Pyrolysis of Iron(III) porphyrin coated Pt/C toward oxygen reduction reaction in acidic medium
Yng Lv ,Huiyun Liu ,Jiqi Qin ,Rui Go ,Yunlong Zhng ,Yn Xie,c ,Ji Li,d,Yujing Song,∗
a State Key Laboratory of Fine Chemicals,School of Chemical Engineering,Dalian University of Technology,2 Linggong Road,Dalian,116024,China
b Department of Mechanical and Mechatronics Engineering,University of Waterloo,Waterloo,ON N2L 3G1,Canada
c Dalian National Laboratory for Clean Energy,Dalian Institute of Chemical Physics,Chinese Academy of Sciences,457 Zhongshan Road,Dalian,116023,China
d College of Engineering and Applied Sciences,Nanjing University,22 Hankou Road,Nanjing,210093,China
ABSTRACT Design and synthesis of highly active and durable electrocatalysts toward oxygen reduction reaction (ORR) is of particular importance for proton exchange membrane fuel cells(PEMFCs),yet remains a grand challenge.Herein,we report the deposition of iron(III)porphyrin(FeP)on house-made Pt/C by rotary evaporation of the mixture of FeP and house-made Pt/C dispersed in chloroform,followed by pyrolysis at 650°C in argon atmosphere.This approach led to the synthesis of new non-precious metal electrocatalyst (NPME)-Pt/C composites (Pt/C-FeP) with an average nanoparticle diameter of 3.1 ± 1.5 nm without aggregation.According to X-ray photoelectron spectroscopy (XPS),the binding energy of Pt 4f7/2 became larger due to the presence of pyrolyzed FeP.In addition,the electrochemically active surface area(ECSA)of Pt/C-FeP-650 is 65 m2/g less than that of house-made Pt/C(80.2 m2/g).This implies that the pyrolyzed FeP may have partially covered the surface of Pt nanoparticles and thus lowering the ECSA.Interestingly,the mass activity(MA)of Pt/C-FeP turns out to be 349.0 mA/mgPt@0.9 V vs.RHE,which is 2.6 times and 1.5 times of house-made Pt/C and commercial Pt/C,respectively.It is speculated that the electronic interaction and possible synergy between Pt and pyrolyzed FeP as NPME might have contributed to the ORR activity improvement despite of partial loss of ECSA.During accelerated durability tests (ADTs),the MA of Pt/C-FeP-650 degrades 64.3% inferior to commercial Pt/C(52.2%).The main reason likely arises from the degradation of pyrolyzed FeP,which is a bottleneck problem confronting NPMEs.
Keywords:Proton exchange membrane fuel cells Oxygen reduction reaction Electrocatalysts Mass activity Durability
1.Introduction
PEMFCs are highly promising power sources for fuel cell vehicles(FCVs)owning to the high power density,high energy conversion efficiency,and low operation temperature as well as low pollution emission[1-4].Despite that FCVs have launched commercialization during the past several years,the sluggish kinetics of ORR at the cathode side needs a large quantity of Pt to accelerate the reaction rate[5-8].Commercial Pt/C comprised of 2-5 nm Pt particles supported on carbon is state-of-the-art ORR electrocatalyst[9,10].However,commercial Pt/C is currently confronting the inter-correlated issues of activity,cost,and durability in the scenario of widespread commercialization of FCVs [2,7,11].In this regard,extensive studies have been focusing on advanced Pt-based electrocatalysts,including alloys[12-17],core-shell structured electrocatalysts [18-22],shape-controlled electrocatalysts [23-33],as well as porous carbon layer coated electrocatalysts [34-37].Especially,the Pt/C electrocatalysts coated with carbon layer have attracted much attention due to significantly improved ORR activity and durability induced by the functional coating layer.
In order to form a carbon layer on Pt,for example,polymer coating layer on Pt was firstly formed viain situpolymerization of a monomer on Pt like aniline or dopamine,and then pyrolyzed at an elevated temperature [34,36,38,39].The nitrogen-containing carbon layer can usually bring in three effects [34,38].It is possible to function like a physical protective layer,somehow preventing Pt/C from direct contact with a corrosion environment and mitigating detachment and agglomeration of Pt nanoparticles.Meanwhile,the N dopant in the carbon matrix can modulate the electronic structure of Pt and enhance ORR activity of Pt/C.In addition,certain types of N dopants and metal-N-C sites are active toward ORR and may interact with Pt sites,boosting the overall activity of carbon layer coated Pt/C.
Aiming to come up with a simple method for the synthesis of N-doped carbon layer decorated Pt/C,we selected 5,10,15,20-tetrakis (4-methoxyphenyl)-21H,23H-porphine iron (III) chloride (FeP) as the coating material that is relatively inexpensive and readily available.Previously,we demonstrated that FeP can uniformly cover carbon surface during evaporation induced self-assembly(EISA)process in light of van der Waals and π-π interaction between FeP and carbon surface [9,40].The usage of FeP instead of polymer can avoid the polymerization procedure.In this study,we coated house-made Pt/C with FeP via EISA and then heat-treated the mixture at 650 °C to achieve a hybrid electrocatalyst,Pt/C-FeP-650.We studied the effect of FeP loading on the ORR activity and possible reasons for the improvement of ORR activity of Pt/C-FeP-650.In addition,we found that the durability of Pt/C-FeP-650 is inferior to that of commercial Pt/C.A higher pyrolysis temperature(800°C)was employed to improve the durability at the expense of ORR activity.Regardless of the shortcoming in terms of durability,this study demonstrates a new avenue for the synthesis of NPME-Pt/C hybrid electrocatalysts with potential applications.
2.Experimental
2.1.Chemicals
Potassium tetrachloroplatinate (K2PtCl4,99 wt%) was purchased from Fengchuan Chemical Reagent Co.(Tianjin,China).Cetyltrimethylammonium bromide (CTAB,99%) and sodium borohydride (99.99%) were obtained from Sigma-Aldrich(USA).Chloroform(Analytical reagent,AR)was ordered from Sinopharm Chemical Reagent(Shanghai,China).5,10,15,20-tetrakis(4-methoxyphenyl)-21H,23H-porphine iron(III)chloride(FeP,Fig.S1)was purchased from Frontier Scientific(USA).Commercial Pt/C(20 wt%,HiSPEC 3000) was ordered from Johnson Matthey-JM (UK).Nafion resin solution was obtained from DuPont(DE520,5 wt%in the mixture of lower aliphatic alcohols and water,45% water,USA).Deionized water was obtained from Dalian University of Technology (Dalian,China).All of the chemicals were used as received without additional treatment.Carbon black (Ketjenblack EC600 JD) was obtained from Akzo Nobel (Netherlands).All aqueous solutions were prepared with ultrapure water (18.2 MΩ cm at 25 °C) produced from a Millipore water system(Synergy®UV,France).
2.2.Synthesis
2.2.1.Pt/C
House-made Pt/C was synthesized by a phase transfer method developed by our group [19,41,42].Briefly,10 mL of 20 mM K2PtCl4aqueous solution was added into 10 mL of chloroform solution containing 40 mM CTAB.The mixture was left under mild stirring at 25°C for 1 h to guarantee the complete transfer of the platinum complexes to aqueous interior of CTAB reversed micelles formed in the chloroform phase,obtaining Pt (II) ion-containing reversed micelles.Next,certain amount of pretreated EC600 JD carbon black [9] was dispersed in 10 mL of Pt(II)ion-containing reversed micelles in chloroform with the aid of sonication for 5 min.Next,90 mL of ultrapure water was added in and then 10 mL of 300 mM NaBH4was added under stirring at 1600 rpm.Finally,this house-made Pt/C was purified simply by washing with copious amount of hot water,and then dried at 65 °C.
2.2.2.Pt/C-FeP composites
FeP (28.6 mg) was dissolved in 70 mL chloroform,and then housemade Pt/C(100 mg)was added to the solution,followed by sonication for at least half an hour in a water bath to ensure well dispersion of Pt/C.The solvent was removed by rotary evaporation at 40 °C and a rotation rate of 100 rpm.Subsequently,the FeP coated Pt/C was heattreated in argon by ramping up temperature from room temperature to 650 °C with a rate of 10 °C/min and held at 650 °C for 2 h.To remove unstable species,the heat-treated sample was leached in H2SO4aqueous solution (0.5 mol/L) at 80 °C for 6 h.Finally,the sample was washed with deionized water until the filtrate was pH neutral,and then dried at 65°C.For comparison,different loadings of FeP on Pt/C(14.3-57.2 wt%) were also synthesized.The obtained electrocatalyst with 28.6 wt%of FeP loading at 650 °C was denoted as Pt/C-FeP-650.
2.3.Materials characterizations
Powder X-ray diffraction (XRD) patterns were collected on a Rigaku Smartlab 9 powder diffractometer with a Cu Kα radiation source (45 kV,200 mA)in a 2θ range of 10°-90°at a scanning rate of 8°/min.Transmission electron microscope (TEM) analysis was carried out on an FEI Tecnai G2 Spirit microscope or a HT7700 EXALENS microscope.High angle annular dark-field scanning transmission electron microscopy (HAADF-STEM) and energy-dispersive X-ray spectroscopy (EDX) analyses (JEM-2100F) were performed to record the mapping of Fe and Pt in electrocatalyst.) XPS measurements were performed on a Thermo ESCALAB XI+spectrometer with an Al Kα radiation source.Thermogravimetry analysis (TGA,SDT Q600)was conducted in a dried air atmosphere(50 mL/min)with a heating rate of 10 °C/min.In order to quantify the Pt and Fe content of samples,each sample was pyrolyzed under air atmosphere and the residue was dissolved in aqua regia.The concentration of Pt and Fe was measured by inductively coupled plasma-mass spectroscopy (ICP-MS,PerkinElmer Nex ION 300D).
2.4.Electrochemical measurements
All the electrochemical measurements of electrocatalysts were carried out on an Autolab potentiostat/galvanostat (Echo Chemie BV Model PGSTAT-302 N,Netherlands) in a standard three-electrode electrochemical cell with a glassy carbon rotating disk electrode (RDE,5 mm in diameter,Pine) coated with electrocatalysts as the working electrode (WE),a saturated calomel electrode (SCE) as the reference electrode(RE),and a graphite rod as the counter electrode(CE).The RE is separated from the WE compartment by a salt bridge fabricated with agar gel containing saturated KNO3.All potentials in this work were referred to reversible hydrogen electrode (RHE) [43,44].
An electrocatalyst ink (1 mg/mL) was prepared by mixing certain amount of electrocatalyst with ultrapure water,ethanol,and Nafion resin solution(5 wt%)at a volumetric ratio of 1:9:0.06 under sonication for 3 min.10 μL of an electrocatalyst ink was transferred onto the RDE and then evaporated the solvent in air.The platinum loading on the RDE is in the range of 7.1-10.2 μgPt/cm2.
Cyclic voltammetry (CV) curves were recorded at 25 ± 0.5 °C in N2-saturated 0.1 mol/L HClO4aq.with a sweep rate of 50 mV/s from 0 to 1.2 V(vs.RHE).The electrochemical surface area (ECSA) was estimated by measuring the charges associated with Hupddesorption (Q) between 0.05 and 0.5 V (vs.RHE) after double-layer correction and assuming a value of 210 μC/cm2for the adsorption of a monolayer of hydrogen onto a Pt surface(qH).ECSA was calculated based on the following equation (1):

where Q is the charge associated with Hupddesorption,m is the mass of Pt on the RDE,and qHis the charge required for the desorption of a monolayer of hydrogen on Pt.
The ORR polarization curves of electrocatalysts were recorded at 25 ± 0.5 °C in O2-saturated 0.1 mol/L HClO4aq.from 0 to 1.2 V (vs.RHE) with a positive sweep rate of 10 mV/s and a rotating rate of 1600 rpm.Correction of background currents was applied to all electrochemical data.Current densities were normalized to the geometric area of the RDE.The kinetic current density was calculated based on Koutecky-Levich equation (2):

where j is the experimentally measured current density at 0.9 V (vs.RHE),jkand jdare the kinetic current density and measured diffusionlimited current density,respectively.The kinetic current density was normalized to the platinum mass in order to obtain MA.
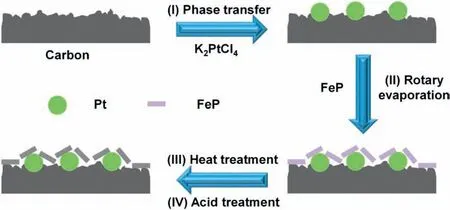
Fig.1.Schematic illustration of the synthesis of Pt/C-FeP.
We performed accelerated durability tests (ADT) on the RDE at 25 ± 0.5 °C in O2-saturated 0.1 mol/L HClO4aq.within a potential range from 0.6 to 1.2 V(vs.RHE)at a scan rate of 100 mV/s.After 200,600,1000,1500,2000,and 2500 potential cycles,corresponding CV and ORR polarization curves in N2-or O2-saturated 0.1 mol/L HClO4aq.were recorded to monitor the degradation process.
3.Results and discussion
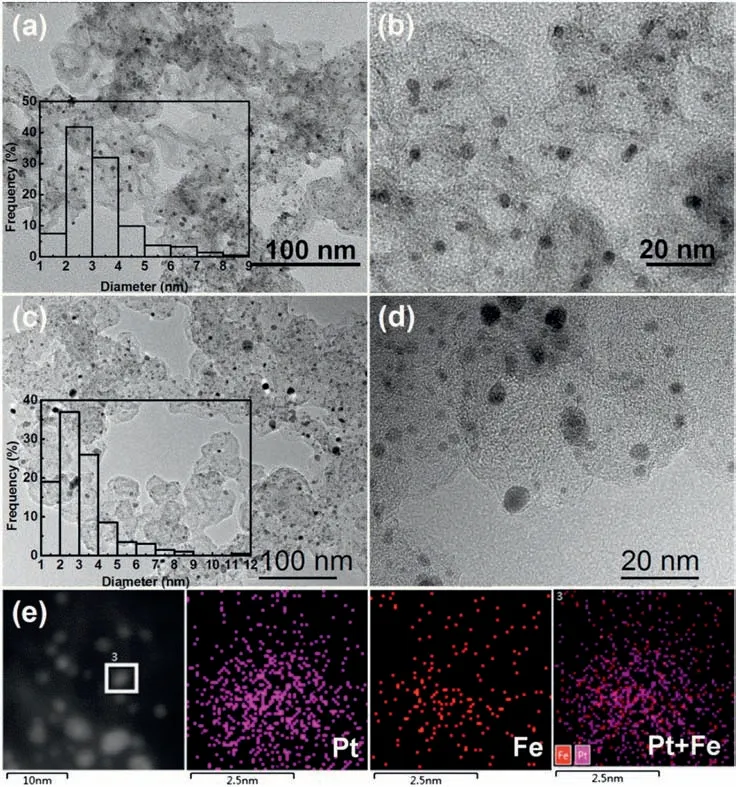
Fig.2.(a-b)TEM images of house-made Pt/C at different magnifications;(c-d)TEM images of Pt/C-FeP-650 at different magnifications;(e)HAADF-STEM image of Pt/C-FeP-650 and elemental mapping of Pt,Fe and PtFe for selected area in 3(Insets:size distribution histogram of nanoparticles by manually measuring at least 200 individual nanoparticles in a randomly selected area.).
The simple synthetic procedure of electrocatalysts is illustrated in Fig.1.Initially,house-made Pt/C was synthesized by phase transfer method reported by our previous studies [19,41,42].The reduction of Pt complexes was confined within the separate reverse micelles absorbed on carbon,preventing nanoparticles from growing into large ones or agglomeration,and thus leading to small Pt nanoparticles(3.3 ± 1.2 nm) uniformly dispersing on carbon without obvious agglomerations(Fig.2a and b).Next,house-made Pt/C was coated by FeP via rotary evaporation.Lastly,the FeP coated Pt/C was heat-treated under argon atmosphere at 650 °C for 2 h,followed by incubation in 0.5 mol/L H2SO4aq.at 80°C for 6 h to remove unstable Fe species and other byproducts.Previously,it has been identified that the temperature of 650 °C is high enough to carbonize FeP [9,45,46].
TEM images reveal that Pt/C-FeP-650 had an average particle size of 3.1 ± 1.5 nm as determined by manually measuring at least 200 randomly selected individual nanoparticles (Fig.2c and d).Most of the nanoparticles were small and uniformly disperse on carbon.Meanwhile,it is worth pointing out that a few large nanoparticles also formed besides the protection of coated FeP.However,after heattreating the house-made Pt/C in argon atmosphere at 650°C for 2 h,the average size of Pt nanoparticles was 3.9 ± 2.5 nm (Fig.S2),slightly higher than that of Pt/C-FeP-650(3.1 ± 1.5 nm)and house-made Pt/C(3.3 ± 1.2 nm)before heat-treatment.In addition,there were some large particles up to 26.9 nm in diameter.This implies that the coated FeP efficiently protected Pt nanoparticles from Ostwald ripening and coalescence during pyrolysis.Overall,due to the protection of coated FeP layer,the pyrolysis process did not cause much increase of particle size,which is beneficial for attaining a high ORR activity.It is also noticeable that no obvious graphene-like layer structure could be identified on the surface of Pt/C-FeP-650 as expected.The influence of FeP loading on ORR performance was also studied,and the results are shown in Fig.3a-c.It appears that the ORR activity was improved with the increase of FeP loading from 14.3 wt%to 28.6 wt%and afterwards the ORR activity did not change much with FeP loading.A reasonably high ORR performance reached at 28.6 wt%of FeP loading,which was considered to be an optimum parameter.
In order to ensure that pyrolyzed FeP still existed,Pt/C-FeP was burned in dried air.The residues were dissolved in aqua regia solution.The content of Fe and Pt in the solution was analyzed using ICP-MS.The loading of Fe and Pt in Pt/C-FeP-650 was determined to be 1 wt%and 15.2 wt%,respectively.This verifies that the pyrolyzed FeP had been successfully deposited on Pt/C.The resulting Pt/C-FeP-650 electrocatalyst was characterized by HAADF-STEM in conjunction with EDX analysis (Fig.2e).The elemental mapping of Pt and Fe for a selected nanoparticle shows that both elements overlapped with each other,indicating the formation of PtFe alloy or a Fe-N-C layer on Pt nanoparticle.In order to figure out if PtFe alloy had formed,Pt/C-FeP-650 was subjected to XRD measurement.The diffraction peaks of Pt/C-FeP-650 slightly shifted to higher angles with respect to those of the housemade Pt/C.This indicates that a small amount of Fe atoms might have diffused into the Pt lattice forming PtFe alloy,but not much.In addition,there is no diffraction peaks assignable to other Fe-containing species,implying that the other Fe species might be non-crystalline and more than likely existed as Fe-N-C sites.Actually,it cannot be ruled out that some Fe species might also exist as clusters.
XPS was collected to further investigate expected carbon layer formed on Pt/C after pyrolysis of FeP [36].The XPS of Pt/C-FeP-650 shows the peaks of N1s and Fe2p (Fig.4a),again corroborating the successful deposition of the residues of heat-treated FeP on Pt/C.The high-resolution N1s spectra can be de-convoluted into five peaks,namely pyridinic N(398.2 eV),Fe-N(399.3 eV),pyrrolic N(400.1 eV),graphitic N (401.5 eV),and oxidized N (403.7 eV) with corresponding percentage of 27.4%,34.6%,18.2%,10.4% and 7.6%,respectively(Fig.4b).As shown in Fig.4c,the binding energy difference between Fe2p1/2and Fe2p3/2is 13.6 eV,proving that Fe3+existed in Pt/C-FeP-650[47].The relatively high Fe-N content(34.6%)and low graphitic N content (10.4%) suggest that the carbonization of the FeP was mild at 650 °C and thus Fe species might have a good chance to exist in the form of Fe-N-C sites like the original FeP structure after heat treatment.Pt4f5/2and Pt4f7/2peaks of Pt/C-FeP-650 locate at 74.28 and 70.94 eV,respectively,as shown in Fig.4d.Compared with Pt/C,there is an evident positive shift (0.32 eV) of Pt 4f7/2peak of Pt/C-FeP-650,implying that the electron transferred from Pt to FeP residues containing N and Fe.The modulated electronic property of Pt would have an important effect on the ORR activity.
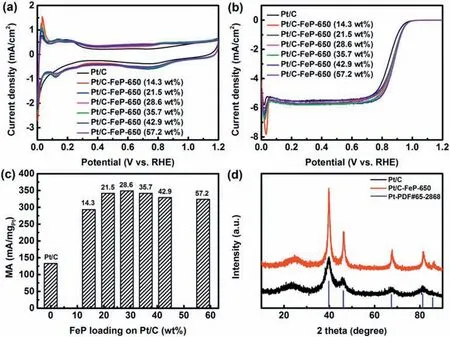
Fig.3.(a) CV curves and (b) ORR polarization curves of house-made Pt/C and Pt/C-FeP-650 synthesized with 14.3-57.2 wt%FeP loading on house-made Pt/C measured in N2-or O2-saturated 0.1 mol/L HClO4 aqueous solution;(c) corresponding MA values at 0.9 V (vs.RHE);(d) XRD patterns of Pt/C-FeP-650 synthesized at 28.6 wt%of FeP loading along with house-made Pt/C and fcc Pt (JCPDS PDF 65-2868) as comparison.
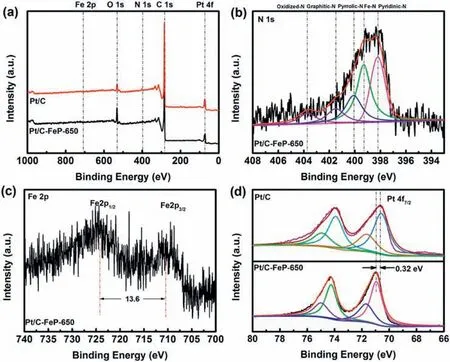
Fig.4.(a)XPS full spectra of house-made Pt/C and Pt/C-FeP-650;(b)high-resolution N1s spectrum of Pt/C-FeP-650;(c)high-resolution Fe2p spectrum of Pt/C-FeP-650;(d) high-resolution Pt4f spectra of house-made Pt/C and Pt/C-FeP-650.
To investigate electrochemical performance,Pt/C-FeP-650 was deposited on glassy carbon RDE with house-made Pt/C and commercial Pt/C as comparison.Fig.5a shows CV curves of Pt/C-FeP-650,housemade Pt/C,and commercial Pt/C.ECSA was estimated by measuring the charges associated with Hupddesorption between 0.05 and 0.5 V(vs.RHE)after double-layer correction and assuming a value of 210 μC/cm2for the adsorption/desorption of monolayer hydrogen on Pt.The ECSA values of Pt/C-FeP-650,house-made Pt/C,and commercial Pt/C were 65.0,80.2,and 100.5 m2/gPt,respectively (Fig.5b).The ECSA of Pt/C-FeP-650 decreased 19.0% relative to house-made Pt/C.This decrease may arise from expected formation of carbon layer partially covering Pt nanoparticles together with the enlargement of a few Pt nanoparticles as shown in Fig.2.The coverage by expected carbon layer,assuming it existed,should be incomplete or the assumed carbon layer might be porous.Otherwise,Pt/C-FeP-650 should have a much lower ECSA value.Actually,partial coverage and/or porous layer coverage are what we exactly needed,namely to improve the performance of Pt/C while retaining or not damaging the ECSA value significantly.Taken together TEM,XRD,XPS,and CV measurements,we tend to assume that the carbon layer containing N and Fe have likely formed on Pt/C after pyrolyzing FeP coated Pt/C.

Fig.5.(a) CV curves and (b) ECSA values of commercial Pt/C,house-made Pt/C,and Pt/C-FeP-650 recorded in N2-saturated 0.1 mol/L of HClO4 aq.with a sweep rate of 50 mV/s at 25 ± 0.5 °C;(c) ORR polarization curves and(d) MA values at 0.9 V (vs.RHE) of commercial Pt/C,house-made Pt/C,and Pt/C-FeP-650 collected in O2-saturated 0.1 mol/L of HClO4 aq.with a positive sweep rate of 10 mV/s and 1600 rpm at 25 ± 0.5 °C.
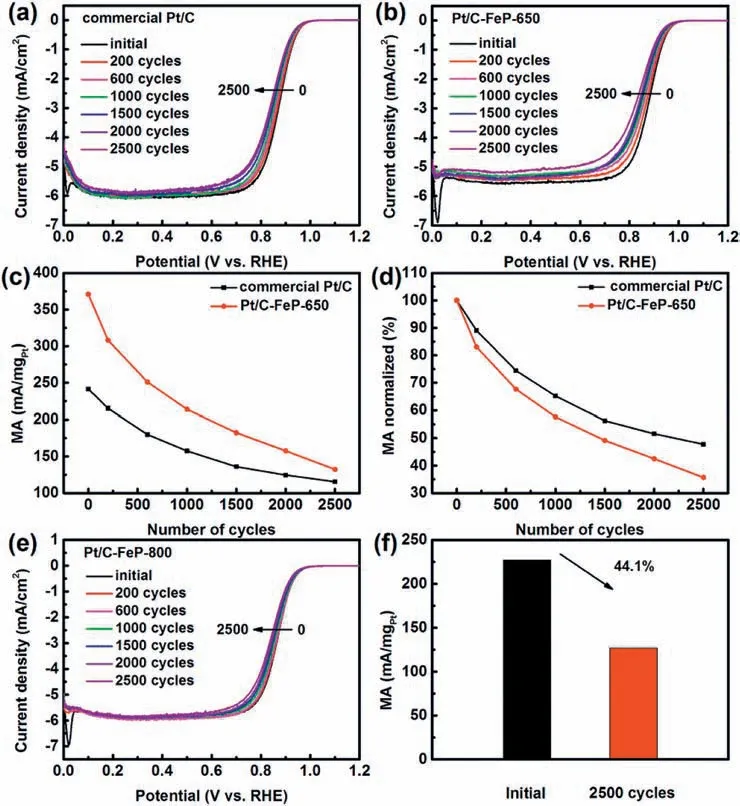
Fig.6.ORR polarization curves of (a) commercial Pt/C and (b) Pt/C-FeP-650 measured during ADT in O2-saturated 0.1 mol/L of HClO4 aqueous solution at 25 ± 0.5°C;(c)MA values and(d)corresponding normalized MA values of commercial Pt/C and Pt/C-FeP-650 recorded during ADT;(e)ORR polarization curves of Pt/C-FeP-800 and (f) corresponding MA values measured during ADT in O2-saturated 0.1 mol/L of HClO4 aqueous solution at 25 ± 0.5 °C.
The ORR activity of Pt/C-FeP-650,house-made Pt/C,and commercial Pt/C was evaluated in O2-saturated 0.1 mol/L HClO4aq.with a rotation speed of 1600 rpm at 25 ± 0.5 °C (Fig.5c).The half-wave potential of Pt/C-FeP-650 toward ORR positively shifted about 29 mV and 4 mV compared with house-made Pt/C and commercial Pt/C,respectively.The ORR mass activity at 0.9 V (vs.RHE) was calculated to be 349.0 mA/mgPtthat is 2.6 and 1.5 times of house-made Pt/C and commercial Pt/C,respectively (Fig.5d).This demonstrates that Pt/C-FeP-650 had a much higher ORR activity than house-made Pt/C and commercial Pt/C.The high ORR activity of Pt/C-FeP-650 may result from the N-C and/or Fe-N-C active sites of FeP residues,which have been regarded as active sites for ORR[2,48-50].In addition,PtFe alloy and modified electronic property of Pt by FeP residues should also contribute to the enhanced ORR activity.
Besides ORR activity,the durability is another critical factor for practical applications of electrocatalysts.The durability of commercial Pt/C,house-made Pt/C and Pt/C-FeP-650 was performed by applying continuous potential sweeps between 0.6 and 1.2 V (vs.RHE) for 2500 cycles (100 mV/s) in O2-saturated 0.1 mol/L HClO4aqueous solution.As shown in Fig.6a and b and Fig.S3a,the half-wave potential of Pt/C-FeP-650,house-made Pt/C,and commercial Pt/C shifted negatively gradually during the potential cycling process.After ADT,the MA value of Pt/C-FeP-650 was 132.3 mA/mgPt@0.9 V vs.RHE,higher than that of house-made Pt/C (80.0 mA/mgPt@ 0.9 V vs.RHE) and commercial Pt/C(115.4 mA/mgPt@0.9 V vs.RHE)(Fig.6c and Fig.S3b).Basically,the design and synthesis of the protective and functional layer coated Pt/C with enhanced ORR activity and acceptable durability have been realized.However,Pt/C-FeP-650 suffered 64.3% loss in MA during ADT,larger than that of house-made Pt/C(46.4%)and commercial Pt/C (52.2%) (Fig.6d and Fig.S3c).The possible reason is that the assumed incomplete and/or porous FeP residues layer is likely unstable in acidic media,which is a great challenge confronting NPMEs toward acidic ORR.As expected,the ECSA decreased(Fig.S3d)and the average size of Pt based nanoparticles grew into 5.0 ± 1.9 nm after ADT(Fig.S4).
In order to enhance the durability with a high degree of graphitization [51],the pyrolysis temperature was increased from 650 to 800 °C (denoted as Pt/C-FeP-800).The loading of Fe and Pt in Pt/C-FeP-800 was determined to be 1.1 wt% and 16.0 wt%,respectively.XRD pattern shows that diffraction peaks of PtFe alloy appeared,implying that Fe atoms diffused into the Pt lattice at 800 °C (Fig.S5).Similar to Pt/C-FeP-650,N species in Pt/C-FeP-800 also included pyridinic N,Fe-N,pyrrolic N,graphitic N,and oxidized N.As shown in Fig.S6a-b,the relative percentage of pyridinic N,Fe-N,pyrrolic N,graphitic N,and oxidized N was 32.9%,21.5%,19.3%,14.9% and 9.7%,respectively.Compared with Pt/C-FeP-650,the graphitic N content did increase,verifying that the degree of carbonization was improved for the case of Pt/C-FeP-800.The Pt4f7/2peak of Pt/C-FeP-800 positively shifted 0.65 eV (Fig.S6c).
The CV and ORR polarization curves of Pt/C-FeP-800 are shown in Fig.S7.The durability of Pt/C-FeP-800 got obviously improved due to the increased degree of graphitization.The MA of Pt/C-FeP-800 only degraded by 44.1% during ADT,much better than Pt/C-FeP-650 and commercial Pt/C (Fig.6e-f and Fig.S3b-c).However,the ECSA of Pt/C-FeP-800 was merely 39.5 m2/gPt,much less than that of Pt/C-FeP-650.Additionally,the ORR activity of Pt/C-FeP-800 decreased by 36.0% compared with Pt/C-FeP-650.Apparently,more efforts are needed to better balance the activity and durability issue in the future.
4.Conclusions
NPME-Pt/C composite has been successfully synthesized by pyrolyzing FeP coated Pt/C.Due to the co-existence of NPME and Pt sites and possible synergy between Pt and NPME,the MA of Pt/C-FeP-650 reaches 349.0 mA/mgPt@ 0.9 V vs.RHE,which is 2.6 times and 1.5 times of house-made Pt/C and commercial Pt/C,respectively.During ADT,the durability of Pt/C-FeP-650 is basically acceptable as compared with commercial Pt/C.We also tried to increase pyrolysis temperature to further improve the degree of carbonization of FeP and thus improve the durability.This study provides a new approach for the synthesis of functional carbon layer coated Pt/C with potential applications.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
This study was partially supported by National Key Research and Development Program of China (Grant No.2019YFB1504501),the Fundamental Research Funds for the Central Universities (Grant Nos.DUT19ZD208 and DUT20ZD208),Science &Technology Innovation Funds of Dalian (Grant No.2020JJ25CY003),and Special Funds for Guiding Local Scientific and Technological Development by the Central Government (Grant No.2020JH6/10500021).
Appendix A.Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.pnsc.2020.08.012.
 Progress in Natural Science:Materials International2020年6期
Progress in Natural Science:Materials International2020年6期
- Progress in Natural Science:Materials International的其它文章
- Stability of PGM-free fuel cell catalysts:Degradation mechanisms and mitigation strategies
- Understanding of free radical scavengers used in highly durable proton exchange membranes
- Electrolyte membranes for intermediate temperature proton exchange membrane fuel cell
- Key technologies for polymer electrolyte membrane fuel cell systems fueled impure hydrogen
- Electrolyte materials for intermediate-temperature solid oxide fuel cells
- Carbon-free nanoporous gold based membrane electrocatalysts for fuel cells
