Towards mass applications:A review on the challenges and developments in metallic bipolar plates for PEMFC
Zhutian Xu,Diankai Qiu,Peiyun Yi,Linfa Peng,Xinmin Lai
State Key Laboratory of Mechanical System and Vibration,Shanghai Jiao Tong University,Shanghai,200240,People’s Republic of China
ABSTRACT The industrial applications of proton exchange membrane fuel cell (PEMFC) technology have attracted tremendous capitals and research attention in the last ten years.However,there are still many bottlenecking problems before the progress triumphs.Among them,a core challenge is the improvement of bipolar plates(BPs).As a key component of PEMFC,the BPs undertake the crucial functions of distributing reactant gases and cooling medium,collecting the electricity generated,exporting waste products,etc.Due to the unique advantages of ultra-thin,high strength,easy-to-mass-produce,etc.,metallic BPs have been considered as the promising solution for the automotive and aerospace application scenarios with a strong requirement of power density.However,many challenges remain to be conquered in metallic BPs to fully meet all the requirements in those industrial applications such as corrosion,toxic ion emission and fabrication.The aim of this work is to provide an overview of the important researches in recent years dealing with the most critical problems of metallic BPs before industrial applications.The future studying directions in material and processing methods development are also given towards the wide application of metallic BP PEMFCs.
Keywords:Metallic bipolar plate Proton exchange membrane fuel cell Base material Coating material Processing method Mass application
1.Introduction
The proton exchange membrane fuel cell (PEMFC) has been profoundly studied in recent years as one of the most promising solutions for the global energy crisis and environmental problems with near-zero emission and high energy conversion efficiency[1].A schematic picture of the PEMFC structure is shown in Fig.1.Hundreds of single fuel cell are held by two end plates.Each cell consists two bipolar plates (BPs)and one membrane electrode assembly(MEA)in between.The assembly of BPs and MEAs constructs the flow fields for hydrogen,oxygen and coolant.Chemical reaction occurs in the MEA with the help of catalyst.The generated electrons are transferred from anode to cathode by the BPs to complete the electrical circuit.To prevent the whole system from overheating,coolant recycles inside the flow channels of BPs.
Although PEMFC has received particular attention in decades,its wide commercialization is still impeded by the high manufacturing cost,low volumetric power density and short life spam.According to the estimations of DOE,the BPs take 20-30%of the total cost and 60-80%of the overall weight and volume in the fuel cell stack [2].Owing to the harsh acidic environment inside fuel cells,the corrosion resistance of BPs is a dominating factor affecting the durability of PEMFC [3].Moreover,the mass production capability of BPs is also a critical issue to be addressed for the commercialization of PEMFCs.Taking the Toyota Mirai as an example,there are 370 BPs(separators)in one fuel cell stack[4].The rapidly increasing application of PEMFC naturally leads to the massive requirements of BPs.According to the DOE fuel cell application roadmap,the cost of BPs should also be reduced to $3 and $2 kW-1in 2020 and 2025 respectively [2].
In those regards,many candidates have been proposed for BPs with the development of PEMFC.By now two major categories are widelyaccepted,namely the metallic and the carbon-based BPs.The carbonbased BPs feature satisfying corrosion resistance and acceptable conductivity.However,strong brittleness,high financial and time cost of manufacturing hinder their application,especial in automobiles [5].In recent years,composite carbon-based BPs with polymer resin and carbon conductive fillers are attracting much attention due to their better strength and manufacturability[6].However,a balance of conductivity,strength,airtightness and cost remains a critical issue.Metallic BPs have shown unique advantages such as high strength,low thickness,easy-to-mass-produce and potential to strongly-reduce the manufacturing cost in many prior researches,which make them promising candidates in the scenarios with volume and weight requirements.Successful applications of them have also been realized in fuel cell vehicles (FCVs) developed by Toyota,Hyundai,Honda etc.However,the development of high-performance metallic BPs towards the massive application of PEMFC remains a challenging issue.
The materials of metallic BPs are strongly associated with the durability,electric conductivity,strength,manufacturability and cost,which are one core challenge during the development of BPs.Various materials have been investigated such as stainless steels,titanium alloys and aluminum alloys [7].The stainless steels are attracting the most attention due to their balanced performances and low cost,which have been utilized in Hyundai,Honda and GM FCVs [8].Titanium alloys feature strong corrosion resistance and little ion damage to MEA.However,the high contact resistance and cost are the major drawbacks for their application.Some researchers also proposed aluminum alloys which are attractive by their satisfying ductility and relatively-low cost.However,their ion toxicity to catalyst makes them not suitable unless coating is applied [9].Up to now,coating has been vastly applied to meet the low electric conductivity and high corrosion resistance demands of BPs even though it could increase the total cost[10].Finding a proper coating material with both reasonable cost and satisfying properties for BPs remains a critical task.Details for the development of materials associated with metallic BPs are presented in Section 2.
The manufacturing of metallic BPs is another major challenge for the mass application of PEMFC.Forming and coating are the two major processes in the manufacturing procedure.Several prior review works were conducted by Peng et al.[5],Leng et al.[8]and Song et al.[11]on the forming processes,and by Asri et al.[10]and Wilberforce et al.[12]on the coating processes.Different from prior reviews,the capability and performance of different manufacturing methods were overviewed with emphasis on the challenges of manufacturing cost and efficiency.The details can be found in Section 3.
With the booming development of PEMFC using metallic BPs in recent years,this work tries to provide a thorough review on the state-ofart of materials and corresponding fabrication technologies of metallic BPs emphasizing on the discussion of how to meet the multifunctional,economic and production requirements towards mass application of PEMFC.The major challenges and future research trends are also identified.
2.Materials
Due to the multifunctional requirements of metallic BPs,finding a proper metal material with high corrosion resistance,low toxic ion emission,high electric conductivity,satisfying formability and low cost is the key.A number of candidates have been proposed in those regards.A common solution is by depositing a functional film onto the base material.
2.1.Base materials
Table 1 gives the chemical compositions of the mostly investigated and applied materials for metallic BPs in recent years.
2.1.1.Electrochemical properties
The electrochemical properties of base materials for BPs can be evaluated by ICR and corrosion current density.The former describes the conductivity of the material and the lateral reflects the corrosion resistance.Table 2 and Fig.2 summarize the ICR and corrosion current density with operating conditions of bare metals.
According to Fig.2,the average corrosion resistance of the three classes of metals is ranked as follows:titanium alloys
2.1.2.Formability
The mechanical properties of commonly used metal materials are summarized in Table 3.A histogram of the tensile strength and elongation of different metals is illustrated in Fig.3 for comparison.
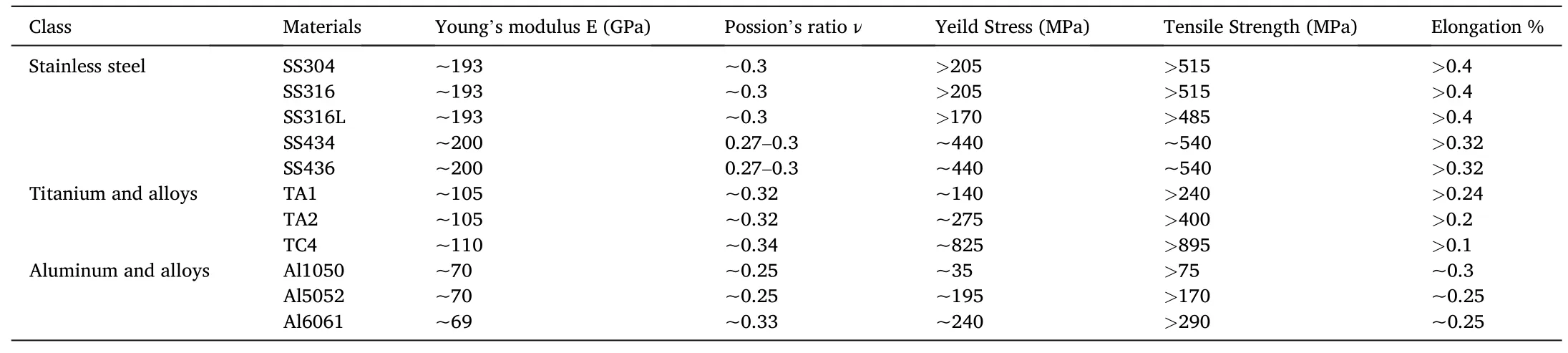
Table 3 Mechanical properties of materials for BPs[31].
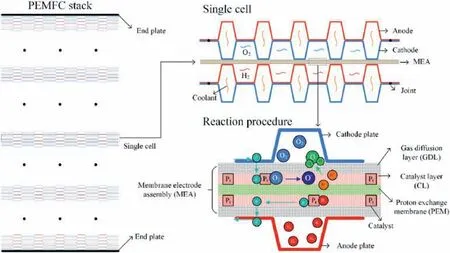
Fig.1.Schematic of the PEMFC and the main parts.
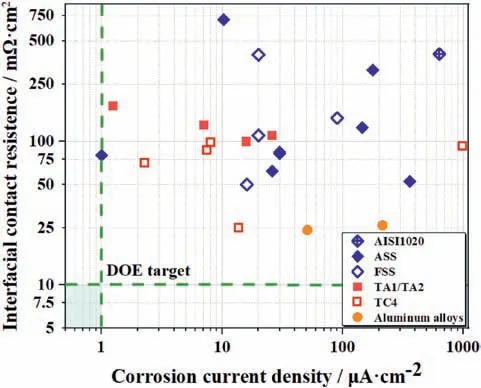
Fig.2.Electrochemical properties of bare materials for BPs.
ASS materials such as SS316 and SS316L with proper tensile strength and high elongation are promising candidates for BP fabrication.Regarding FSS,however,the elongation rate is smaller than ASS owing to the body-centered cubic (BCC) structure.For titanium alloys,the tensile strength can meet the manufacturing requirements of BP,but the elongation is rather low.Among the titanium alloys explored,TA2 with balanced tensile strength and elongation may be the best choice at the current stage.TC4 with high tensile strength and low elongation may pose a challenge to the subsequent fabrication process.
Based on the analysis above,the summary of various metals for BPs can be given as follows.For stainless steel,SS316/SS316L is considered as an optimal choice in recent years due to low cost and reasonable formability.However,the electrochemical properties are still not satisfying.Two solutions could be adopted.The first one is to deposit a protective coating,which has been employed a lot in recent studies.However,an evident increase of the manufacturing cost makes the application of fuel cell more difficult.The other solution is by developing a novel non-coated material with strong corrosion resistance,satisfying electric conductivity,good formability and low cost.One instance reported was the POS470FC stainless steel developed by POSCO [32].Titanium alloys have good corrosion resistance,which makes them satisfactory candidates for durable metallic BPs.Toyota Mirai employed the titanium alloy BPs which enables a 5000 h service life for the first time in commercial FCVs.However,the titanium alloys are expensive,which makes them only applicable in cost-insensitivescenarios.The corrosion resistance and formability of aluminum are comparable to the stainless steel.However,the aluminum BPs rely on the development of coating to prevent the release of the toxic Al3+.
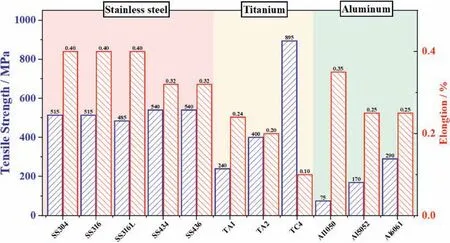
Fig.3.Mechanical properties of materials for BPs.
2.2.Coating materials
At present,the main performances of metallic BP coatings,including high corrosion resistance and low ICR,can meet the ex-situ technical targets established by the DOE [5].However,there are still limitations that should be improved.Firstly,due to a low deposition rate and expensive coating materials and equipment,both the economic and time costs of preparing coating are too high.Another problem is their durability under PEMFC working environments.
Metallic BPs operate at an acidic (i.e.,pH 2-3),warm and humid(65-90°C) PEMFC environment [33].The running conditions constantly alter among load changing,idling,start-up/shutdown,and rated power conditions in automotive applications,thus leading to high voltages on the metallic BPs[34].The harsh environments and corrosive electrolytes can result in significant active and passive corrosions of metallic BPs,leading to the release of toxic ions such as Fe3+,Fe2+and Cu2+and the growth of passivation layers on bare metals.The emission of toxic ions could significantly degrade the efficiency and lifetime of PEMFCs by poisoning catalysts adhered on MEAs [35].While the growing passivation layers also result in the evident increase of ICR at the interface between metallic BPs and gas diffusion layers(GDLs)[36].As a result,protective and functional coatings need be deposited on metallic BPs to further enhance the corrosion resistance and reduce the ICR.Typical coating materials synthesized in recent years are listed in Table 4 and Fig.4.

Table 4 A summary of recent materials used as coatings on metallic BPs.
2.2.1.Pure carbon films
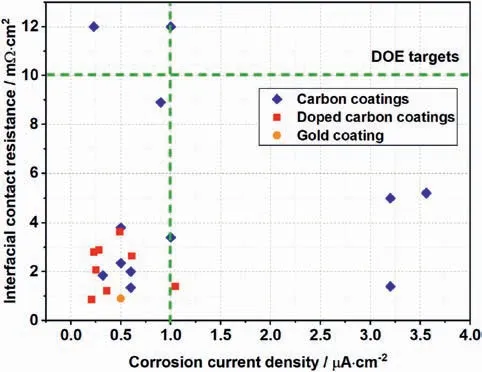
Fig.4.A summary of the interfacial contact resistance and corrosion current density of typical coatings,i.e.,carbon coatings,doped carbon coatings and gold coatings.
Many groups have investigated the pure carbon films including amorphous carbon (a-C) films and nanocrystalline carbo films for the application of metallic BPs.In recent years,the carbon films have attracted great attention due to their lower cost and better performance.Furthermore,the amorphous carbon films exhibit excellent corrosion resistance and electrical conductivity in the operating environment of PEMFCs.Carbon has many allotropes and the performance of carbon materials depends on their unique microstructures.Composite carbon films with both a-C and microcrystals have been revealed to offer better performance.For instance,Afshar et al.[50]prepared a-C films via DC magnetron sputtering method on SS316L and studied the influences of substrate temperature on the structure and performance of the films.They found a higher deposition temperature was beneficial to the graphitization of a-C films.The corresponding corrosion current density of the film deposited at 100°C in the simulated PEMFC environment of 1 M H2SO4and 5 ppm F-at 70°C was 6.4E-6 A/cm2.Show et al.[38]coated Ti BPs with a-C film and studied the influence of temperature on the performance of a-C.The output power of the stack with a-C coated Ti BPs was 1.4 times higher than that of the stack with uncoated Ti BPs.Yi et al.[51] prepared a-C films on SS316L and investigate the effects of film thickness on the graphitization of a-C films.They pointed out the a-C with the thickness of 69 nm contained nanographite clusters and exhibited excellent performance.The corrosion current density of the films in the simulated environment of pH 3H2SO4and 0.1 ppm HF at 80°C was less that 5E-7 A/cm2.In addition,the ICR of the films at 140 N/cm2was 2.35 mΩ cm2.
2.2.2.Doped carbon films
Although pure carbon films have been widely studied,the carbon films prepared via sputtering method or filtered arc deposition often have high residual stress,which can significantly degrade their adhesion strength.Metal doping has been proven to be effective in reducing the internal stress in pure carbon coatings [52].Among the many researches,Cr-doped carbon films have been attracting much attention.Yi et al.[53] and Zhao et al.[54] fabricated Cr-doped a-C coatings on SS316L.At a simulated environment of PEMFCs,namely 0.5 M H2SO4solution with 5 ppm HF at 70°C,the corrosion current density was 0.28 μA/cm2at a cathodic running voltage(i.e.0.6 VSCE)and the ICR is 2.89 mΩ cm2obtained at a pressure of 1.5 MPa.Then,the ICR measured after the potentiostatic testing of the Cr doped a-C films increased to 4 mΩ cm2.Wang and colleagues[46]electroplated Cr-doped a-C coatings with the thickness range from 1.4 to 11.4 μm.They found the optimized film had an ICR of 16.54 mΩ cm2under a pressure of 1.50 MPa and a stable passivation current density of 9.36E-8 A/cm2in a 0.5 M H2SO4solution.
Regarding other elements,Bi et al.[55] synthesized a multilayered zirconium doped a-C (Zr-C/a-C) coating and tested in a pH=3H2SO4and 0.1 ppm HF solution at 80°C.The coating significantly improved the corrosion potentials and reduced the passivation current density of bare stainless steels.Zhang et al.[40] studied the Ag,Cr-co-doped a-C coatings which have an ultra-low ICR of 0.87 mΩ cm2.In addition,with proper amounts of Ag and Cr doped,the stability of film was also found to significantly improved after durability tests.
2.2.3.Transition-metal carbide (TMC) coatings
In addition to the a-C and doped a-C coating candidates,the TMC coatings have also drawn increasing attention with their applications in PEMFCs in recent years.Novel materials such as MAX phase [56] and MXene [57] have excellent anti-corrosion properties and electrical conductivities,exhibiting great potentials applying to the coated metallic BPs.Lu et al.[58] prepared Ti3SiC2and Ti3AlC2coatings on SS304 substrates for BPs with the deposition temperature of 300-350°C and the heat treatment temperatures ranging from 800 to 1000°C.Both satisfying corrosion resistance and ICR were revealed.However,the high deposition and heat treatment temperatures could lead to many issues such as energy consumption,complexity of equipment and manufacturing inefficiency.To deal with that,many researchers are looking for solutions to reduce the deposition and heat treatment temperatures.Andersson et al.[59]investigated the corrosion resistance of amorphous Cr-C carbide (a-Cr-C) films in the 1.0 M H2SO4solution at 80°C.They found the corrosion current density was mainly originated from the oxidation of Cr and partially of carbon phases.In Cr-C carbide coatings,the corrosion properties were up to the Cr-C phases.For low-carbon samples,Nygren et al.[60] indicated that higher temperatures could improve the corrosion resistance of Cr-C carbide coatings.They also concluded that Cr-C carbide coatings with higher carbon contents exhibited better oxidation resistance.However,there is still not sufficient researches addressing the durability of TMC coatings.
2.2.4.Conductive polymer coatings
Conductive polymers are regarded as possible candidate for BP coatings due to their excellent corrosion resistance.Deyab and Mele[61] developed a polyaniline/Zn-Porphyrin (PANI/Zn-Pr) composites coating for SS303 BPs.They found the Zn-Pr plays an evident role in improving the coating resistance of PANI.A TiN layer over TiN-PANI composite coating was developed by Cooper and Kharouf [62] onto SS316L substrates.After evaluation,the potentiostatic corrosion currents and ICR were found to be 0.024 μA/cm2and 11.2 mΩ cm2according to the DOE standard test conditions.A PPY-graphene oxide(PPY-GO)/PPY-camphorsulfonicacid (PPY-CSA) composite coating was prepared onto SS304 BPs [63].PPY-GO was employed to enhance the adhesion while PPY-CSA to improve the conductivity.Galvanostatical electrodeposition process was employed to fabricate the composite layers.The PPY-GO/PPY-CSA composite coating is revealed to maintain a satisfactory conductivity and sustained anodic protection during the 696 h of immersion in 0.3 M HCl solution.Current researches indicate the polymer coatings can effectively decrease the corrosion,but also lead to a high ICR.Solving the conductivity issue is an important task for the development of polymer coated metallic BPs.
2.2.5.Metal nitride films
Many different metal nitride films were also widely investigated for BPs such as CrN,TiN,NbN,TaN,etc.In recent years,the combination of different metal nitride and various elements to achieve even better performance has been an important research trend.Wang et al.[64]doped a Ti/(Ti,Cr)N/CrN multilayer coating on the surface of SS316L by Arc ion plating (AIP).The ICR between the coated sample and carbon paper was 4.9 mΩ cm2under 150 N/cm2and the corrosion current density 0.12 μA/cm2in 0.5 M H2SO4at 70°C.A Ti/TiN multi-layer coating was deposited via PVD onto SS316 by Jannet et al.[65].The ICR of coated samples was 18 mΩ cm2after 1 h in 0.5 M H2SO4with 2 ppm HF and the corresponding corrosion current density was 0.93 μA/cm2at +0.6 VSCE.Ingle et al.[66] investigated an amorphous Al-Cr-Mo-N coating on SS316L.The electrochemical corrosion behaviors were explored under both H2-purging and air-purging environment with 0.5 M H2SO4+2 ppm NaF at 70°C.The corrosion current density was 0.02 μA/cm2and the ICR after polarization was around 30 mΩ cm2comparing to the bare material value of over 80 mΩ cm2.In addition,the contact angle was found to increase from 71°to 106°after deposition,suggesting a better draining performance by utilizing the coating.
2.2.6.Noble metals
Noble metals such as gold have outstanding electrochemical performance and stability.Reducing the cost and film thickness is the critical issue for their application in metallic BPs.Avasarala et al.[67]prepared Au films with the thickness of 1 μm on stainless steel via electroplating method.The ICR value of the Au film was 30 mΩ cm2at 140 N/cm2,which did not meet the DOE target(<10 mΩ cm2).Yun et al.[68] used an electron beam evaporator to deposit gold films ranging from 0.1 to 2 μm.The electrochemical tests results showed that the gold films exhibited excellent corrosion resistance in the simulated environment.And the ICRs of the gold films with different thicknesses were 5-8 mΩ cm2.Kumar et al.[49] prepared Au films with the thickness of 10-20 nm on SS316L.The corrosion current density of the films in the solution of 0.5H2SO4at 80°C was less than 1.0E-6 A/cm2.Furthermore,the corrosion current density after 1.6 V (vs.NHE) potentiostatic polarization for 6 h was 5.0E-5 A/cm2.Wang et al.[69] investigated nano-thick gold and polypyrrole films on SS316L.The nano-thick Au layer was coated on SS316L prior to the polypyrrole.The concentration of metal ions after electrochemical test reduced significantly due to the excellent protection of Au layer.
In summary,many different coatings for metallic BPs have been developed in decades to reduce the corrosion,ICR,harmful ion emission of base material so that the fabricated BPs can sustain a high performance in PEMFC environments.Many promising candidates such as doped carbon,metal nitride and noble metal films have been proved offering satisfying properties.However,according to the DOE 2015 requirements,the cost of BPs must be reduced to $3/kW for commercialization.The coating materials and their fabrication still take a large proportion of cost in the currently-employed BPs.Finding an appropriate coating material with both excellent performance and low cost remains a critical task.
3.Fabrications
The fabrication is another critical issue in the development of metallic BPs.Three major challenges exist:(1) Accuracy.The fuel cell stack consists hundreds of layers of large-area BPs with complex micro features.To guarantee the successful assembly of stack,BPs must be fabricated with extreme consistency.In addition,the coating process also requires high accuracy and homogeneity to avoid local defects.(2)Quality.Due to the high corrosion resistance and electrical conductivity requirements of metallic BPs,defects such as evident surface roughness,scratches and coating defects need to be eliminated during the fabrication.(3)Manufacturability.The improvement of fabrication methods is still urgently required to satisfy the elevating function requirements of PEMFCs such as even finer micro channels with a higher aspect ratio,better durability,etc.(4) Cost and efficiency.The development of PEMFC towards mass application requires metallic BPs manufactured with extreme low cost and high production capacity.To achieve the mentioned goals,recent development of metallic BP forming and coating processes are reviewed as follows:
3.1.Forming
Many fabrication methods have been explored previously including Electrical discharge machining (EDM),Electrochemical machining(EMM),LIGA,selective laser melting (SLM),etc.Among them the forming processes attract the most attentions due to high efficiency,low cost,near-net-shaping,satisfying accuracy,etc.,which make them more suitable for mass production [70].A compare of various metallic BP forming processes are illustrated in Table 5.The aspect ratio (i.e.,the channel height divided by the width)is an important factor affecting the performance of a fuel cell stack.Recent researches indicate the increase of aspect ratio could improve the PEMFC performance by promoting the uniform flow distribution and water removal[71].However,the aspect ratio is limited by the formability of material and the forming process.The limit drawing ratio (LDR) of each forming process is also summarized in Fig.5.According to the reviewed works,the aspect ratio of channels fabricated by different methods is mostly between 0.4 and 1.0.
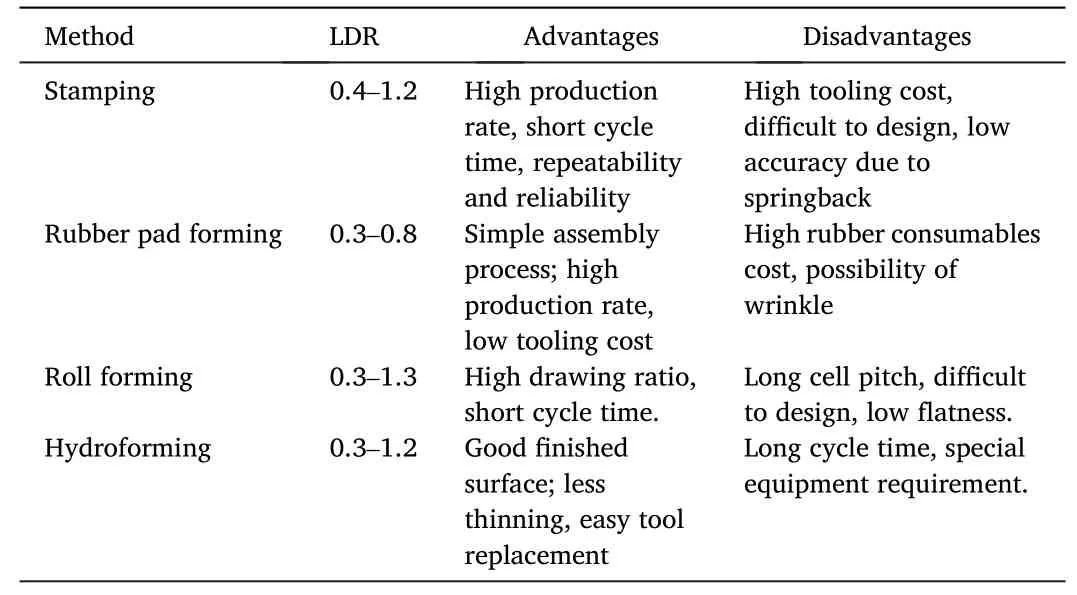
Table 5 Comparisons of different metallic BP forming methods.
3.1.1.Stamping
Stamping has been vastly employed in various applications due to the high production rate and low unit cost.In metallic BP manufacturing scenario,the stamping process is also widely accepted as a promising method and applied in the manufacturing of commercial-available FCVs such as Toyota Mirai,Hyundai NEXO,etc.However,the stamping of metallic BPs needs special attention on thinning,wrinkles,surface roughness and springback to realize the accurate forming of the designed complex micro features.The size effect of material,which is defined to represent the evident deviation of plastic and frictional behaviors from traditional theory predictions in the forming of miniaturized features,also affects the process design and optimization [72,73].In addition,due to the uneven loading of rigid dies and punches,the LDR of micro channels of stamping is restricted due to the easy strain localization [74].
To address the problems aforementioned,many efforts have been made.Mahabunphachai et al.[75] concluded that increasing the stamping force not only led to a higher aspect ratio,but also yielded higher surface roughness.Nylon propylene was revealed to be able to improve the forming uniformity and LDR [76].Multi-stage stampingwas an effective method to improve the LDR by reducing the deformation localization tendency[77].The method has been proved efficiency in the manufacturing of metallic BPs for Mirai.

Fig.5.Aspect ratio of BPs manufactured by various forming methods.
As the most attractive method,the large-scale manufacturing of metallic BPs via stamping process was analyzed thoroughly to meet the DOE cost target of 3 $/kW by Huya-Kouadio et al.[78].Three major challenges could be identified:
(1) The smaller and more complex flow channels require greater stamping tonnage,which leads to the substantial increase of the machine capital cost(~$1000/ton)and the stamping cycle time(2.5 s per stamp).In addition,the high accuracy and aspect ratio requires multiple stamping cycles,which further leads to high equipment cost and long manufacturing time.
(2) The machining time and cost for the fabrication of die sets are considerable.According to Huya-Kouadio et al.,the time and cost can be several thousand hours and$600,000 respectively per die set.
(3) The production rate of metallic BPs is still a bottlenecking problem.Considering the slow stamping cycle,each stamping line can only supply 5100 FCVs by operating 3360 h per year.The estimated cost is $6.50/kW which is over twice of the DOE target.
The stamping process of metallic BP still needs extensive improvement regarding the listed problems towards an even lower cost and shorter cycle time so that the mass application of PEMFC could be possible.
3.1.2.Other forming methods
Rubber pad forming attracts many attentions since only one die is required,the cost of die set fabrication can be reduced.The surface condition of formed sample is also better than the stamped one.Many prior researches were conducted revealing different factors such as the punch speed and force,friction,fillet radius,rubber thickness and hardness,etc.[79].However,with the increasing amount of rubber entering the die cavities in the experiments,the pressing load as well as the friction force between the sample and rubber increase substantially,leading to earlier fracture.Hence the aspect ratio in the rubber pad forming process is lower than the others.
Roller forming is a highly-efficient forming method which has been employed a lot in industry.Abeyrathna et al.[80] developed a roller forming experimental setup to study the feasibility of forming micro channels for metallic BPs.Huang et al.[81]also investigated the roller forming of micro channels in longitudinal and transverse directions respectively.They both reported that the roller forming was more complicated than stamping or hydroforming process due to the dynamic forming process.The wrinkle and springback behaviors need further attention before the method can be applied.
Hydroforming of metallic BPs is also an attractive solution,which was employed by Borit [82] in commercial manufacturing of metallic BPs.In a hydroforming process,the sheet metal is formed into the cavities of die set under a high hydraulic pressure to obtain the desired shape.The unique advantages of hydroforming include higher LDR,better surface condition and only one die needed to be machined,simple application,etc.Pioneering attempts were made by Koç et al.[83] to explore the feasibility of hydroforming of metallic BPs.Following that a high-pressure hydroforming apparatus was developed by Hung and Lin[84].However,the cycle time of hydroforming is about 7-10 s per forming step,which is much longer than stamping.To compete with stamping,four BPs should be formed simultaneously in one hydroforming step [78].
3.2.Coating process
The composition,structure,electrical conductivity,and corrosion resistance of coatings are closely related to the fabrication process.In the last two decades,a lot of researches on the coating processes has been conducted.The typical ones are summarized in Table 6.
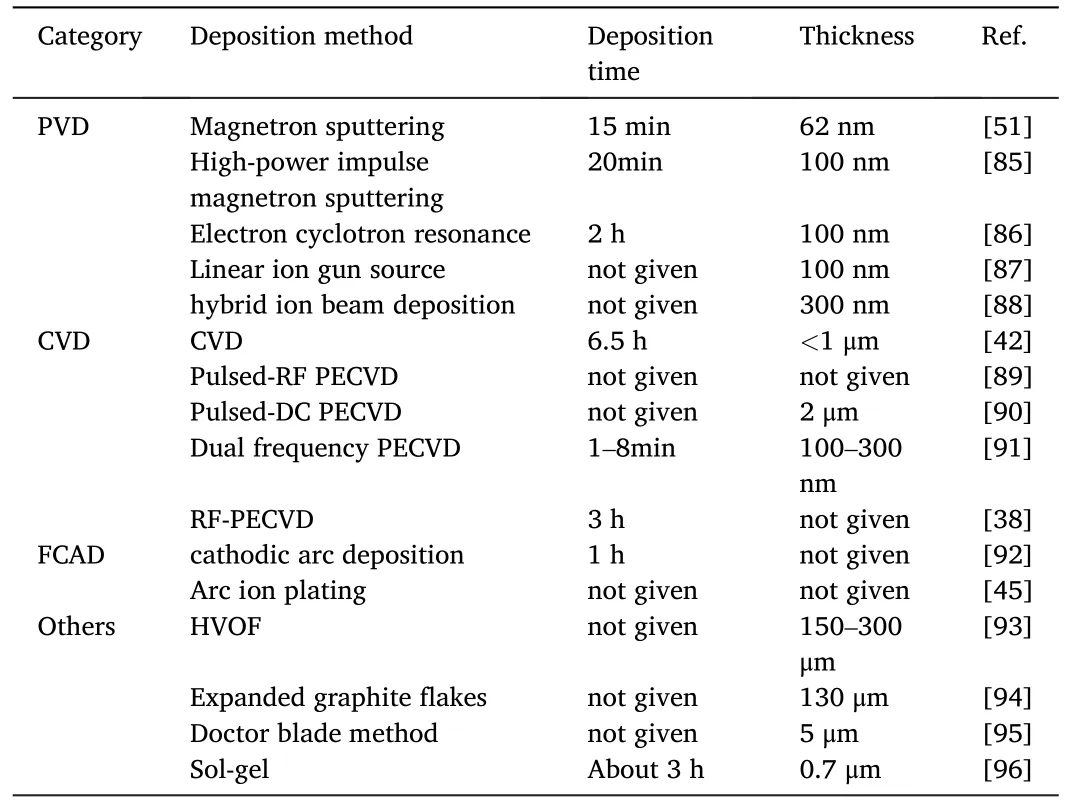
Table 6 Categories,deposition methods,typical deposition time,and thicknesses of carbon-based coatings used for PEMFC applications.
PVD has been widely employed due to its low temperature with high ion energy,low pollution,and the ability to preparation highperformance coatings.CFUBMSIP system is one of the most commonly-employed PVD processes.This process deposits coatings by bombardment with a high-density of low-energy ions,which leads to a dense layer with low internal stresses.Yi et al.investigated the performance of CreC/a-C:Cr coated SS304 BPs deposited via CFUBMSIP[53].They revealed the 300 V bias voltage was an optimized parameter for both the ICR and corrosion resistance.The coatings prepared by the PVD process usually have a satisfying compactness of coating,and various composite multilayer coatings can be obtained by regulating the target.However,the PVD process also has shortcomings such as low deposition rate,low ionization rate of sputtered particles,etc.which are regarded as a critical issue to be solved for the efficient manufacturing of coated BPs.
Chemical vapor deposition (CVD) has also been studied a lot to fabricate different coatings.However,the excessively high temperature in CVD could cause the deterioration of metal BPs.PECVD has been proved efficiency in reducing the treatment temperature of CVD.Show[38] studied the electrically conductive a-C coating on Ti BPs by using the radio frequency plasma enhanced chemical vapor deposition(RF-PECVD)method using ethylene as the reaction gas.They found a-C:H coatings deposited above 500°C have the lowest contact resistance of 2.5 mΩ cm2.However,the voltage required to excite the plasma with a DC power supply could be rather high while the plasma intensity is still not sufficient leading to a low gas ionization rate.To address that,high-energy power sources,including RF power sources,have been employed in the PECVD technology.Improving the deposition rate and coating performance at a lower temperature is still the current development direction of PECVD.
FCAD features satisfying deposition rate,temperature,bonding strength,etc.,which is another attractive coating process.In the FCAD process,excited plasma on the surface of the cathode target is first generated.Following that the uncharged large particles are filtered by using the magnetron elbow.Hence the plasma with a certain energy reaches the substrate surface and condenses to form a film.Mani et al.[92] investigated the TiN-coated SS316L BPs prepared by cathodic arcdeposition(CAD).They revealed that the FCAD has a higher deposition rate than magnetron sputtering under low temperature.Jannat et al.[65] prepared nanometric Ti/TiN multi-layer coating on SS316L.The coated BPs were revealed to satisfy the DOE requirements of ICR and corrosion resistance with a multi-layer coating of 2.75 μm-thick and each mono-layers of around 50 nm.However,further explorations are still required to find out how the coating process affects the durability of BPs.
3.3.Precoating and preforming methods
The coated metallic BPs can be fabricated not only by coating on preformed plates,but also via forming precoated foils.By now most of the research and engineering efforts have been focusing on the previous method since one can expect fracture of coated layers could occur during the severe plastic deformation in BP forming.Those fractures may lead to earlier deterioration of the protective films.However,from the viewpoint of manufacturing efficiency,forming precoated BPs is the better solution due to the following considerations:
(1) Possibility of fast roll-to-roll.Coating of the undeformed strips is much easier than that of the individual BPs.The strips can be coated in a fast roll-to-roll manner with the line speed over 10 m/min [78].
(2) Less handling and simpler work flow.Instead of the frequent loading and unloading of singulated plates between forming,joining and coating,the precoated strips can be easier formed and joined in a simpler work flow.
(3) Higher manufacturing speed and lower cost.Easier automation of the simpler production line of precoated strips can be established with shorter cycle time and less stock.
As a pioneering research,Turan et al.[97] investigated the possibility of forming precoated plates.Three coatings of CrN,ZrN and TiN on SS316L were investigated by forming-then-coating and coating-then-forming for comparison.Significant cracks of coatings were observed by coating-then-forming.However,the ICRs were similar,or even smaller comparing to forming-then-coating.Sandvik Co[98].developed a graphite-like carbon (GLC) coating with metallic interlayers via PVD on 304 L.After a corrosion test of 100 h,the ICR of biaxially-stretched pre-coated foils becomes even lower.However,up to now,there is still a lack of research revealing how durable the BPs formed after precoating are and how to improve the precoating process.Thorough investigations evaluating the applicability of the method is expected.
4.Conclusions
With the development of fuel cell technologies,the mass application of PEMFC is becoming more practical and attracting considerable attentions.Durability,cost and mass-producibility are three major issues to be conquered.The improvements of metallic BPs in corrosion resistance,cost reduction and production rate are critical challenges determining the commercialization progress of PEMFC.This review discusses those challenges in the materials,designs and fabrication methods of metallic BPs in detail and the following conclusive remarks are drawn:
(1) Base material-coating is the major solution to realize the critical functional requirements of corrosion resistance and electric conductivity.Even lower cost base and coating materials with superior electrical conductivity,corrosion resistance,durability,etc.are key targets in the material scenario.
(2) The development of forming and coating technologies of metallic BPs in recent years have enable the fabrication of BPs with reasonable accuracy and reliability.However,with the higher requirements of BPs such as more complicated flow field design,micro channels with a higher aspect ratio,longer coating time,the fabrication method needs further improvement in cost reduction and producing efficiency.To deal with the aforementioned challenges in mass application,the improvement of fabrication methods should be coordinated with the innovation progress made in the material scenario as well as optimized designs considering both multifunctional and manufacturability requirements.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was carried out within the projects supported by the National Natural Science Foundation of China (No.51975363,No.51905342,No.51835011,and No.U1809220).It was also funded by the Scientific and Innovation Action Plan of Shanghai (No.19511107903).Their supports are gratefully appreciated by the authors.
 Progress in Natural Science:Materials International2020年6期
Progress in Natural Science:Materials International2020年6期
- Progress in Natural Science:Materials International的其它文章
- Stability of PGM-free fuel cell catalysts:Degradation mechanisms and mitigation strategies
- Understanding of free radical scavengers used in highly durable proton exchange membranes
- Electrolyte membranes for intermediate temperature proton exchange membrane fuel cell
- Key technologies for polymer electrolyte membrane fuel cell systems fueled impure hydrogen
- Electrolyte materials for intermediate-temperature solid oxide fuel cells
- Carbon-free nanoporous gold based membrane electrocatalysts for fuel cells
