Low-dimensional catalysts for oxygen reduction reaction
Xin Tong ,Xinxing Zhan ,Diane Rawach ,Zhangsen Chen ,Gaixia Zhang ,Shuhui Sun,∗
a School of Chemistry and Material Science,Guizhou Normal University,Guiyang,550001,China
b Institut National de La Recherche Scientifique (INRS),Centre Énergie,Matériaux et Télécommunications,1650,Boulevard Lionel-Boulet,Varennes,QC,J3X 1S2,Canada
ABSTRACT Developing highly efficient electrocatalysts to facilitate the sluggish cathodic oxygen reduction reaction(ORR)is a key challenge for high-performance fuel cells.Low-dimensional materials have attracted great attention recently because of their unique structure and properties.In this review,the application of zero-dimensional(0D),one-dimensional (1D),and two-dimensional (2D) materials in ORR are discussed and particular attention is given to the relationship between their structure and the ORR activity.Graphene-based materials,transition metal dichalcogenides,transition metal oxide,nanotubes,nanoribbons,nanowires,and single-atom ORR catalysts are introduced and classified by their geometric dimension.
Keywords:Oxygen reduction reaction Low-dimensional catalysts Graphene Transition metal dichalcogenides Transition metal oxide Single-atom catalyst
1.Introduction
Climate change caused by the burning of fossil fuels is one of the major global problems.The development for clean and renewable energy resources to ensure the sustainable development of the world is in urgent need[1,2].Proton exchange membrane fuel cells(PEMFCs)that can convert the chemical energy (from clean and renewable fuels such as hydrogen) directly into electrical power have attracted tremendous attentions[3].In the fuel cell system,oxygen reduction reaction(ORR)proceeds at the cathode while hydrogen oxidation reaction (HOR) occurs at the anode.The catalysts are essential to lower the activation energy,thus making these reactions kinetically-activated [4,5].Moreover,the kinetics of ORR is five-orders-of magnitude slower than that of HOR,which leads to more catalyst usage in the cathode.Therefore,developing highly efficient cathodic ORR electrocatalysts is highly desired for high-performance and low-cost fuel cells.
ORR can be catalytically achieved through two different mechanisms:(i) four-electron (4e−) pathway to directly produce water in acid media or hydroxide anion in alkaline media;(ii) two-electron (2e−)pathway to create the intermediate compound (hydrogen peroxide) in acid media or peroxide anion in alkaline media [6].Compared with 2e−pathway,the 4e−process is more attractive because it could ensure higher operating potentials and current efficiency in fuel cells.Currently,the carbon black supported platinum(Pt/C)catalysts are the state-of-the-art 4 e−catalysts for ORR in PEMFCs.Due to the scarcity of Pt,finding alternative catalysts with less cost is one of the central tasks for the large-scale commercial implementation of the fuel cell industry.Low-Pt catalysts,non-precious metal catalysts,and metal-free catalysts have been widely investigated as substitutes for the commercial Pt/C[7-9].
Graphene is a 2D layer with sp2-bonded carbon atoms,which has attracted worldwide attention in both metal-free catalysts and supporting materials for ORR.Inspired by the successful development of graphene,a variety of studies of using other types of 2D materials as cost-effective ORR catalysts have started to appear,such as transition metal dichalcogenides (TMDs),phosphorene,hexagonal boron nitride(h-BN) and graphitic carbon nitride [10-12].Besides,the 2D graphene can be split into smaller particles such as 0D quantum dots and 1D nanoribbons[13].With this revelation,low-dimensional materials have recently emerged as new types of electrocatalysts [14-17].
2.Dimensionality classification
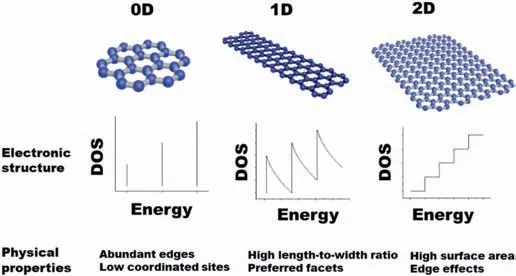
Fig.1.The classification,electronic structure,and physical properties of lowdimensional materials:0D materials (no dimensions are outside the nanoscale,e.g.Nanoparticles,quantum dots),1D materials (one dimension is outside the nanoscale, e.g.nanotubes,nanorods,and nanowires) and 2D materials (only one dimension in nanoscale, e.g.nanofilms,and nanosheets) [15].
When the materials are smaller than 100 nm in at least one dimension,their electronic structure and surface properties change drastically,resulting in unique physical and chemical properties due to their quantum size effect and large surface area.In low-dimensional materials,the motion of electrons is restricted in zero,one,and two dimensions.As shown in Fig.1,the polts of the density of states (DOS)versus energy in low dimensional structures are distinct from each other.It's a staircase for 2D materials,a fence for 1D material,and a pack of discrete lines for 0D materials.In 0D materials,such as nanoparticles or quantum dots,the electrons are confined in all three directions (x,y,z-axis) and cannot move anywhere [15].The 0D material shows abundant edges and low coordinated sites.In 1D materials,such as nanotubes,nanorods,and nanowires,the electrons are restricted in two directions and can only move in one direction.The 1D material displays a high length-to-width ratio with preferred facets.In 2D materials,such as thin nanofilms and nanosheets,only one direction is restricted for the movement of the electron.And they exhibit high surface area and edge effects.Dimensionality plays a vital role in depicting the fundamental properties of a material.Due to the quantum mechanical effects,the properties of these low-dimensional materials are significantly different from those of bulky materials.
Low-dimensional materials with designated thickness,size,and morphology show many advantages for catalysis[15].On the one hand,the high surface area could increase the number of active sites on the surface,which enhances the atom utilization.On the other hand,their unique electronic structure and low coordinated environment benefit the interactions between catalysts and reactants.According to the Sabatier principle,the adsorption energy of the reactants and intermediates on the catalyst surface should be neither too small nor too large.The interactions between catalysts and reactants are largely dependent on the electronic structure of the catalyst,affecting the catalytic activity and stability of the catalyst.Therefore,the controlled synthesis process of low-dimensional catalyst materials with desirable electronic structures is the key to the promotion of catalytic performance.
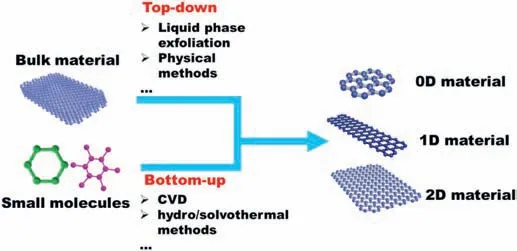
Fig.2.Schematic diagram of the top-down and bottom-up strategies for the synthesis of low-dimensional materials and GNRs.
The synthetic strategies of low-dimensional materials can be divided into two main categories:(i)top-down and (ii) bottom-up,as shown in Fig.2.Briefly,the top-down approach is to reduce the bulk material to the nanoscale by liquid-phase exfoliation or physical methods.For example,graphene can be obtained from graphite by breaking the van der Waals forces between the graphite layers using adhesive tape[18].The general idea of the bottom-up strategy is using small building blocks made of atoms or molecules to synthesize nanomaterials.Chemical synthesis methods such as chemical vapor deposition(CVD)and hydro/solvothermal methods are typical examples [19].In general,top-down approaches are simple and easy for scale-ups but lack the precise control of the morphology.The bottom-up method is good at synthesizing nanomaterials with precisely controlled size,shape,and morphology.The synthetic approaches of low-dimensional materials have been well summarized in many recent papers [20,21].This review will focus on the relationship between the structure and the ORR activity of the low low-dimensional materials.
3.2D electrocatalysts for the oxygen reduction reaction
2D materials have emerged as the promising catalysts for ORR since the 2D-graphene was explored experimentally in 2004 [23].As shown in Fig.3,a wide range of 2D materials such as graphitic carbon nitride(g-C3N4),metal dichalcogenides (TMDs,e.g.MoS2,MoSe2),layered metal,layered metal-organic frameworks (MOFs),MXenes (transition metal carbides,nitrides,and carbon nitrides),layered transition metal oxides (TMDs,e.g.MnO2),and hexagonal boron nitride (h-BN) have also been successfully prepared and applied in electrochemistry in recent years.These 2D materials are of single or a few layers in thickness.This distinct structure along with the extraordinary physical and chemical properties such as mechanical flexibility and high specific surface area make 2D nanomaterials promising as the catalyst support in electrochemical energy conversion.Moreover,the electrocatalytic activity of 2D material can be tailored by inducing intramolecular charge transfer with heteroatom-doping or defect engineering.The unique electronic structure and increased active sites render 2D material appealing candidates for the ORR catalyst [11].However,the active sites of these 2D materials differ significantly,which will be discussed in the following section.
3.1.Graphene-based materials
Graphene-based catalysts have attracted great attention for ORR[24-26].Graphene exhibits many excellent chemical and physical properties as well as the unique graphitic basal plane structure that could supply numerous active sites and facilitate the electron transport.Unfortunately,the ORR activity of pristine graphene is very poor due to the lack of free electrons for the reaction and a limited number of active sites [27].The electroneutrality of pristine graphene should be broken down to create charged sites that are favorable for O2adsorption.Introducing dopants and generating defects on graphene could modulate the surface energy,the chemisorption energy of O2,as well as the local electronic properties and thus enhance the catalytic properties [28].
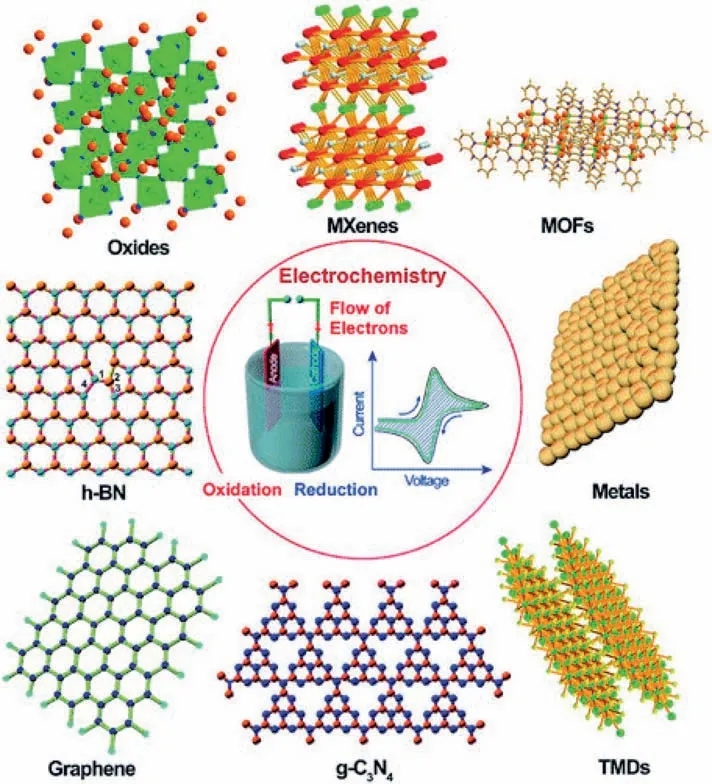
Fig.3.Schematic illustration of typical 2D nanomaterials such as graphene,graphitic carbon nitride(g-C3N4),metal dichalcogenides(TMDs),layered metal,layered metal-organic frameworks(MOFs),MXenes,layered metal oxides,and hexagonal boron nitride(h-BN)used in electrochemistry.Reprinted with permission from Ref.[22].
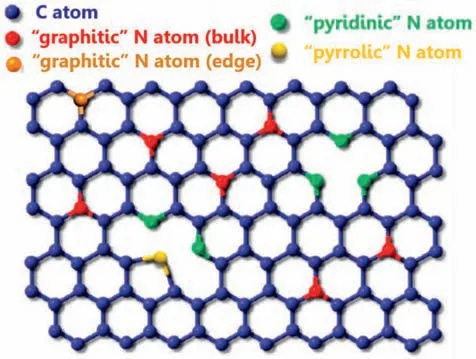
Fig.4.Schematic representation of three types of nitrogen species:pyrrolic,pyridinic,and graphitic N atoms.Modified with permission from Ref.[29].Copyright © 2009,American Chemical Society.
Among various possible dopants,N-doped graphene is by far the most investigated material.The atomic radius of N is close to C.So it is relatively easy to incorporate N into the backbone of the graphene material.The carbon atoms that are adjacent to the more electronegative nitrogen atoms are supposed to be the active sites for ORR[30].The interaction with oxygen molecules will be facilitated because of the favorably changed charge profile.As shown in Fig.4,there are three types of nitrogen dopants (pyridinic,pyrrolic,and graphitic).Each type of these nitrogen dopants influence the catalytic property differently and the exact role of the specific N specie in ORR is still under debate [31,32].Pyridinic nitrogen is believed to be the most active site for ORR[33,34].The lone electron pair of pyridinic N could donate to the π-bond of the carbon matrix,making pyridinic N atom itself electron-attractive and catalytically active.Satoshi Yasuda et al.[35] used temperature-induced surface polymerization of nitrogencontaining aromatic molecules to get two types of N-doped graphene:one is mainly composed of pyridinic nitrogen and the other is graphitic nitrogen.It was revealed that pyridinic nitrogen could catalyze the ORR via the 4e−process,whereas graphitic nitrogen reduced oxygen via a 2e−pathway.However,different conclusions were proposed by other researchers.[36,37],Luo et al.[38]employed a pyrolysis method using methane (CH4) and ammonia (NH3) as carbon and nitrogen sources,and the graphene doped with nearly 100%pyridinic N was obtained on the surface of a Cu substrate.The results showed that the pyridinic N doped graphene prefers a 2e−pathway for ORR,indicating that the pyridinic N couldn't effectively promote ORR by a 4e−dominated process.This result may play a vital role in tailoring the electronic properties for the improvement of the ORR performance of the pyridinic N.Ruoffet al.[37] prepared the N-doped graphene with controlled N doping species by selectively annealing graphene oxide (GO)with different N-containing precursors.Higher ORR activity and larger limiting current density could be obtained by graphitic N-dominated catalysts.In contrast,more positive onset potential of ORR could be found in the pyridinic N-dominated catalysts.However,the catalytic ability of doped graphene regarding the ORR depends on many complicated factors of the coordination environment including surface area,morphology,crystallographic structure,oxidation state,and chemical composition.It cannot be simply summarized over a few studies.The comprehensive and profound research for ORR is needed to discuss the real mechanism.
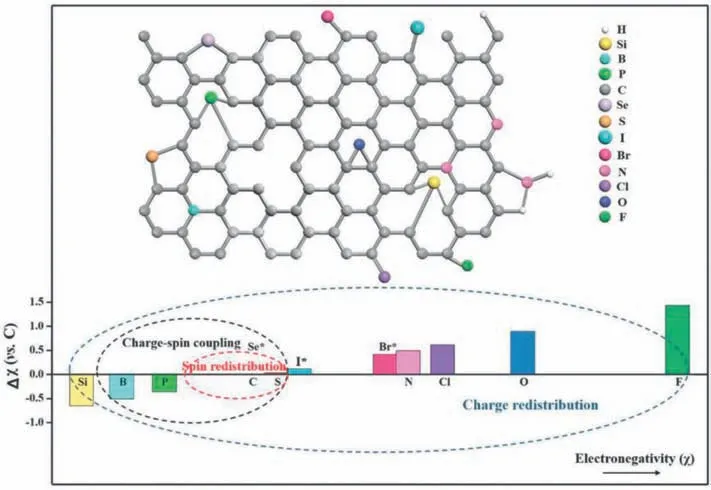
Fig.5.Illustration of different heteroatoms doped graphene materials.Reprinted with permission from Ref.[14].Copyright©2018 WILEY-VCH Verlag GmbH&Co.KGaA,Weinheim.
Meanwhile,as shown in Fig.5,the dopping of heteroatoms in graphene lattice can cause a charge density redistribution because of their distinct electronegativity.In practice,the atomic radius of heteroatoms should be considered.Large atoms such as Br,I,and Se cannot be incorporated into the carbon lattice.Boron (B),sulfur (S),and phosphorus (P) have been widely used for the heteroatom-doped graphene [30,39,40].The catalytic activities will be enhanced because of the spatial distortion and charge redistributions.Since the electronegativity of B atoms is less than that of carbon atoms,when they are introduced into graphene,the p-type conductivity can be induced.The B-doped graphene is believed to be a good catalyst for promoting the 4e−process [41].As for S,the electronegativity of S is close to that of carbon.However,the size of the S atom is larger than that of the C atom.Spatial distortion of S-doped graphene is a key factor to enhance the ORR activity.The carbon atoms near doped sulfur atoms and the zigzag edges are the catalytic active sites in S-doped graphene [42].In terms of P doping,the P3p and C2p orbital are hybridized by sp [3]-orbital configuration,which is shown as a pyramidal structure.These structures are easily oxidized to generate C-P-O bonds.The active sites of P-doped graphene are positively charged carbon atoms because the O atoms in the C-P-O structure can polarize the P and the adjacent C atoms.Graphene doped with two or more kinds of heteroatoms have also been proven to be interesting because of the increased number of dopant heteroatoms and the synergistic effects between the dopants.B-N co-doped,P-N co-doped,S-N co-doped graphenes are the most widely studied materials for ORR[43,44].The intermediate adsorption on the surface of doped graphene can be enhanced by tailoring the electron-donor properties with controlled dopant types and their amounts.Elucidating the underlying ORR mechanism of the doped graphene will be one of the top priorities for future research.
3.2.2D Transition-metal dichalcogenide (TMD)
Transition-metal dichalcogenide (TMD or TMDC) monolayers are thin-layer materials and their general chemical formula is MX2.The M represents transition-metal atoms (e.g.Mo,W,etc.) whereas the X represents chalcogen atoms (e.g.S,Se,Te).In the MX2catalysts,a single layer of transition metal atoms is situated between two layers of chalcogen atoms where a strong covalent bond between the M and X atoms is observed [45].The 2D TMDs show adjustable bandgaps that are decided by the stacking order between layers and the different coordination models between M and X atoms,which will lead to different surface properties and electronic structures and thus varied catalytic activities will be observed [46].
Among the known sixty TMDCs thus far,2D molybdenum disulfide(MoS2) has attracted much attention for the ORR catalyst.Their active sites are the edges and corner atoms with low-coordination.In particular,Mo atoms passivated by S atoms that are found at the edges are determined to be the most active sites because of their sulfide rich and unsaturated coordinated environment as well as their dangling bonds[47].However,the pristine MoS2shows poor electron transport properties and limited active sites.Various attempts have been made to expose more active edge sites and improve conductivity.For example,heteroatom doping can be used to activate the atoms on the basal plane.Similar to the doping process of the graphene,B,N,P,and O were introduced to the MoS2[48,49].Moreover,metallic atoms such as Fe,Co,Ni are proved to be effective for improving the ORR activity as well[50,51].In addition,conducting nanomaterials,such as graphene,are used as the support to improve the electron transport properties of the catalysts.As shown in Fig.6,the N-MoS2/C composite materials were obtained by thermal treatment with the mixture of ammonium molybdate,thiourea,melamine,and Pluronic F127[52].The ORR activity was enhanced as a result of the abundant active sites that were derived from rich-defects on the N-doped MoS2/carbon materials and the high electron conductivity.
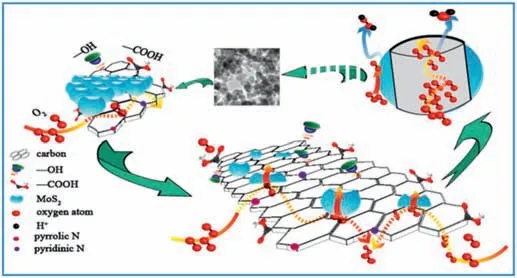
Fig.6.Illustration of the ORR on the interfaces of N-doped MoS2/carbon materials.Reprinted with permission from Ref.[52]©2016 Elsevier B.V.All rights reserved.
3.3.2D Transition-metal oxides (TMOs)
Transition-metal oxides (TMOs) consist of oxygen atoms with transition-metals from the d-block of the periodic table(such as Mn,Fe,Co,Ni,Ti,etc) [53].The 2D layered structures have extra advantages such as high surface area and edge defects.Quasi-2D sheets of the TMOs are stacked together by van der Waals interactions.Transition metal atom possesses unpaired electrons and dangling bonds,leading to strong surface polarization of the TMOs[5,54].The 2D TMOs also show multiple valence states,various metal oxidation states,and hence a tunable ORR activity.Since the thickness of the 2D TMOs is limited(always less than 2 nm),most of the low-coordinated transition-metal atoms are exposed on the surface and serve as the active sites [55,56].
However,due to the poor conductivity,TMOs exhibit unsatisfactory ORR performance.Highly conductive support material with large surface areas such as graphene is used to form a 2D graphene/TMO heterostructure to address the conductivity issue [58].There are interphase ligand effects and excellent interfacial interactions between TMOs and graphene due to oxygen-containing functional groups found on graphene.Thus,the use of graphene can improve the conductivity while the TMOs on the surface could maximize electrochemically accessible surface area.Therefore,this synergistic effect provides graphene/TMO heterostructure with a better ORR performance.As shown in Figs.7 and 2D manganese oxide nanosheets/graphene composite was synthesized by heating the graphene oxide with KMnO4[57].The nanopores were then introduced into the composites by heating with S powder.The improved ORR activity of the nanoporous MnO2nanosheets was related to the active Mn3+/4+sites,the large surface area,and oxygen vacancies,which reduced the kinetic barriers and facilitated the oxygen adsorption.
Among different crystal structures of TMOs,the perovskite structures with a general formula ABO3have attracted great attention recently because of their non-stoichiometric chemistry and variable crystal structures [60,61].The 2D perovskite oxide consists of stacked layers with edge-sharing octahedra and shows a higher surface.As shown in Fig.8,three mechanisms are proposed to explain the active sites in 2D perovskite oxide for ORR.The oxygen can be absorbed on the catalyst surface by three configurations:(a) end-on adsorption,(b)side-on adsorption,and(c) bidentate adsorption.For both mechanisms in Fig.8a and b,the metal sites are the active sites for ORR.However,the mechanisms in Fig.8c show that the metal sites and oxygen vacancies can both serve as the active sites for ORR.In alkaline media,the OH group near the oxygen vacancies is displaced by one atom of O2while the other atom of O2fills the oxygen vacancy.In this case,the O-O bond is weakened,facilitating the further reaction to form OH−.The oxygen vacancies can also change the electronic structures of perovskite oxides,leading to the presence of redox couples and electron holes,benefiting the ORR by promoting charge transfer and increasing the electrical conductivity.Therefore,the ORR performance of perovskite oxides is largely related to oxygen vacancies.
4.1D electrocatalysts for the oxygen reduction reaction
The 1D electrocatalysts are the ideal candidates with high catalytic activity for ORR,owing to their unique structural superiorities such as rapid electron and mass transport,large surface area,low vulnerability to aggregation,and dissolution.The catalytic activity of 1D electrocatalysts is not only dependent on their composition but also their geometry such as the number of walls,length,diameter,and chirality.Nanotubes,nanoribbons,and nanowires are the most common 1D electrocatalysts that have been widely used for boosting the ORR activity.
4.1.Nanotubes
Nanotubes are made of various materials that take the shape of tubes with their diameters measured in nanometers.Nanotubes are frequently used as ORR catalysts because of their inherent morphologic features such as large surface area and rapid mass transport as shown in Fig.9.Moreover,nanotubes show high ORR stability because they can inhibit carbon corrosion,Ostwald ripening,and aggregation under fuel cell operating conditions [62].
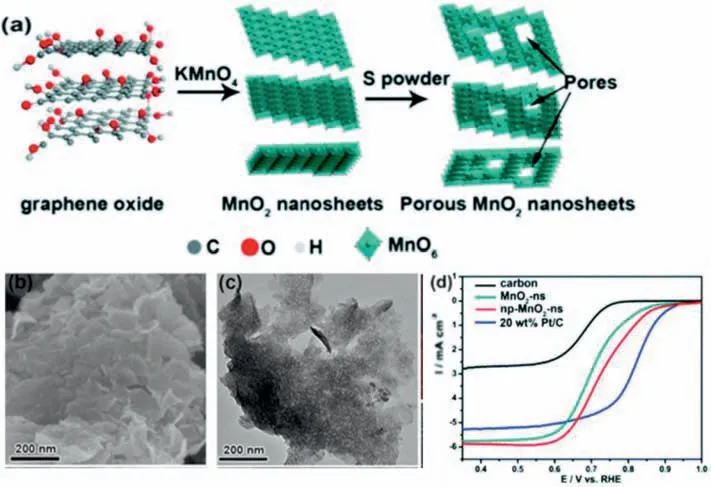
Fig.7.(a)Schematic diagram for the synthesis of nanoporous MnO2 composites.(b)The SEM,(b)TEM images,and(c) LSV curves of the composites in O2-saturated 0.1 M KOH solution.Reprinted with permission from Refs.[57].© 2018 Wiley-VCH Verlag GmbH &Co.KGaA,Weinheim.
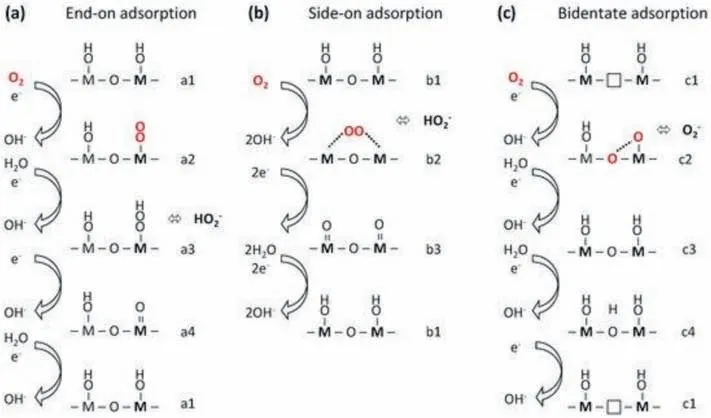
Fig.8.The ORR mechanisms in alkaline media of the perovskites oxide which is set up based on the orientation of oxygen adsorption:(a)end-on adsorption,(b)sideon adsorption,and (c) bidentate adsorption.Reprinted with permission from Ref.[59].
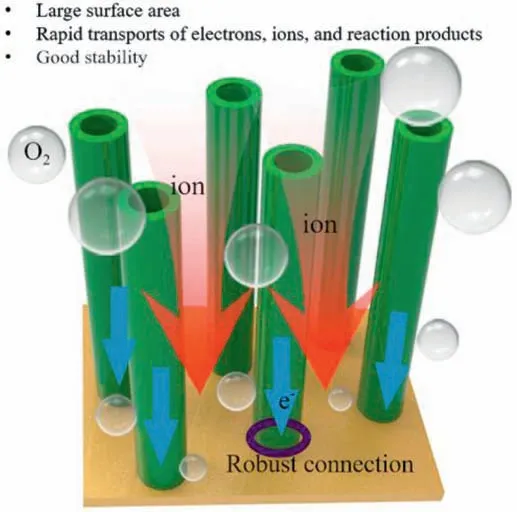
Fig.9.Schematic diagram of the prominent features for 1D nanotubes electrocatalysts.Reprinted with permission from Ref.[62]©2018 Elsevier Ltd.All rights reserved.
Carbon nanotubes (CNTs) can be directly used as the metal-free ORR catalyst or as the catalyst support.However,the electrons in pristine CNTs are too inert for ORR.Surface engineering is essential to modulate the chemisorption energy of O2and increase active sites thereby enhancing the ORR activity.Similar to graphene,the doping of heteroatoms is frequently used to change the surface properties.In particular,the N-doped CNTs are attracting much more attention[63,64].This pioneering work was achieved by L.Dai's group in 2009[6].Vertically aligned nitrogen-containing carbon nanotubes were employed to be the ORR catalyst with improved catalytic activity and high stability compared to commercial Pt/C catalysts.The carbon atoms adjacent to N dopants show high positive charge density and thus serve as active sites.Moreover,N-doped CNTs are used to replace carbon black to build Pt nanoparticles supported by N-doped CNTs material[65,66].Uniform Pt nanoparticles with an ultra-small size can be prepared on the N-doped CNTs and show improved ORR activity and high stability when compared with the carbon black and pure CNTs.
To avoid the carbon-corrosion problem,self-supported Pt nanotubes were developed to address the activity and long-term durability of ORR[67,68].These unique Pt nanotubes show preferential exposure of highly active crystal facets,easy electron transport,and inherent high stability.Also,the built of 1D Pt-based alloy and core/shell nanotubes structure has attracted remarkable attention[69,70].Self-supported Ptbased nanotubes can be served as an alternative to carbon-supported materials and offer a broad materials library of noble metal structures.
4.2.Nanoribbons
Nanoribbons are strips of 2D materials with a narrow width (less than 50 nm) in the plane.Graphene nanoribbons (GNRs) can be regarded as the unzipped CNTs.Compared with CNTs,GNRs show an open structure where the abundant edges are visible.The desirable catalytic activity towards ORR can be obtained by controlling the edge structures and chemical terminations [71].However,the synthesis of the GNRs is not simple with opening the CNTs being one of the most promising approaches.As shown in Fig.10,the longitudinally unzipping of CNTs was used to get GNRs,and after anin-situpolymerization and thermal treatment process,nitrogen doping was achieved[71].The TEM image showed the unzipped CNTs and the as-prepared GNRs demonstrate a thin elongated morphology.The N-doped GNRs showed an enhanced ORR activity with the dominant 4e-pathway.The active sites were the C atoms near the graphitic N and the pyridinic N at the edges.
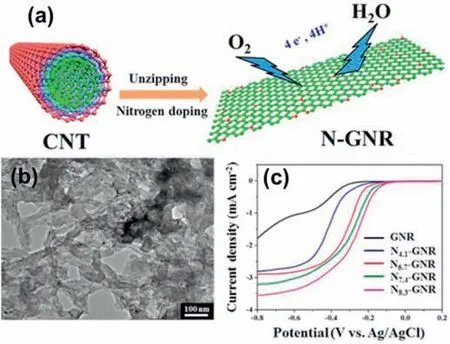
Fig.10.(a) Schematic diagram of the synthesis of nitrogen-doped graphene nanoribbons.(b) TEM images of N-doped GNRs.(c) LSV curves of N-doped GNRs under 1600 rpm in O2-saturated 0.1 M KOH solution.Reproduced with permission from Ref.[71].Copyright © 2014,American Chemical Society.
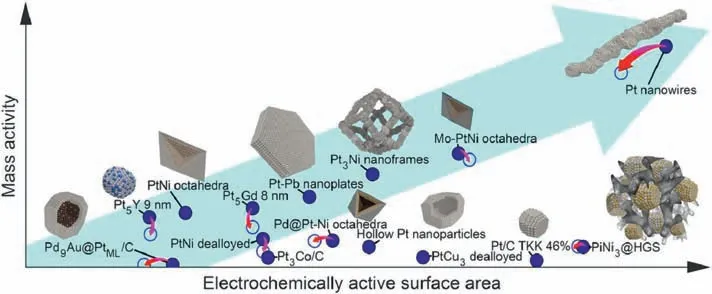
Fig.11.Electrochemical activity towards ORR of different nanostructure catalysts.Reproduced with permission from Ref.[76].© 2018 Elsevier B.V.All rights reserved.
4.3.Nanowires
Compared to the CNTs and GNRs,the nanowires are relatively easier to be synthesized,especially for metal and metal oxide nanowires,such as MnO2,Co3O4[62,72,73].In particular,the building of the nanowire structure has been proved to be an efficient strategy to enhance the mass activity of Pt-based catalyst[74,75].The morphology of 1D nanowires is beneficial to maintain high Pt-surface utilization and thus increases the electrochemical surface area (ECSA).As shown in Fig.11,because of the uncoordinated configuration on the surface,jagged Pt-based nanowires show the highest mass activity compared with other Pt nanostructures such as nanoparticles,nanoframes,etc.Moreover,the Pt-based nanowires display enhanced durability.The unique 1D nature of nanowires can be anchored on the support by multiple points.Therefore,Ostwald ripening and particle agglomeration are not prone to occur.Also,the Pt-based nanowire shows fewer defective sites and lattice boundaries vulnerable to Pt dissolution [76].
5.0D electrocatalysts for the oxygen reduction reaction
The 0D materials are materials having all three dimensions on the nanometer scale.Various materials,such as nanoparticles and quantum dots,are the typical example of 0D materials.Usually,0D electrocatalysts for ORR can be classified into metal nanoparticles,metal alloy nanoparticle,and metal complexes.Compared with bulk materials,the 0D nanostructures always show high surface free energy and low-coordinated atoms,and thus considerably improving their ORR activity[77-79].However,0D nanoparticles always suffer from aggregation.Therefore,the use of the appropriate support material is an efficient way to enhance their stability.In low dimensional materials,the van der Waals forces of the 1D and 2D materials allow them to strongly interact with 0D nanoparticles.Therefore,the 0D/1D or 0D/2D heterostructures are formed and show a synergistic effect on enhanced catalytic activity for ORR [80,81].
When the size of the catalyst reaches the limitation (single atom) it can be considered as the 0D material as well.Single-atom catalysts(SACs) can maximize the atomic utilization,which indicates that ideally,every active single atom can serve as an active site [83-90].Moreover,SACs show excellent activity and exclusive selectivity for ORR because of the unsaturated coordination environments and their unique electronic structure.As shown in Fig.12,a carbon-supported defect-anchored Pt SACs were synthesized and showed high ORR performance with high onset potential and the 4e−ORR process [82].In an acidic H2/O2fuel cell,the Pt SACs with carbon defeat showed superhigh platinum utilization of 0.09 gPtkW−1.The individual Pt atom was anchored by the carbon defects and the Pt dispersion was improved.The Pt atoms anchored by four carbon atoms (Pt/C4) was supposed to be the active sites.Furthermore,the Pt alloy and the other metal SACs were also prepared [89,91,92].The SACs catalyst should be strongly coupled to the support to enhance stability.Furthermore,the catalytical activity is highly related to the coordination enlivenment of the metal atoms [93].So the support effect should be considered when using the SACs.
M-N-C(M=Fe,Co,Cu,Ni,Mn,etc.)based ORR catalysts is another unique class of SACs.In 2009,breakthroughs were achieved by the Dodelet group at INRS that the activity of the Fe-N-C catalyst reached to the level of Pt/C catalyst [94].Since then,M-N-C catalysts have attracted extensive research interests.When the active species of M-N-C catalysts are downsizing into single atoms level,it can maximum atomutilization and thus enhance the intrinsic activity of catalysts[95,96,97].As shown in Fig.13,similar to noble metal SACs,the catalytic performances of these atomically dispersed non-precious metal catalysts are also affected by their coordination environments and geometric configurations.To further elucidate the synthesis−structure−property correlations,more work should be done in the combination of precisely controlled synthetic process,in-situ structural characterizations,simulations,and intrinsic catalytic performance in the future.
6.Conclusions and perspectives
The different categories of low dimensional materials used in ORR have been reviewed.When the size of the materials is reduced to the nanometer scale (at least one of their dimensions),their electronic structure and their surface properties change drastically,resulting in unique physical and chemical properties due to the quantum size effect and large surface area.The electrocatalytic activity of low dimension materials is highly related to their electronic properties and nanostructures.The reaction mechanisms of low dimension materials are discussed by the combination of computational and experimental results.By reviewing the past progress,we expect to extend in-depth research on PEMFC catalysts of different dimensions.The low dimensional material can be used as both the catalysts and the supporting materials.Low-dimensional materials not only show a promising way to enhance the ORR activity but also provide new fundamental insight into ORR.
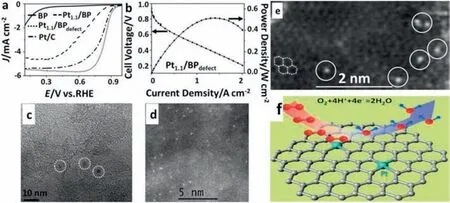
Fig.12.(a)The RDE polarization curves in acid and(b)the polarization and power density curves of Pt1.1/BPdefect.(c,d,e)The TEM images of Pt1.1/BPdefect.(f)The schematic diagram of active sites in Pt1.1/BPdefect.Reproduced with permission from Ref.[82] © 2019 Wiley-VCH Verlag GmbH &Co.KGaA,Weinheim.
Despite much progress,great efforts are still needed to further develop the low dimensional catalysts for ORR.For example,more theoretical and experimental studies are required to develop efficient strategies for alloying,hybridizing,doping (bi-doping and multidoping),and combining different dimensional materials to further improve the thermodynamics and the kinetics of the catalysts.More systematical structure-property studies should be done to quantify the active sites and clarify the corresponding reaction mechanism of ORR.This calls for the employment of advanced characterization techniques,especially in-situ ones(such as XRD,XAS,IR,Raman),to investigate the ORR process based on low-dimensional catalysts in half cells and real fuel cell environments.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We thank the support from the Doctor's Initial Funding of Guizhou Normal University,China (GZNUD[2019]25,GZNUD[2019]29),International Science and Technology Corporation Program of Guizhou Province,China (G[2013]7022),Youth Science and Technology Talent Development Project from Guizhou Provincial Department of Education,China (KY[2016]063),Fonds de Recherche du Québec-Nature et Technologies(FRQNT),the Natural Sciences and Engineering Research Council of Canada(NSERC),Institut National de la Recherche Scientifique (INRS),and Centre Québécois sur les Matériaux Fonctionnels (CQMF).
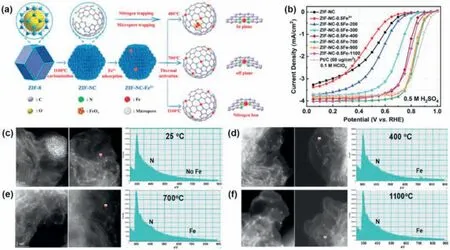
Fig.13.(a)Schematic illustration of the Fe-N bond formation process by adsorbing Fe ions into ZIF-8-derived N-doped carbon.(b)The ORR activity of the catalysts obtained in different stages and pyrolysis temperatures.(c-f)Morphology and atomic structure of Fe-N-C catalyst treated at various temperatures.Reproduced with permission from Ref.[97].Copyright 2019 Wiley-VCH Verlag GmbH &Co.KGaA,Weinheim.
 Progress in Natural Science:Materials International2020年6期
Progress in Natural Science:Materials International2020年6期
- Progress in Natural Science:Materials International的其它文章
- Stability of PGM-free fuel cell catalysts:Degradation mechanisms and mitigation strategies
- Understanding of free radical scavengers used in highly durable proton exchange membranes
- Electrolyte membranes for intermediate temperature proton exchange membrane fuel cell
- Key technologies for polymer electrolyte membrane fuel cell systems fueled impure hydrogen
- Electrolyte materials for intermediate-temperature solid oxide fuel cells
- Carbon-free nanoporous gold based membrane electrocatalysts for fuel cells
