (Fig.6a).This implied that CoNC@KJ600 was more durable than CoNC catalyst,which may be attributed to a lower content of Co metal particles [41].By comparing high-resolution N 1s XPS of the CoNC@KJ600 catalyst before and after the stability test,the pyridinic-N content decreased from 2.56 at%to 1.38%(Fig.6b and Fig.S9),which implied that the disappearance of pyridinic-N content might be highly relevant to the observed instability of CoNC@KJ600 catalyst [42].The disappearance of pyridinic-N maybe due to the attack of H2O2toward the N functionalities [43].
4.Conclusion
A Co-N-C catalyst with high ORR performance has been synthesized by one-step pyrolysis of Co-doped ZIF-8 particles in-situ grown on high-surface-area KJ600 carbon black.The high surface area and porous structure of KJ600 carbon black allow the high dispersion of ZIF-8 particles and minimizing aggregation of ZIF-8 particles during pyrolysis process.In addition,the mesopore structure and high electronic conductivity of KJ600 is retained in the as-synthesized catalyst.As such,CoNC@KJ600 catalyst exhibits high intrinsic ORR activity,fast mass transfer rate and low ohmic polarization loss,and consequently yields a remarkable power density of 0.92 W cm-2in H2-O2PEMFC.
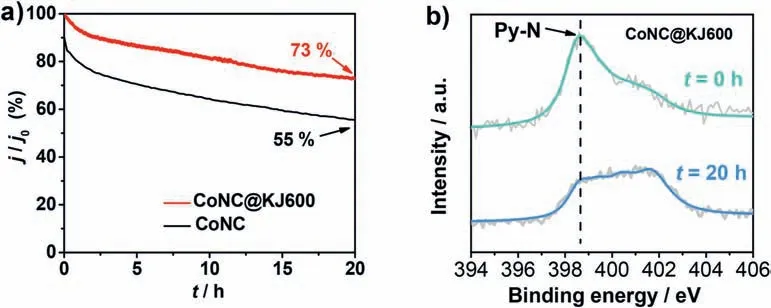
Fig.6.(a) Current-time chronoamperometric response of CoNC@KJ600 at 0.77 V with a rotating speed of 900 rpm in O2-saturated 0.1 M H2SO4 solution.All the data was relative to the initial ORR current density.(b) High-resolution N 1s spectra of CoNC@KJ600 before and after 20-h stability test.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This study was supported by grants from National Key Research and Development Program of China (2016YFB0101200) and Natural Science Foundation of China(21875194).Special statement:the author Y.S.W and K.Z have nothing to do with the program,and they are not supported by the program.
Appendix A.Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.pnsc.2020.09.010.
 Progress in Natural Science:Materials International2020年6期
Progress in Natural Science:Materials International2020年6期
- Progress in Natural Science:Materials International的其它文章
- Stability of PGM-free fuel cell catalysts:Degradation mechanisms and mitigation strategies
- Understanding of free radical scavengers used in highly durable proton exchange membranes
- Electrolyte membranes for intermediate temperature proton exchange membrane fuel cell
- Key technologies for polymer electrolyte membrane fuel cell systems fueled impure hydrogen
- Electrolyte materials for intermediate-temperature solid oxide fuel cells
- Carbon-free nanoporous gold based membrane electrocatalysts for fuel cells
