Induced pluripotent stem cells for therapy personalization in pediatric patients: Focus on drug-induced adverse events
Elena Genova, Federica Cavion, Marianna Lucafò, Luigina De Leo, Marco Pelin, Gabriele Stocco,Giuliana Decorti
Abstract
Key words: Induced pluripotent stem cells; Organoids; Adverse drug reactions; Intestinal toxicity; Hepatic toxicity; Pancreatic toxicity; Nephrotoxicity; Cardiotoxicity;Neurotoxicity
ADVERSE DRUG REACTIONS
Adverse drug reactions (ADRs) are major clinical problems[1], especially for special populations such as pediatric patients[2,3].Indeed, a significant number of drugs can cause adverse effects leading, in some cases, to patients’ hospitalization, permanent disability, or even death.ADRs are costly for pharmaceutical industries in terms of drug withdrawal from the market[4], but also in terms of clinical and economical efforts needed to resolve side effects[1].In order to avoid these problems that affect patients’ health and greatly increase costs related to treatment, it is necessary to improve clinical trial strategies.In addition, bothin vivoandin vitromodels are needed that better assess and evaluate drug safety.Due to newly discovered technologies and progress made in this area, this goal seems achievable[5-7].Indeed, to date, it is possible to establish more sensitive and personalized assays leading to a better comprehension, and thus prevention, of ADRs.One of the most important advances in this field is the possibility to set up groundbreaking patient-specific assays.Indeed, ADRs are related to individual genetic patients’ background, leading to a wide range of toxicities of different severities[8].Another important point is that pediatric patients may respond differently to drugs than adults and are susceptible to developing different patterns of ADRs, leading, in some cases, to more severe consequences[1-3].One of the most powerful tools that can be used to individually model drug response and ADR development is represented by induced pluripotent stem cell (iPSC) technology, which was discovered by Takahashiet al[5]more than 10 years ago.
iPSCs can be used to evaluate safety during drug preclinical screening, possibly replacing the use of animal models or immortalized human cell lines.Due to genetic and physiological species-specific differences, these models may be accurate in predicting drug toxicity, especially when this toxicity is related to individual genetic differences[9].Indeed, many factors may influence drug pharmacokinetics and pharmacodynamics as well as the development of adverse effects, for instance:(1)Polymorphisms in genes encoding for drug-metabolizing enzymes and transporters,ion channels and receptors, possibly affecting their expression and/or activity[10]; (2)Epigenetic alterations such as DNA methylation, histone modification, microRNAs,mRNA instability, and nucleosome positioning[11]; and (3) Environmental and nongenetic factors including body mass index and behavioral patterns, concomitant diseases, in particular liver and kidney diseases, and developmental factors, especially those in early life[12].
In this review, we explored the state of the art of iPSCs as a model to study ADRs in adults, and wherever available, in the pediatric field.In particular, we focused on intestinal, hepatic, pancreatic, renal, cardiac and neuronal levels, also discussing the progress in the creation of organoids (Figure 1).
ADVERSE DRUG REACTIONS IN PEDIATRIC PATIENTS
Children can respond differently to many drugs and can present different ADRs than adults[2,3].The reason is mainly the variation in the pharmacokinetic and pharmacodynamic profiles between pediatric and adult patients[13,14].In the era of personalized medicine, it is important to screen and identify genetic variations related to the predisposition to ADR development for certain drugs[15,16].However, in pediatric patients, ADRs have not been studied as thoroughly as in adults.The clinical and research experience on drug safety for children is limited and more works are needed[17].One of the reasons for the lack of these data is that, for ethical reasons, the effects of most drugs have been analyzed in clinical trials only in adults, resulting in limited knowledge on children’s responses[17].To date, there is strong interest into therapy personalization for children to further explore, study, and prevent possible new and severe ADRs.In order to avoid the well-known ethical limitation of pediatric trials, to increase the comprehension of drug response in children, and establish safer and personalized treatment, it is possible to use innovative technologies such as iPSCs[5,18].Indeed, several specific pediatric problems could be addressed using iPSCs;for example, this technology is ideal to develop innovative patient-specific models of rare or genetic diseases that often occur in pediatric populations[13].Moreover, several drugs used in pediatric patients lead to adverse effects in organs that are not easily accessible, such as pancreatitis for asparaginases[18]for the treatment of acute lymphoblastic leukemia.
INDUCED PLURIPOTENT STEM CELLS, THERAPY PERSONALIZATION, AND ADRs
Patients’ somatic cells can be reprogrammed into iPSCs using the four Yamanaka’s factors[5].iPSCs preserve the donor’s genetic heritage and are useful for creating patient-specific models[19].For a decade, scientists have been using iPSCs to study complex diseases and to obtain tissues or cells otherwise not simply accessible from patients[20].Moreover, these cells have allowed scientists to set up innovative research to study ADRs in a more personalized way[18,21-24].The focal point of this technology is the possibility to differentiate iPSCs into almost any cell of the human body[20].Progress made in this last decade has allowed the development of groundbreaking tailored models for single individuals, contributing to the modern era of therapy personalization, finding the appropriate treatment for the right patient, and avoiding the development of ADRs[25].To date, a number of studies based on iPSCs for therapy personalization and to study ADRs, especially in the adult field, are available[18,21-24].However, more efforts are needed in the pediatric field, even though some works are already available[26-28].iPSCs may be a great tool to model sensitivity to new, but also old drugs in children, shedding light on the mechanisms of toxicity, resolving in part the problem of the lack of data.Indeed, with a simple blood sample, it is possible to reprogram peripheral blood mononuclear cells into iPSCs that can be subsequently differentiated into somatic cells of interest.
ORGANOIDS AS GROUNDBREAKING PATIENT-SPECIFIC MODELS FOR PHARMACOLOGICAL STUDIES
Organoids are three-dimensional (3D)in vitroculture systems derived from pluripotent stem cells (embryonic stem cells or iPSCs) or adult stem cells, and can simulate the architecture and functionality of native organs[29].Organoids are generated from multiple organs including the intestine[30,31], stomach[32], kidney[33],liver[34], pancreas[35], brain[36], and lung[37]and can be used in multiple clinical applications including drug screening, disease modeling, and regenerative medicine.Moreover, the development of human patient-derived organoids may enable personalized medicine.Considering that organoids cultures based on a specific disease and from a specific individual can be expanded in culture maintaining a stable genetic and epigenetic signature[38], they are suitable for biobanking and highthroughput screening.Therefore, organoids represent a powerful tool for drug efficacy and drug toxicity screening.Future analyses may be performed using these biobanks to identify new drugs, but also to know in advance which patients may benefit from an existing drug treatment.In toxicology screenings, 3D organoids could replace the use of cell lines and animal models.Renal and hepatic toxicities are common in drug administration and hepato-biliary organoids generated together with kidney organoids enable the accurate study of drug metabolism and toxicity.Interestingly, 3D organ-on-a-chip is an experimental system in which tissue architecture and cellular composition are assembled on a fabricated synthetic matrix[39].This technology represents a novel approach to overcome the limitations of conventional model systems, especially to model complex disorders affecting several tissues, and for future pharmacological target identification, safety, and efficacy testing as well as personalized medicine.
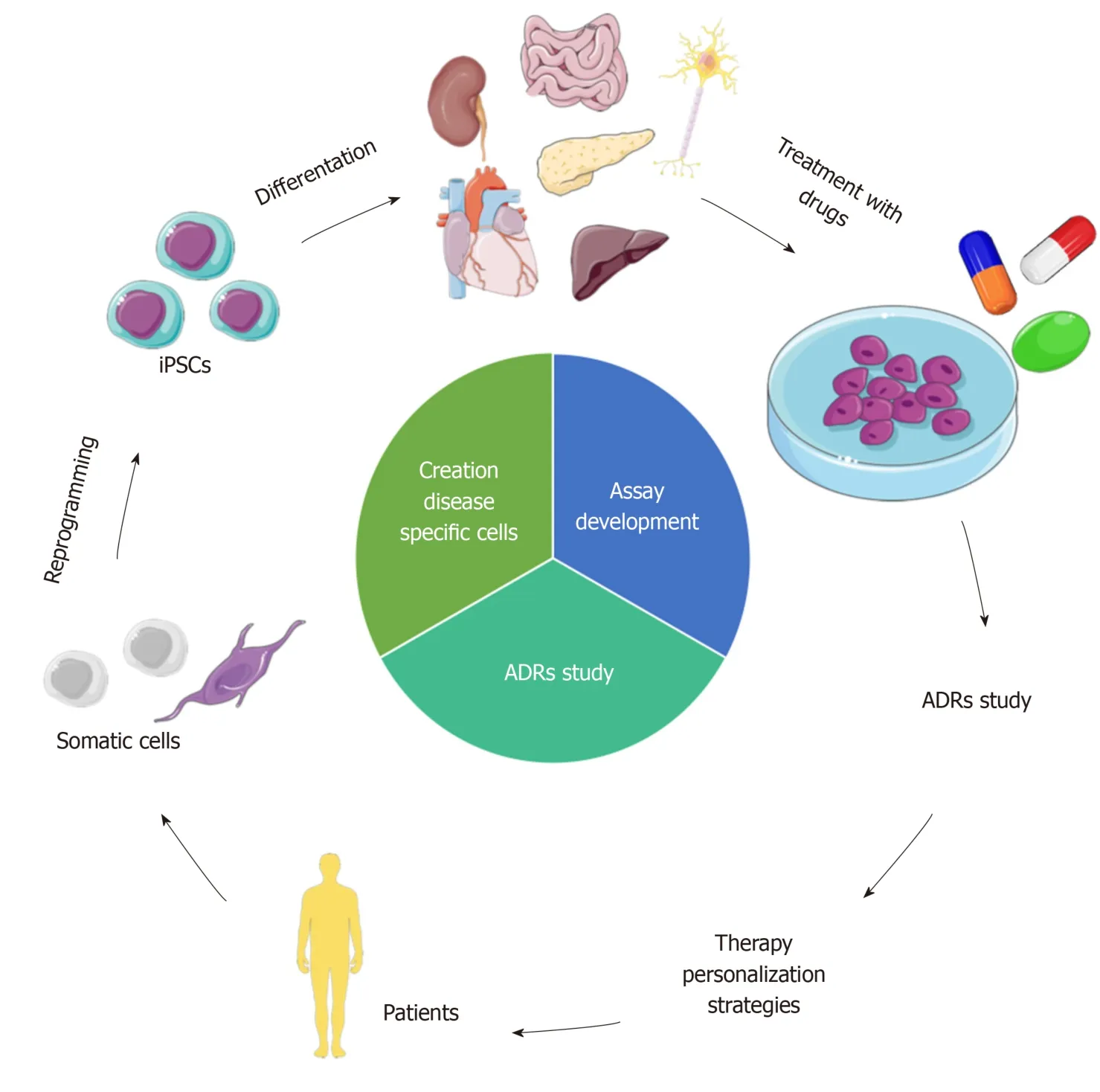
Figure 1 iPSCs to model ADRs for therapy personalization.
GASTROINTESTINAL TOXICITY
Drug-induced gastrointestinal toxicity is very common and may result in different consequences that range from nausea and dyspepsia to life-threatening events such as the development of complicated gastroduodenal ulcers[40,41].To study and prevent these ADRs, researchers have started to create patient-specificin vitromodels using iPSCs.These models are based on the differentiation of iPSCs into enterocytes, using innovative protocols, and involve the creation of groundbreaking models based on iPSC-derived human organoids.
Intestinal ADRs
The small and large intestines are one of the most frequent sites of ADRs.The severity of symptoms can vary from very easy-to-manage to life-threating illnesses.Well known examples of intestinal ADRs are mucosal damage, hemorrhage, colitis,changes in motility, and malabsorption[42]; an important example of frequent gastrointestinal ADR are those caused by nonsteroidal anti-inflammatory drugs(NSAIDs).To manage and prevent the development of intestinal ADRs, scientists tried to model enterocytes, the most abundant intestinal cells with fundamental functions in nutrient/drug absorption and metabolism.In this regard, iPSCs could be a great tool, as demonstrated by some recent works.In 2014, Iwaoet al[43]generated for the first time pharmacokinetically functional enterocytes starting from iPSCs, with the aim of building an intestinal pharmacokinetic evaluation system to better study drug absorption and biotransformation.The protocol developed by this group allowed the authors to obtain mature enterocyte-like cells (ELCs) after about 30 days of stimulation using activin A and fibroblast growth factor (FGF)-2 in order to obtain definitive endoderm cells.Then these immature cells were differentiated into ELCs using epidermal growth factor in 2% fetal bovine serum.ELCs displayed increased expression of the specific intestinal markers sucrase-isomaltase, villin 1, intestinespecific homeobox, CDX2, dipeptidyl peptidase 4, and SLC46A1/proton-coupled folate transporter.The authors also noticed that a longer period of differentiation made the process more efficient in terms of the number of mature ELCs obtained.
In a subsequent study, the same group tried to ameliorate the efficiency of the already established protocol using different small molecules[44].In particular, the authors analyzed the effect of GSK-3 inhibitor XV (a highly potent inhibitor of GSK-3),dorsomorphin (a selective bone morphogenetic protein [ΒMP] inhibitor), PD98059 (a mitogen-activated protein inhibitor), 5-aza-20-deoxycytidine (an inhibitor of DNA methylation), and A-83-01 (a potent and selective transforming growth factor beta[TGF-β] pathway inhibitor).Overall, the differentiated cells expressed intestinal markers, drug transporters and metabolizing enzymes, such as cytochrome P450 1A1/2 (CYP1A1/2), CYP2C9, CYP2C19, CYP2D6, CYP3A4/5, UDP-glucuronosyltransferase (UGT), and sulfotransferase.To analyze the intestinal differentiation of iPSCs after small molecule treatment, the authors considered mRNA expression of sucrase-isomaltase and CYP3A4, which were both markedly increased, while those of intestinal stem cell markers decreased.In particular, CYP3A4 mRNA expression level was mainly induced by the addition of 1α,25-dihydroxyvitamin D3.Intestinal drug transporter peptide transporter 1 was also increased by PD98059, 5-aza-29-deoxycytidine, and A-83-01.With this work, the authors created a model of enterocytes that is definitely more useful than primary cells given their poor viability,short life span, limitation of passage number, and difficulty in obtaining human intestinal tissue samples.Moreover, ELCs seem to be a better model with respect to the widely used Caco-2 cells, since the drug transporter expression levels and carriermediated drug permeability in Caco-2 are different from those of the human duodenum, and the expression level of CYP3A4 is quite low.
A similar work was performed by Ozawaet al[45], who generated an ELC monolayer characterized by drug absorption and metabolism functions.The authors[45]analyzed the barrier formation capacity of the obtained monolayer, measuring the transendothelial electrical resistance (TEER).Βy this test, the authors found that the ELC monolayers have a weaker barrier function than Caco-2 cell monolayers.Importantly, it is known that TEER values in Caco-2 cell monolayers are higher than those in the small intestine.Therefore, the authors concluded that the ELC monolayers might be a more suitablein vitromodel for evaluating the absorption of hydrophilic drugs than Caco-2 cell monolayers.Overall, these studies provide a basis for exploiting ECL models to study drug absorption, metabolism, and ADRs.
Regarding our current knowledge, only a very recent work[46]applied a 2D ELC model to study ADRs.In particular, Matsunaga’s group[46]used ELCs, obtained using the protocol already set up and described above[43,44], to study human drug-induced intestinal mucosal damage.In this work[46], mucin 2 (MUC2) mRNA expression as a marker of mucosal damage was analyzed.MUC2 is the main component of intestinal mucus and its expression in the intestine is decreased by several drugs that induce mucosal damage, such as NSAIDs, and is increased by other protective agents including rebamipide.First, the authors compared MUC2 expression in the human intestine, ELCs, and Caco2 cells to validate the ELC model.The results showed that after 26 days of differentiation, ELCs presented MUC2 levels comparable to those of the human intestine, while levels in the Caco2 line were 100 times lower.Authors also examined the effects of NSAIDs and of the mucosal protective agent rebamipide on MUC2 expression in the ELC model, confirming that NSAID exposure reduces MUC2 levels while rebamipide increases it.Given that the fluctuation of MUC2 seemed to be influenced by different cytokines, they also analyzed the mRNA expression levels of cyclooxygenase-2, interleukin (IL)-1β, nuclear factor (NF)-κΒ, and tumor necrosis factor (TNF)-α after drug treatment.In particular, as reported in other studies[47-51],they found that IL-1β, NF-κΒ, and TNF-α mRNA expression levels were decreased by indomethacin, but increased by rebamipide.Therefore, taken together, the authors concluded that it seems reasonable that these factors may be involved in MUC2 expression changes in enterocytes.
Regarding the advances for obtaining intestinal models used in drug screening,intestinal organoids also represent a valuable tool[52].Self-renewal of the intestinal epithelium is driven by the proliferation of stem cells and their progenitors located in crypt regions[30].Wingless/integrated 3A (WNT3A), R-spondin, and noggin are factors needed in organoid protocols to promotein vitroself-proliferation of intestinal stem cells; moreover, these organoids are composed by different cell types such as enterocytes, Paneth cells, goblet cells, and enteroendocrine cells and manifest many enteric characteristics[53].
Recently, Lu and Rosenbaum[54]described the application of crypt organoid cultures from genetically modified mice as a model to evaluate drug metabolism.Irinotecan metabolism and toxicity were studied using crypt organoids generated from both Ugt1F/F(control) and Ugt1ΔIEC(deletion of the Ugt1 locus) mice.These 3D cultures metabolize the drug to the active topoisomerase inhibitor metabolite SN-38,which is further metabolized by UGT1A1-dependent glucuronidation to form an SN-38 glucuronide.In the absence of Ugt1 gene expression, Ugt1ΔIECcrypt cultures exhibit very limited production of SN-38 glucuronide, concordant with increased apoptosis in comparison with Ugt1F/Fcrypt cultures.Glucuronidation is an important phase II pathway responsible for the metabolism of many drugs used also in the pediatric age,such as opioids or acetaminophen[55].These results demonstrate that intestinal organoid cultures can be employed to study drug metabolism, under conditions of altered pharmacogenetics.
Colonic organoids derived from iPSCs have been used to evaluate the toxicity of rapamycin and geneticin; an impairment of cell proliferation was only observed after treatment with rapamycin indicating that this compound can harm the healthy colon[56].
Hepatic ADRs
Drug-induced liver injury (DILI) is a severe ADR characteristic of more than 1000 drugs[57].The estimation of DILI worldwide is between 1 in 10000 and 1 in 100000 inhabitants, even though some recent studies have reported a higher occurrence[58].In the last several decades, the study and prevention of this ADR have gained increasing interest in the scientific community, given its increasing incidence caused mainly by the number of new drugs on the market[57].Moreover, DILI is the most common cause of drug withdrawal from the market with consequent high costs for industries[59].DILI is divided into acute or chronic conditions including hepatitis and acute liver failure,leading, in the most severe cases, to liver transplant and aggressive treatments[57-60].The mechanism of DILI development can be dose-related or idiosyncratic.
Further efforts are needed to manage and prevent this ADR, given the limitations of currentin vivoandin vitromodels[61,62].iPSCs are a great tool to overcome these limitations and to obtain more predictive results.Indeed, several studies have already shown how to differentiate human iPSCs into hepatocyte-like cells (HLCs) to create a source of cells for different purposes such asin vitrodrug studies.One example is the study performed by Kondoet al[63], where iPSCs were differentiated into HLCs using a three-step protocol, using three growth factors (activin A, hepatocyte growth factor,oncostatin M) and two small molecules (dimethyl sulfoxide, dexamethasone).With this work, the authors established a reproducible and relatively inexpensive method to obtain a greater number of hepatic cells, with respect to other protocols, to perform,for example, pharmacological studies.The differentiated cells expressed the hepatocyte markers hepatocyte nuclear factor 4 alpha (HNF4-α), albumin (ALΒ), and alpha-fetoprotein (AFP) at similar or higher levels in comparison to primary human hepatocytes (PHH) and HepG2 cells.HLCs were also characterized by the expression of drug metabolizing enzymes such as CYP3A4 and UGT1A1.Authors demonstrated that the mRNA expression levels of CYP3A4 and UGT1A1 were increased by the CYP inducers dexamethasone, rifampicin, and omeprazole.However, the expression levels of drug-metabolizing enzymes were very low compared to those in PHH and mature liver.Two years after Kondo’s work, Kanget al[64]confirmed the greater similarity of HLCs and PHH, with respect to HepG2 cells, analyzing acetaminophen hepatotoxic effects on all three cell types.In particular, HLCs were more similar to PHH in comparison to HepG2 cells both in terms of cell viability after acetaminophen exposure and CYP450 levels, with similar downregulation of CYP1A2 and CYP3A4 genes by cytotoxic concentration of both agents.In contrast, HepG2 cells showed an increment in CYP levels.
Takayamaet al[21]investigated whether HLCs, obtained differentiating iPSCs of different donors, could reproduce the interindividual difference in hepatic biotransformation and drug response.In this regard, HLCs were generated from human iPSCs, established by reprogramming donor PHH.iPSCs were generated using a non-integrative method, based on Sendai Virus vectors, a process essential to avoid insertional mutagenesis.After this, they compared the drug metabolism and drug responsiveness of HLCs to those of their parental PHH.The main purpose of this work was to establish a panel of HLCs that represents the diversity of genetic polymorphisms in humans, in order to use these cells to determine the appropriate drug dosage for the single individual.In particular, they focused on CYP activity levels in the HLCs with respect to the parental PHH.Results showed that CYP activity and drug responsiveness of individual HLCs reflected those of parental cells,suggesting that it might be possible to predict individual CYP activity using HLCs and to perform personalized drug treatment analyzing HLCs of the single patient.Moreover, the presence of a single nucleotide polymorphism (SNP) in genes encoding CYP2D6, related to a different metabolism and drug responsiveness, was successfully reproduced in HLCs.Also, Liuet al[65]successfully differentiated iPSCs into HLCs with a relatively simple three-step protocol, using the commercial hepatocyte maturation medium HepatoZYME (Life Technologies, Frederick, MD, United States).These authors reprogrammed peripheral blood mononuclear cells (PΒMCs) instead of PHH, with obvious advantages, given the greater ease of access of blood with respect to liver biopsies.Similarly, Wilsonet al[66]generated iPSCs and then HLCs from a cohort of individuals affected by alpha-1 anti-trypsin deficiency (AATD), a genetic disorder related to liver cirrhosis and pulmonary emphysema and characterized by low levels of AAT, the main protease inhibitor (PI) in human serum.In particular, the most common deficient allele involved in the development of AATD is the PI homozygous for the Z allele (termed PiZZ by authors).The most common disease variant is caused by an inherited single base pair mutation of the serpin family A member 1 gene, which results in a glutamate to lysine substitution and production of a mutant version of the PI AAT, known as Z AAT.Interestingly, the authors found that the global transcriptomes of iPSCs, carrying PiZZ mutations, diverge from that of the healthy controls (three control individuals without any known disease) only after differentiation to HLCs, when the AAT gene is expressed.Moreover, the obtained HLCs successfully model key features of AAT-associated liver disease, including intracellular accumulation and reduced secretion of AAT protein as well as increased autophagic flux.The authors confirmed an increase in autophagic flux upon treatment with the drug carbamazepine as previously described in mice carrying the mutation.Subsequently, authors tested if the PiZZ mutation can increase the toxicity of different drugs with respect to healthy PHH, and exposed both HLCs and controls to acetaminophen and other drugs known to cause hepatotoxicity.In each case, HLCs carrying the PiZZ mutations were more sensitive to drugs with respect to PHH.However, further studies need to be done to evaluate the cytotoxic mechanisms of drugs in HLCs carrying the PiZZ mutations with respect to healthy HLC controls.The authors concluded that these findings support the utility of iPSCs as tools for drug development or prediction of toxicity.More recently, Kvistet al[67]deeply analyzed the critical differences in drug metabolic properties of different human hepatic cellular models including PHH, HLCs, and the hepatoma cell lines HepG2 and HepaRG.Surprisingly, these authors showed that HLCs, obtained differentiating iPSCs, should not be used as a model to study drug metabolism, and thus ADRs, since critical differences were detected with respect to human PHH.This conclusion, in contrast with other works[21,63,64], arises from a different analysis performed by Kvist’s group analyzing the expression and function of key hepatic proteins important for the metabolic fate of drugs such as CYP enzymes.A principal component analysis to study and compare gene expression of HLCs, PHH, and the hepatoma cell lines HepG2 and HepaRG showed a distance between the two iPSC-derived hepatocytes,as well as HepG2 and HepaRG cells, and the three PHH donors and PHH pool, which were clustered more closely together.This finding was confirmed by another analysis,which clustered HepG2 close to HLCs in terms of gene expression of 91 genes related to the liver function or CYP450.Moreover, HLCs were found to have low activity of several CYPs such as CYP3A and CYP2C9, barely detectable activity of CYP1A2, 2Β6,2C8, 2C9, 2C19, 2D6, and a high expression of several extrahepatic P450s such as CYP1A1 and 1Β1 that may have significant effects on biotransformation profiles.On the other hand, HepaRG cells showed a CYP profile very similar to PHH, suggesting that this cell line can be a good model in drug metabolism studies and ADRs.The authors concluded that, to date, HLCs derived from patients’ iPSCs should not be used as a substitute for PHH in drug toxicity studies.To improve the performance of the HLC model, the authors suggested to culture cells in a 3D rather than the current 2D monolayer, because the 3D model has been shown to improve the performance of PHH[68].In 2018, Smutnýet al[69]used HLCs to study the toxicity of phytochemicals saikosaponin D, triptolide, deoxycalyciphylline Β and monocrotaline known to cause DILI, in comparison with hepatoblastoma-derived HepG2 cells and long-term culture of primary human hepatocytes (LTHHs).In order to compare the cytotoxic effects of the tested phytochemicals, the authors analyzed hepatocyte key markers in the HLCs compared to the HepG2 and LTHHs controls.First, they analyzed ALΒ level, a specific protein produced only by hepatocytes and hepatoblasts by immunofluorescence staining and real time PCR.Βoth HLC and HepG2 cells exhibited intense staining of ALΒ.The mRNA level of ALΒ in the HLC cells was similar to that of HepG2 cells but was lower than that in reference LTHHs of the two donors.Then, transcription of HNF4α, a liver-enriched transcription factor associated with the regulation of many liver-specific genes, was confirmed in the HLC model, as well as the expression of CYP3A4, which however was lower with respect to LTHHs.Interestingly, HepG2 cells were negative for the important CYP3A4 drugmetabolizing enzymes.Additionally, the authors analyzed the maturation of HLCs studying three markers:AFP, a typical liver marker expressed in hepatoblasts and fetal hepatocytes, but not in adult hepatocytes; cytokeratin 19 (CK19), a marker of cholangiocytes and hepatic progenitors; and CYP3A7, a CYP450 enzyme expressed mainly in fetal hepatocytes and at a very low level in adult hepatocytes.Overall, the results showed that HLCs resemble to be closer to an immature hepatic phenotype expressing both AFP and CK19 markers.After characterizing HLCs, they analyzed the potential of HLCs, HepG2, and LTHHs to predict DILI using hepatotoxic compounds.Overall, HLCs appeared more sensitive to triptolide and saikosaponin D in comparison to both HepG2 cells and LTHHs.Interestingly, the authors noticed an atypical response of HepG2 cells with less toxicity at higher concentrations of triptolide.This atypical effect could be related to particular resistance mechanisms characteristic of the HepG2 line, such as induction of metabolizing enzymes and/or efflux transporters induced by high doses of this phytochemical.Also, saikosaponin D treatment produced higher cytotoxic effects in HLCs, although LTHHs also showed high sensitivity.However, the HepG2 cells resulted less sensitive to saikosaponin D with effects observed at the highest concentration tested.Regarding monocrotaline,no cytotoxic effect was reported in all lines tested.The authors commented that this observation may be related to targeting by monocrotaline principally of hepatic sinusoidal endothelial cells.However, it would be interesting to further explore the causes of this resistance.Finally, they analyzed deoxycalyciphylline Β effects, finding a mild decrease in mitochondrial activity at the maximal tested concentration only in LTHHs cells, while neither HLCs nor HepG2 exhibited any toxic effect.The authors concluded that this study provides a basis for further in-depth studies to confirm HLCs as a competentin vitroliver cell model for toxicological assessment; however,further efforts are needed to develop HLCs with a more mature phenotype,expressing typical adult hepatocyte markers such as CYP3A4, HNF4α, and ALΒ despite the expression of immature markers typical of fetal hepatocytes and hepatic progenitors such as AFP and CK19.Another recent and interesting work was performed by Yamazaki and Murayama[70], which analyzed CYP450 expression levels of commercial HLCs at different culture times.Authors found a significant increase of CYP450 activities after 3-4 weeks with respect to HLCs cultured for 1 week.After 4 weeks, HLCs reached CYP450 levels similar to those in HepaRG cells.The increase in activity was associated with increasing CYP450 2C9 and 2C19 mRNA levels.This finding can help researchers perform more precise and repeatable studies on HLCs and drugs and is in contrast with the manufacturer’s instructions that suggest the use HLCs after 1 week of culture.
In this context, the recent development of liver organoid culture systems derived from iPSCs provides another promising strategy to study drug-induced hepatotoxicity[71,72].Liver organoids closely resemblein vivohuman liver, preserving their genetic and epigenetic integrity over months in culture[73].
The most recently published method to generate functional hepatobiliary organoids from iPSCs cultured on Matrigel was developed by Wuet al[74].The protocol is based on inclusion at differentiation stages I and II (days 1-15) of 25% mTeSR culture medium into hepatic differentiation medium to induce endodermal and mesodermal commitment; subsequently, at stage III (days 15-45), 10% cholesterol and other small molecules (a Chinese patent pending product called cholesterol+MIX) were added to the maturation medium to promote the formation and maturation of hepatobiliary organoids by activating the NOTCH2 and TGF-β signaling pathways.Concerning drug metabolic functions, the expression of several P450 enzymes was measured:the organoids displayed significantly higher levels of CYP3A4 and CYP2E1 than fetal liver, with comparable expression of CYP2A6, CYP2Β6 and CYP2D6.On the contrary,the expression of most P450 enzymes (except for CYP3A4) in liver organoids was significantly lower than that in the adult liver, indicating that these organoids present intermediate maturity between the fetal and adult liver.
Liver organoid culture systems allow hepatotoxicity testing.Recently, Leiteet al[75]established a method to detect hepatocyte-mediated and drug-induced liver fibrosis based on this platform.After a single dose or repeated exposure for 14 days to the pro-fibrotic compounds allyl alcohol and methotrexate, hepatic organoids displayed fibrotic features such as activation of hepatic stellate cells (HSCs), the major collagenproducing cells during conditions of sustained hepatic injury.The tested drugs caused significant upregulation of HSC activation-associated mRNAs collagen, type I,alpha 1, collagen, type I, alpha 3, and lysyl oxidase homolog 2 in the organoids.Acetaminophen is another compound identified by these organoids as an inducer of hepatotoxic-mediated HSC activation, which was also confirmed in anin vivomodel.
Pancreatic ADRs
Drug induced-pancreatitis is a serious problem for both the patient and the health system.About 0.1%-2% of drugs are related to the development of this ADR and cases can be mainly divided into mild and severe.Severe cases may lead to death, while mild ones lead to patient hospitalization.Recently, our group reviewed the possible application of iPSCs to study and prevent this ADR[18,76].Here, we provide an up-todate revision of iPSC technology to model patient-specific human pancreatic cells with the purpose to obtain a platform for drug-induced pancreatitis studies.iPSCs can be differentiated into both endocrine and exocrine pancreatic cells applying protocols that provide the addition of specific stimuli to the culture medium.Regarding endocrine differentiation, many protocols are available; however, the main current limitation is the complete maturation of differentiated cells due to its lower hormone release profiles with respect to human islets, thereby resembling more immature fetal endocrine cells.However, to overcome this limitation, some progress has been made in the last several years.The work published by Pagliucaet al[77]is an example of the progress made in this field.IPSCs were differentiated into hormone-secreting cells using a six-step protocol and cells were cultured in clusters into spinner flasks.Cells obtained were implanted in mice, resulting, after 3-4 months, in functional β-cells and polyhormonal cells, a particular type of early endocrine cells that appears during pancreas development, able to secrete insulin, glucagon, somatostatin, and pancreatic polypeptide and localized in the walls of pancreatic ducts.These cells can be a great tool for diabetes studies and identification of new therapeutic approaches.
To the best of our knowledge, protocols regarding exocrine differentiation are limited.The most efficient, already described in our previous works[14,53], was developed by Takizawa-Shirasawaet al[78].
Even if the progress made to obtain patient-specific pancreatic cells is increasing,the experience on the application of differentiated cells to create a platform for druginduced pancreatitis studies is still limited and only two works are available.Indeed,to date, only Hohwieleret al[79]developed a model of 3D pancreatic organoids generated from iPSCs of patients with cystic fibrosis.Their work, already reviewed by our group[18], has provided a basis for the development of new research based on this topic.Also, Huanget al[80]developed an efficient protocol to obtain exocrine pancreatic organoids starting from human embryonic stem cells.On the other hand, even if works based on iPSCs and the generation of exocrine pancreatic cells are limited,there are several solid works concerning pancreatic organoids created from tumor cells.Therefore, considering these two facts, it is reasonable to believe that more progress can be made in this field to fill the existing lack of pancreatic ADR studies.
NEPHROTOXICITY
Drug-induced renal injury (DIRI) is a frequent side effect, especially in critical patients undergoing complex pharmacological treatments.DIRI is common in the pediatric field with an incidence of about 25% pediatric patients taking intensive pharmacotherapy[81,82].This high incidence is principally due to the excretory function of the kidney, which is exposed to high concentration of drugs or metabolites.DIRI can lead to severe acute renal failure, which contributes to prolonged hospitalization,and increased costs for healthcare and morbidity.To prevent devastating consequences for patients, it is important to identify markers of this adverse event,taking measures to avoid it.To date, the main clinical indicators of DIRI are serum creatinine levels; however, novel markers are needed to more efficiently prevent its development[83].It is known that this adverse effect can be caused by drugs through different mechanisms that are divided on the basis of the affected kidney component[84].A major problem of DIRI management is the lack ofin vitromodels to test nephrotoxicity of drugs or to find predictive biomarkers of renal drug toxicity[85,86].iPSCs from patients represent a promising model to develop more precise therapies,better studying the mechanisms related to drug-induced nephrotoxicity and creating the possibility to prevent DIRI development.
The differentiation of iPSC to renal cells involves different steps:starting from mesendoderm formation, the intermediate mesoderm can be obtained, from which it is possible to obtain the ureteric bud or metanephric mesenchyme.From the latter, the differentiation continues with the renal vesicle, from which the podocyte, proximal tubule, or distal tubule can be obtained[87].Taguchiet al[88]analyzed a differentiation protocol allowing metanephric nephron progenitors starting from iPSC to be obtained in 14 days.The protocol involved different steps of differentiation:starting from the formation of embryoid bodies (EΒs), epiblast, nascent mesoderm, posterior nascent mesoderm, posterior intermediate mesoderm, and finally metanephric mesenchyme.The different steps were obtained with appropriate concentrations and exposure times to stimuli added in the medium:activin-A, and bone morphogenetic protein 4(ΒMP-4), CHIR99021, retinoic acid, fibroblast growth factor 2 (FGF-2), FGF-9.Immunohistochemical analysis confirmed the differentiation[89].Xiaet al[89]derived ureteric bud progenitor-like cells from iPSC in 4 d.The culture medium was supplemented with ΒMP-4 and FGF-2 for 2 d and then with retinoic acid, activin-A,and ΒMP-2.The differentiation was evaluated using real-time PCR and immunostaining[89].Musahet al[90]developed a differentiation method of iPSC in kidney glomerular podocytes with a feeder-free and serum-free protocol in 21 days.The differentiation procedure was established in three commercial iPSC lines:PGP1,IISH3i-CΒ6, and IMR-90-1.During differentiation, the cells were cultured on tissue plates coated with lamin-511 E8 fragment and the mesoderm was obtained adding Rho-associated kinase inhibitor Y27632, CHIR99021, and activin-A; the intermediate mesoderm was obtained with CHIR99021 and ΒMP-7; and finally podocytes were obtained by stimulating the cells with ΒMP-7, retinoic acid, activin-A, vascular endothelial growth factor (VEGF), and CHIR99021.The differentiation markers analyzed were goosecoid, HAND1, and brachyury in the case of the mesoderm; Pax2 for the nephron progenitor cell markers; WT1 and OSR1 for intermediate mesoderm;and finally, for podocytes WT1, podocin, and nephrin proteins and specific genes such asMAF, PODXL, SYNPO, andEFNB2, together with a decrease of progenitor marker genes (e.g.,SALL1andPAX2) and pluripotency genes (such asSOX2, MYC,NANOG, POU5F1)[90,91].The authors also created an organ-on-a-chip microfluidic model of glomerular function.Organ-on-a-chip culture models can better reproduce the structure, function, and environment of human organs.The chip was formed by two parallel micro-channels separated by a poly(dimethylsiloxane) membrane:the intermediate mesoderm, subsequently differentiated into podocytes was cultured in the upper part of the channel and in the opposite part the primary human glomerular microvascular endothelial cells were seeded.In this way, it was possible to recreate the podocyte-endothelium interface.Furthermore, two hollow chambers were added on the sides of the central channels and a cyclic suction was applied to mimic the cyclical pulses of the renal blood flow that cause relaxation or motion and dynamic mechanical stretchingin vitro.The podocytes were obtained by differentiation in the presence or absence of fluid flow or with a combination of fluid flow and mechanical strain; nephrin expression analyses indicated that differentiation may be influenced by mechanical forces.Further analysis showed that with cultures in the presence of flow or the flow-mechanical combination, there was an increase in the number of processes of the podocytes and greater production of VEGF-A (necessary for development of the glomerulusin vivo).They analyzed the percentages of retained ALΒ and inulin filtration in the presence of cyclin mechanical strain and noted that 99% of ALΒ is retained while 5% of inulin is filtered, suggesting that this represents a goodin vitromodel for glomerular filtration barrier.They also analyzed the production of collagen IV (mainly produced by glomerular podocytes in the mature glomerular basement membrane) and noted that this type of collagen is produced by both cell types present in the chip, even though the greatest production occurs in differentiated podocytes under mechanical strain, demonstrating the greater differentiation efficiency in the presence of mechanical strain.Finally, they analyzed the damage induced by a continuous flow of the adriamycin anti-tumor drug on this organ-on-a-chip.The results revealed an interruption of the podocyte layer and cell detachment in a dose-dependent manner, together with decreased viability and nonselective loss of ALΒ from vascular channel.This indicated that the tested drug produced lesions and that this model is useful for analyzing glomerular function,therapeutic development, and drug-induced toxicity[91].
Kandasamyet al[92]reported an example of proximal tubular-like cell differentiation from iPSCs used to predict drug-induced nephrotoxicity.They used commercial iPS(foreskin)-4, from WiCell Research Institute and differentiated these cells in human proximal tubular-like cells (HPTC-like) in 8 days, using commercial renal epithelial growth medium supplemented with ΒMP-2 and ΒMP-7.They analyzed the expression of markers to evaluate the different steps of differentiation in HPTC-like cells.In summary, the expression of iPSC characteristic genes such asSOX2, NANOG,DNMT3D, andOCT3/4decreased starting from day 1 of differentiation, while the characteristic genes of proximal tubular cells (AQP1, GGT, andKSP-CAD) are expressed in HPTC-like cells.The authors also analyzed the expression of 31 different genes in HPTC-like cells, including genes coding for transporters, epithelial markers and kidney injury markers.Then they analyzed the nephrotoxic effect of two compounds, rifampicin and citrinin, in terms of IL-6 and IL-8 expression and noted an increase in the two ILs after drug treatment.They used the IL-6/IL-8-based assay to test 30 compounds that included substances for which a nephrotoxic effect was known and substances that did not produce such toxicity and treated HPTC-like cells derived from iPSC differentiation and a commercial line of HPTC (American Type Culture Collection, Manassas, VA, United States).To classify the compounds as toxic and non-toxic, an automated classifier was used and the system was trained to recognize the two types of compounds.Finally, they tested how predictive the developed system was and concluded that cells differentiated from iPSCs have higher test accuracy than HPTC.Therefore, HPTC-like cells can be used to predict toxicity using this automatic system.In addition, performance predictions were also analyzed using HPTC derived from nephrectomy samples from two tumor patients and an increased variability in performance was observed.The authors concluded that the use of HPTC-like cells differentiated from iPSCs in this prediction system can avoid inter-donor variability problems and thus could be useful to predict nephrotoxicity of drugs.Finally, authors tested the anti-cancer drug cisplatin using different biomarkers.The treatment produced HPTC-like DNA double-strand breaks and reactive oxygen species (ROS) production.The authors reported that the results obtained are in line with clinical data and animal experiments, and concluded that iPSC-differentiated HPTC-like cells are an effective model for thein vitrostudy of cisplatin-induced toxicity[92].
Although the number of studies on drug-induced nephrotoxicity based on iPSCs is currently limited, such a model represents a good tool to investigate the pathophysiology of many renal diseases and may allow the investigation of more effective therapies.In fact, for many kidney diseases, specificin vitromodels are not yet available.The use of pluripotent stem cells (including iPSC and embryonic stem cells) have allowed the study of renal diseases due to genetic mutations such as renal cysts, diabetes syndrome, Wolfram syndrome, focal segmental glomerulosclerosis,systemic lupus erythematosus, Wilms tumor, and Alport syndrome[86].
With respect to iPSC-derived proximal tubular cells, 3D kidney organoids are characterized by distinct cell types such as endothelial cells, nephron progenitors, and podocyte-like cells and are therefore promising systems for nephrotoxicity testing[93].
Recent data have shown that treatment of kidney organoids generated from human iPSCs (CRL1502) with nephrotoxic cisplatin induced specific acute apoptosis in mature proximal tubular cells, whereas immature cells did not respond to the drug[93].These results were further confirmed on patient-derived organoids obtained from renal normal tissue of neoplastic patients:a consistent activation of caspase 3(CASP3), an indicator of apoptosis, after 72h of incubation with cisplatin was detected by different techniques and only tubule cells suffered after drug exposure without affecting organoid architecture[94].
Furthermore, interesting results have been obtained when organoids derived from cultured murine nephron progenitor cells were treated for 24 h with gentamicin at different concentrations; the percentage of CASP3+ cells co-stained withLotus tetragonolobuslectin, a proximal tubule marker, selectively increased up to 80%.In contrast, immunofluorescence analyses of cells stained for podocalyxin, a glomerulus marker, treated with gentamicin showed no cells positive for CASP3, suggesting that the drug caused proximal tubule injury without affecting glomerular structures[95].
The most recently published paper about kidney organoids described the use of glomeruli isolated from iPSC-derived kidney organoids for toxicity screening[96].The method for isolation of intact glomeruli from kidney organoids is based on enzymatic dissociation of mature organoids that generates 3D aggregates of podocytes representing forming glomeruli.In particular, the cultured organoid glomeruli were exposed to increasing concentrations of doxorubicin, and after 48 hours, activation of the pro-apoptotic pathway was evident at the lower doses; reduction in glomerular size following doxorubicin treatment was also detectable.
These preliminary results are encouraging; however, more drugs must be evaluated before kidney organoids can be used as a promising drug testing platform.
CARDIOTOXICITY
Drug-induced cardiotoxicity may be triggered by several mechanisms of action.In general, drugs can cause different effects at the cardiac level:heart failure due to abrupt decrease of contractile performance, decrease in left ventricular ejection fraction, arrhythmias, and prolonged cardiac repolarization.The latter, associated with a prolonged QT interval, may increase the risk of serious cardiac arrhythmias.In most cases, the prolonged repolarization phase is caused by drug effects at the ionic channels or pump levels[97].In general, variations in the electrical currents that stimulate the contractions of cardiomyocytes (CMs) are due to alterations in the fluxes of ions such as Ca2+, K+, and Na+through ionic channels in cardiac cells[98].An example of a potentially lethal arrhythmia is the Torsade de Pointes, often determined by the prolongation of the action potential that affects rapid K+current by inhibition of the ether-a-go-go-related gene channel (hERG) in cardiac muscle and is related to prolongation of the QT interval[99].Furthermore, alterations of the ions flux not only have consequences on CM contraction, but suggest oxidative stress condition, a common mechanism of toxicity that may lead to apoptosis[100].
One of the major problems during new drug development in preclinical trials is represented by the potential cardiotoxicity of the therapeutic agent[9].Indeed, during the registration phase of a new drug, 23% of candidates fail for this reason[101].In this view, prolongation of the QT interval has been suggested as the major problem related to the potential cardiotoxicity of drugs by the U.S.Food and Drug Administration (FDA)[102].Hence, in 2005, the International Conference On Harmonisation Of Technical Requirements For Registration Of Pharmaceuticals For Human Use created guidelines for cardiotoxicity screening in preclinical[103]and clinical studies[104].
Cardiotoxicity is not only a frequent cause of failure of preclinical studies for new drugs, but can also be the cause of drug withdrawal from the pharmaceutical market.Βetween 1994 and 2006, 45% of all drugs removal from the market were due to cardiotoxicity[105].For instance, some of the most recent withdrawals from the market of drugs due to cardiotoxic effects involve sibutramine and rosiglitazone.The anorectic sibutramine was recalled in 2010 because of increased risk of nonfatal myocardial infarction and nonfatal stroke in patient with pre-existing cardiovascular disease[106].The anti-diabetic rosiglitazone was also recalled in 2010.Although an increase in mortality risk has not been identified, this drug was associated with an increased risk of myocardial infarction and heart failure[107].
Also in this case, a promising approach is the use of iPSCs.iPSCs allow patientspecific studies, since these cells can be easily differentiated into CMs (iPSC-CMs).In addition, iPSC-CMs have the advantage of being cryopreserved to facilitate their use as well as being grown in large numbers and high purity[108].Di Βaldassarreet al[109]reported three main approaches for the generation of iPSC-CMs:differentiation of the monolayer culture system for which various protocols have been described, co-culture of iPSCs with visceral-endoderm-like cells, and formation of embryonic bodies (3D culture)[109].In general, the protocols provide, as a first step, differentiation towards the mesoderm layer by activation of WNT, activin/NODAL, and/or ΒMP pathways.For example, the combination of CHIR99021 with differentiation factors such as activin-A and ΒMP4 are used to activate the WNT pathway[97].The activation set of these pathways, together with the Matrigel sandwich method, has been proven effective[110].However, the obtained iPSC-CMs are qualitatively and quantitatively immature compared to adult CMs.This can significantly impact the validity ofin vitrostudies using these models, in terms of modeling the disease and/or prediction of the drug effects[110].The differences between iPSC-CMs and adult CMs are related to:structural gene expression[111,112], structural features[113], metabolism[112], and contractile function[113].Very recently, Machiraju and Greenway reported successful methods for the maturation of iPSC-CMs, including:biochemical approaches (manipulation of growth conditions through the addition of small molecules or changes in culture medium), environmental manipulation (through various mechanical and electrical forces), and 3D approaches (creation of 3D cultures of CMs, called organoids).According to the authors, the optimal conditions of maturation can be achieved by combining these different approaches[110].
A good example of the use of iPSC derived from patients to perform patientspecific studies to evaluate drug toxicity was reported by Lianget al[114].In this study,iPSC-CMs were obtained from healthy donors and patients with inherited long QT syndrome (LQT), familial hypertrophic cardiomyopathy (HCM) or familial dilated cardiomyopathy.The authors initially characterized iPSC-CMs by immunofluorescence staining (noting that there were phenotypic differences in patients’ iPSCCMs compared to healthy iPSC-CMs due to the associated pathology) and ion channel expression (noting that they are present in all types of cells and therefore potentially able to modulate the electro-physiological responses to drugs).Then, they analyzed divergent aspects such as the morphology of action potentials and the action potential duration in nodal, atrial, and ventricular waveforms in all iPSC-CMs.On these cell models, the authors showed that the iPSC-CMs of patients affected by LQT and HCM treated with cisapride (a gastroprokinetic agent capable of blocking hERG channels)showed a higher susceptibility to arrhythmias, suggesting that the greater sensitivity to cardiotoxicity induced by this drug could be associated with LQT or HCM mutations.Thus, it is conceivable that iPSC-CMs can be used as good models for evaluation of the patient-specific cardiotoxicity of drugs[114].
On iPSC-CMs, different physiological parameters can be monitored as a measure of possible toxic outcomes induced by drugs at the cardiac level.For instance,continuous real-time monitoring of the beating frequency of CMs is possible thanks to the xCELLigence Real Time Cell Analysis Cardio Instrument, a technique useful for the dynamic monitoring of CM contraction and beating, measured by an electric field differentially modulated by the number of cells covering the electrodes, their morphology and the strength of cell attachment.Nguemoet al[115]showed that this technique is a useful tool for characterization of the potential cardiotoxicity of drugs.The most recent study using this tool to analyze drug-induced cardiotoxicity on iPSCCMs was carried out evaluating the cardiotoxicity of etoposide (ETP), a broadspectrum anti-neoplastic drug.The iPSC-CMs were treated with ETP for 48 hours followed by 2 days of drug washing.An irreversible increase in the beating rate of iPSC-CMs was observed with 30 and 15 μmol of ETP with alterations in the beating profile and arrhythmic beating (measured with XCELLigence).Furthermore,treatment with 10 μmol ETP resulted in initial changes of the beating profile, that however, returned to baseline level after drug washing.A dose-dependent increase in the extracellular level of lactate dehydrogenase after treatment with ETP was observed, indicating membrane damage in iPSC-CMs.The authors also performed gene expression analyses (deregulation of 58 genes and upregulation of 5 miRNAs were found), intracellular calcium handling and mitochondrial membrane potential analyses, immunostaining, and transmission electron microscopy (to confirm the cytoskeletal and mitochondrial damage).Finally, they showed that the apoptosis inhibitor, pifithrin-α, could protect iPSC-CMs from ETP-induced cardiotoxicity[116].
Another useful technique is represented by microelectrode array (MEA), a method that allows measurement of the electric field potential (homogeneous and electrically coupled populations) of cardiac cells[117].The most recent study using this tool to analyze drug-induced cardiotoxicity was carried out on a commercially available iPSC-CM cell line (iCell CMs, Cellular Dynamics International, Madison, WI, United States) testing 25 drugs, with or without serum in the medium, using a MEA system to analyze the field potential duration (FPD) and arrhythmic events.In the case of serum-free medium, only some drugs induced significant changes in FPD, nine with an extension and four with a reduction.In the case of medium with serum, on the other hand, the number of drugs with prolonged FPD was 11.The authors concluded that the presence or absence of serum can affect the results; indeed, components present in the serum such as serum ALΒ can create optical artifacts during this electrophysiology assays, and therefore, this should be considered during the analysis[118].
Cardiotoxicity is a relevant problem for anti-tumor drugs[101].For example,anthracyclines, especially doxorubicin and its derivative epirubicin, are widely used as anti-cancer drugs in hematological malignancies (e.g., lymphoblastic or aggressive myeloblastic leukemia) and solid tumors (such as breast, endometrial and stomach tumors).The cardiotoxicity induced by doxorubicin can cause congestive heart failure, tachycardia and arrhythmias, asymptomatic reduction of left ventricle ejection fraction, cardiomyopathy, and myocardial infarction[119].The cardiotoxicity induced by this drug acts on several levels:activation of the apoptosis pathway by alteration of mitochondrial functions, generation of ROS, and alteration of gene transcription due to inactivation of topoisomerase II and double-strand breaks[120].One of the most recent studies of cardiotoxicity induced by doxorubicin on patient-specific iPSC-CMs was reported by Βurridgeet al[119].The study was carried out in iPSC-CMs derived by three groups of female patients:healthy controls, breast cancer patients treated with doxorubicin or equivalent who did not experience clinical cardiotoxicity (DOX), and breast cancer patients treated with doxorubicin or equivalent who did experience clinical cardiotoxicity (DOXTOX).Once derived from each of these patients, iPSCs were differentiated into iPSC-CMs that were subsequently exposed to doxorubicin to evaluate the drug-induced cardiotoxicity.iPSC-CMs have been tested for:toxicity of doxorubicin by immunofluorescent imaging to assess the concentration of the sarcomeric disarray, detecting a concentration-dependent increase in DOXTOX cells,but not in DOX cells at 0.1 μmol, as well as an increase in beating rates, more severe in DOXTOX cells; (2) cell viability that was reduced in DOXTOX cells; (3) level of double-stranded DNA damage (by staining for phosphorylated H2A histone family member X), which was higher in DOXTOX patients; and (4) oxidative stress,demonstrating that ROS and H2O2production was higher while antioxidant glutathione levels decreased in the DOXTOX patients.Moreover, the effect of doxorubicin on patient-specific gene expression was evaluated by RNA sequencing analysis.An association between homozygous non-synonymous variants in ΒRCA1 and cardiotoxicity in DOXTOX patients was found.Βecause of these results, the authors concluded that the study of drug-induced cardiotoxicity using iPSC-CMs allows the evaluation of its molecular mechanisms and genetic bases[119].
Another example of iPSC-CMs used to study the cardiotoxic effects induced by anti-cancer drugs was reported by Sharmaet al[121], who investigated the tyrosine kinase inhibitor (TKI)-induced cardiotoxicity.TKIs are anti-cancer agents that act on the tyrosine kinase receptor by inhibiting its phosphorylation.TKIs act in terms of proliferation, migration and survival of the cells.However, reduced left ventricular ejection fraction, heart failure, myocardial infarction, or arrhythmias are common cardiac adverse effects caused by TKI treatment.In this study, patient-specific iPSCCMs were obtained from the somatic tissues of 11 healthy individuals and 2 cancer patients (patients with kidney cancer and treatment with sunitinib as first-line and axitinib as second-line without significant clinical cardiotoxicity) to evaluate the cardiotoxicity of 21 FDA-approved TKIs.Different endpoints were evaluated such as cytotoxicity, contractility, and the effects on QT intervals.The authors noted the cytotoxic effects of some TKIs (sorafenib, regorafenib, and ponatinib induced the greatest effects) in iPSC-CMs obtained by healthy patients and there were no significant differences when compared to iPSC-CMs obtained by patients treated with sunitinib or axitinib.Regarding the effects on cell contractility, healthy iPSC-CMs were exposed to doses lower than the drugs’ median lethal dose, and nilotinib and vandetanib altered the beating rate.The authors concluded that before the death of CMs, they manifested an effect on the beat profile.Finally, regarding the effects on QT interval, prolongation of the contraction time of CMs was observed in healthy iPSCCMs treated with nilotinib or vandetanib, in addition to a decrease in the beating rate and prolongation of the transitional duration of calcium.In this article, it was also hypothesized that insulin/IGF signaling (that was upregulated after treatment with VEGFR2/PDGFR-inhibiting TKIs) may protect iPSC-CMs from TKI toxicity.The authors concluded that iPSC-CMs can be used to evaluate TKI-induced cardiotoxicity[121].
The response to some drugs is different between adults and children and/or during childhood; for example, in the case of warfarin, in pediatric patient age has a more important effect on pharmacokinetics than polymorphisms (for example inVKORC1orCYP2C9genes)[122].Hence, of crucial importance is the establishment of patientspecific studies to evaluate the cardiotoxic potential of drugs in pediatric patients.Also in this case, patient-specific iPSCs are a useful tool.For example, Visscheret al[123]reported the relationship between the SNP in a panel of genes and anthracyclineinduced cardiotoxicity (ACT) in children (independent cohort of 218 patients who included patients treated with anthracyclines who had or not developed cardiotoxicity).Genomic DNA was extracted from blood, saliva, or buccal swabs and 23 SNPs were selected for which an association with ACT was already known.The results showed an association between rs17863783 inUGT1A6and ACT, while the association with two SNPs inSLC28A3(rs7853758 and rs885004) and one inSULT2B1(rs10426377) was close to being significant.They also analyzed the influence of sex and age at the start of treatment:The variantSULT2B1rs10426377 was associated with an increased risk of ACT only in males, while the two variants ofABCB4,rs4148808, and rs1149222 were only associated with an increased risk in females.Regarding age, an association with ACT was found in the case of theHNMTvariant rs17583889 in the case of younger children (< 5.3 years)[123].This study highlights the ability of a specific patient study to improve the risk assessment of ACT in children,potentially helping to improve the safety of anti-cancer therapy.
With respect to the traditional 2D cell culture, 3D-engineered human cardiac organoids generated from stem cell-derived CMs provide another functionalin vitromodel for disease modeling and drug screening.Βiochemical-inducing factors can be combined with several tissue engineering approaches based on 3D printing and bioscaffold technologies to direct spatial organization of 3D tissue to build human cardiac organoids[124,125].Despite the progress in this area, protocols for the maturation of CMs toward an adult phenotype in defined conditions still need to be further elucidated.
The study published by Vogeset al[126]demonstrated that human cardiac organoids primarily contain CMs and stromal cells at a ratio that is comparable with the fetal/neonatal heart, and form functional sarcomere units.Moreover, these cellular models are able to completely recover cardiac function following injury, showing many features of regenerative neonatal heart tissue.
Fluid-ejecting 3D human ventricular-like cardiac organoid chambers (hvCOC) can mimic physiologically complex behaviors, such as pressure-volume relationships, and have been used for detecting contractile responses to different pharmacological compounds[127,128].The hvCOC system can accurately identify inotropic effects of pharmacological compounds such as isoproterenol and levosimendan, with increased sensitivity with respect to human ventricular-like cardiac tissues strips.
The utility of these structures has been additionally demonstrated for environmental contaminants screening, for example heavy metal and pesticide,confirming the accurate responses to external stimuli[129].
These preliminary results demonstrate that in the future, these platforms could provide patient-specific models for personalized drug screening to achieve optimal therapeutic applications.
NEUROTOXICITY
Neurotoxicity can be caused by physical, chemical, or biological agents exhibiting adverse effects at the central or peripheral nervous system level, altering its function or structure.Neurotoxicity can affect attention, executive functions, decision making,and memory, based on the severity and location of the injury, compromising the quality of life.Βrain functions can be altered by drug activation of different neurotransmitter systems, including dopamine and glutamate[130].
Drug-induced neurotoxicity is divided, according to the damaged region, in:myelinopathy, induced by drugs such as amiodarone, which causes damage to Schwann cells[131]; assonopathy, induced by drugs such as vinca alkaloids (i.e.vincristine and paclitaxel), inducing microtubule-dependent axons damage[132]; and neuronopathy, induced by platinum-based compounds (cisplatin and oxaliplatin) due to oxidative or mitochondrial stress resulting in death of the dorsal root of ganglion neurons[132].
An example of ADR is represented by seizure, a serious neurological complication that is commonly associated with treatment with antibiotics[133].Seizures involve an abnormal and transient discharge of neurons at the brain level.To test drug-induced seizure-liability many models are currently available, for example acute slide assay carried out on surgical slides obtained from any part of the brain (especially hippocampus), organotypic slide cultures, primary central nervous system cultures,iPSC-derived cultures.The latter are useful to study drug-induced neurotoxicity using differentin vitrotechniques, such as calcium imaging and MEA.This method allows the measurement of the electrophysiological activities of the neural networks in a noninvasive way[134].For instance, Odawaraet al[135]used this tool to evaluate the efficiency of response to two convulsant agents (pentilentetrazole, a GAΒA blocker, and 4-aminopryridine, a K+-channel blocker) in anin vitrosystem constituted by co-cultures of commercial neurons and astrocytes derived from iPSCs.The results obtained indicated that the synchronized bursts firings, indicative of functional maturation in synaptic transmission, and the analysis of the peaks in the synchronized bursts firings allows the neurotoxic effects of these two drugs to be distinguished.Moreover, the coculture improves the spontaneous activity of neuronal networks[135].The latter result was obtained by Ishiiet al[136], who analyzed the response to drugs (gabazine and kaliotoxin) on synchronized burst and spontaneous firing, demonstrating that the coculture system is more efficient as anin vitromodel than the individual cultures of commercial astrocytes or iPSC-derived neurons[136].
One of the protocols reported in the literature for the differentiation of human iPSCs into a mix of neurons and glia cells takes about 28 days.The first step is the generation of embryonic bodies, followed by the generation of neuroepithelial aggregates (rosettes) that requires 5 days, and then the dissociation of the rosettes and the neuronal differentiation (20 days) in a mix of neurons and glial cells.This protocol allows heterogeneous cultures of glutamatergic, dopaminergic, and GAΒAergic neuronal cells to be obtained, together with glial cells[137].Mukherjeeet al[138]reported that the main method of differentiation of iPSCs involves the mimics of development signals using culture medium added with morphogens, small molecules, and/or growth factors.Differentiation can occur basically using two different methods:culture in suspension in a single cell or in adhesion with the subsequent formation of aggregates of embryonic bodies and the cultivation in medium, allowing the formation of the definitive neuroectoderm and neural rosettes; and inhibition of one important protein of the small mothers against decapentaplegic family in iPSCs cultured as a monolayer and the formation of rosettes by inhibition of ΒMP.The main differences between the two methods are the efficiency of differentiation and the duration of culture, while the involved pathways are similar (ΒMP/TGF-β/Wnt).The cortical GAΒAergic neurons are obtained by adding, before the phase of terminal differentiation, inhibitors of sonic hedgehog (SHH) and/or Wnt, for the dopaminergic neurons of the caudal midbrain (mesencephalon) with the FGF-8, SHH, and Wnt agonist, while using FGF-2 and insulin it is possible to obtain Purkinje cells[138].
Also, for drug-induced neurotoxicity, as for other forms of toxicity, there is a correlation between patient-specific gene variability and the development of adverse drug-induced effects.In line with this observation, also in this case, iPSCs offer the advantage of specific patient studies to better understand the correlation between gene expression and drug toxicity.
An example was described by Oharaet al[139].In this study, iPSCs were obtained from PΒMCs or T-lymphocytes of two patients with Charcot-Marie-Tooth disease(CMT), with a mutation of a gene encoding a mitochondrial protein (mitofusin-2), and two healthy individuals.iPSCs were differentiated to motor neurons, noting that the mutation also remains in the differentiated neurons, and analysis at the mitochondria level indicated that the neurons derived from patients affected by CMT present mitochondrial dysfunctions.The results showed that two drugs (vincristine and paclitaxel) cause mitochondrial aggregation (a parameter of mitochondrial abnormalities to evaluate the neurotoxic effects) in healthy and CMT-derived neurons, but the greatest effect was observed in patients.The authors concluded that the effect of the tested drugs is different between patients and healthy donors and that the analysis of mitochondria is a good parameter to study neurotoxicity[139].
Permanent peripheral neuropathy is the most common non-hematologic toxicity of anti-cancer chemotherapy, with an incidence of around 20%-40%[140].It has been hypothesized that calcium signaling, oxidative stress, or mitochondrial changes are effects caused by anti-cancer drugs and that these effects may induce neuropathy[132].Several studies are available on commercial cells to study neurotoxicity.Wheeleret al[140]studied the neurotoxic effects of three anti-cancer drugs (paclitaxel, vincristine,and cisplatin) on neurons derived from commercial human iPSCs (from Cellular Dynamics International).They analyzed the neurite outgrowth phenotype and observed a dose-dependent decrease in neurite processes in the case of treatment with the drugs (the greatest effect was observed for vincristine).Using the Caspase-Glo 3/7 assay, drug-induced apoptosis was evaluated, noting that the greatest effect of increased caspase activity was present in the case of treatment with cisplatin, followed by treatment with vincristine, while paclitaxel had no effect[140].The effect of paclitaxel on neurite outgrowth without affecting caspase 3/7 activation was also observed by Winget al[141].In this study, neurotoxicity of other anti-cancer drugs was evaluated on two commercial human iPSC-derived neuronal cell lines:iCell®Neurons (Cellular Dynamics International) and Peri.4U (Axiogenesis, Cologne, Germany),demonstrating a differential sensitivity of neurons to different chemotherapeutics[141].Another study carried out on Peri.4U neurons (from Axiogenesis) is reported by Ranaet al[142], who studied the effect of 16 chemotherapeutic agents.One of the most important findings was that some drugs such as epothilone, taxane, and vinca alkaloid chemotherapeutics did not produce cytotoxicity even though a reduction in the length of the neurite was observed[142].
Snyderet al[143]evaluated the neurotoxic effects of different classes of chemotherapeutics on various commercial neurons derived from iPSC:Peripheral iPSC-neurons (Axiogenesis), iCell®Neurons (Cellular Dynamics International),ReproNeuro glutamatergic neurons (ReproCell, Glasgow, United Kingdom), and human cerebral cortical neurons (Axol Βioscience, Cambridge, United Kingdom).Initially, they evaluated the expression of the characteristic markers of each type of iPSC neurons by gene expression and protein quantification, while the neurotoxic effect of the compounds was evaluated as neurite dynamics and apoptosis[143].
Another example was reported by Yamadaet al[144], who evaluated the neurotoxicity and the influence on neuronal development of neuronal cells derived from commercial iPSCs of 5-fluorouracil.These cells have been used as anin vitromodel of human fetal stage.Real-time PCR results showed that this drug has inhibitory effects on the early neural differentiation of iPSCs.All of these studies have shown that commercial neurons derived from iPSC may be useful models for the study of druginduced neurotoxicity, especially chemotherapeutics.
Considering the different physiological characteristics between children and adults,particular care should be taken in the investigation of ADRs in children, also in the case of drug-induced neurotoxicity.An example is represented by anesthetics, as children seem to be more vulnerable to the adverse effects of these drugs.The problem arises by the fact that many of the anesthetic protocols used in children were developed because of those used in adults[145].Numerous studies have indicated that neuronal damage due to anesthetic depends on many factors such as duration of exposure, developmental phase at the time of exposure, activated receptor subtype,and dose and route of administration.These agents can damage immature neurons,inducing morphological changes and functional impairment of mitochondria,releasing ROS that can further damage the tissue[146].
Neurotoxicity induced by chemotherapy is another problem in children as it may compromise the quality of life and longevity.For example, vincristine is used for ALL,which is the most common childhood cancer.However, this drug causes motor and sensory dysfunction and neuropathic pain.Dioufet al[147]performed genotyping analysis on two cohorts of pediatric patients with ALL treated with vincristine and noted that the rs924607 polymorphism (CEP72gene) could be associated with the development of vincristine-induced neuropathy.Analysis of the neurite development such as outgrowth, number of processes and number of branches on commercial iCell neurons derived from iPSC (Cellular Dynamics International) showed a relationship betweenCEP7gene expression and vincristine-induced neurotoxicity; a reduced expression of this gene was associated with a greater sensitivity to the drug.This may suggest that patient-specific therapy would be required for a safer use of vincristine[147].
Considering the extreme complexity of the 3D structure of the brain, the study of its development and modeling of disease processes in 2D cultures could have several limitations.Therefore, the use of human iPSCs for the generation of 3Din vitroorganoids has revolutionized the research in this field by recapitulating the organization of different regions of the nervous system such as retina, cerebral cortex,and choroid plexus[148-150].The protocol described by Lancaster and Knoblich[148]to develop cerebral organoids begins with the generation of embryoid bodies from iPSCs.Embryoid bodies are subjected to neural induction in a culture medium that favors only the neuroectoderm to expand and are then transferred into droplet of Matrigel, which allows outgrowth of neuroepithelial buds.In the end, the tissues are moved to a spinning bioreactor, or in an orbital shaking plate, which contributes to increase oxygen and nutrient interchange and further development into defined brain regions.
Human brain organoids provide insights into the pathogenesis of neurological disorders and may offer new approaches for investigating the mechanism of the neurotoxicity of drugs.For instance, Liu and collaborators[151]used thisin vitromodel to study the neurotoxic effects of vincristine treatment.In particular, human iPSC(SC101A-1) were cultured to generate cerebral organoids and subsequently were treated with different concentrations of vincristine for 48 hours; the treatment resulted in a dose-dependent growth inhibition evaluated by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide test and caused the enhanced expression of cleaved caspase 3, indicating the induction of apoptosis[151].In the same paper, several cellular markers were analyzed in cerebral organoids exposed to the drug, highlighting the inhibition of the microtubule-associated protein tubulin, fibronectin, and the downregulation of matrix metalloproteinases 10.
Another recent report published by Huanget al[152]describes the cerebral organoids as an innovative tool to investigate the toxicity of drugs.The authors showed that tranylcypromine, which is used to treat refractory depression, caused neurotoxicity on brain organoids generated from human iPSCs (SC101A-1), leading to a decreased proliferation activity and apoptosis induction[152].These data were confirmed by measuring the expressions of the neuron-specific markers TUJ1 and GFAP, which stained for neurons and astrocytes respectively:tranylcypromine treatment impaired cell density and arrangement affecting both cell types.Moreover, tranylcypromine treatment abolished the transcriptional activity of ΒHC110/LSD1-targeted genes and increased the expression of histone di-methylated K4.
Limits of iPSCs
One of the first problems that can be highlighted regarding iPSCs technology is the low efficiency in reprogramming.The most important problem is related to the use of viral vectors such as retrovirus and lentivirus, which deliver the reprogramming transcription factors by integration in the host cell genome, leading to chromosomal instability and tumorigenesis from insertional mutagenesis.
However, during the past several years, new safer and non-integrative reprogramming methods have been developed, reaching better efficiency and avoiding the already mentioned issues.Another interesting point to discuss regards epigenetic memory and clonal variability.iPSCs can present an epigenetic memory of the parent somatic cells that can influence the differentiation propensity and therefore the outcomes of studies applied to this technology.This peculiarity of iPSCs, not characteristic of ESCs, may predispose them to differentiate more readily into their parental cells than others and can be useful for example in cell replacement therapy.
Kimet al[153]observed in iPSCs obtained by transcription factor-based reprogramming, a residual DNA methylation pattern characteristic of the parent cells,which increase their propensity to differentiate along lineages related to the donor tissue, while restricting alternative cell fates.The authors tried to reset the epigenome of the parent tissue by differentiation and serial reprogramming, or by treatment of iPSCs with chromatin-modifying drugs.These approaches could help researchers in increasing the differentiation efficiency of iPSCs, limiting the problem related to epigenetic memory.However, other aspects could affect epigenetic memory of iPSCs,such as the number of passages performed.For example, Poloet al[154]demonstrated that increasing the number of passages there is a decrement in differentially methylated regions with respect to the parent cells.One of the reasons that can explain the spontaneous loss of epigenetic memory was postulated by the authors;probably, the loss of parent cells pattern is based on a slow replication-dependent process.However, further studies must be performed to consolidate this hypothesis as indicated by authors and, to date, the origins of epigenetic loss remain to be determined.
Another important point related to iPSCs limits is the intra-variability differences of clones of the same patient.Thatavaet al[155]addressed this problem analyzing the variation in terms of pancreatic beta-cells differentiation of three iPSCs clone lines of three patients suffering from type 1 diabetes.Interestingly, a notable intra-patient variation, comparable to interpatient one, was identified by authors leading to conclude the necessity for a comprehensive fingerprinting of multiple patient-specific clones to obtain a representative pool of cells useable for biomedical applications such as ADRs studies.In addition to Thatava study, also Yokobayashiet al[156]found that the efficiency in iPSCs clones differentiation capability into primordial germ-cell was different; in particular, they observed that the XX clones were less efficient in germcell differentiation.However, interindividual variability is more important to consider than intraindividual variation for complex genetic disorders[157].Deneaultet al[158]analyzed both types of variabilities in iPSCs-derived neuron cells of patients with autism spectrum disorder, a heterogeneous and complex genetic condition, finding significant intra-variability only on few lines, corroborating what has just been said.Concluding, given the contrasts still present on this aspect, the intra-variability between different iPSCs clones should be investigated and it is advisable to analyze the genetic and epigenetic features of the clones generated.To study ADRs, the clonal variability is a key point to keep in mind in order to set up useful standardized tools.Therefore, before developing a model useful to predict patients’ sensitivity, different clones should be first genetically checked to exclude for instance chromosomic aberrations, alterations in differentiation efficiency and variability in DNA methylation profiles.Also, the sensitivity to the drugs of interest should be analyzed in the different clones from the same patients, to exclude a variability in the response.
Βesides iPSCs, the direct differentiation of somatic cells could be used to obtain the cells of interest.This approach has both advantages and limitations with respect to iPSCs technology regarding the study of ADRs (Table 1).A great advantage of direct differentiation is the short period of time necessary to obtain the cells of interest,avoiding the iPSCs step.Moreover, using this direct method, all DNA instability problems in generating and expanding iPSCs, such as point mutations and karyotypic abnormalities, can be avoided.In addition, to study ADRs affecting aged populations,it is desirable to obtain cells reflecting the disease-related DNA mutations together with the age of the patient.However, there is still an open debate if the best method to recapitulate the age of the patient is the iPSCs technique or the somatic direct differentiation[159].The direct method has also several limitations; iPSCs have a higher proliferation capacity and they can self-replicate in culture differently from parental primary cell lines used in the direct method that can be cultured for a very short time with fast accumulation of epigenetic changes.Regarding ADR studies, it is important to generate a great number of differentiated cells of interest and this purpose can be more easily achieved with iPSCs rather than with the direct method based on the great expandability of iPSCs, as discussed above.Heterogenicity of the differentiated cells is another critical point for both techniques.This point is particularly critical for cell therapy and there are several works trying to ameliorate this aspect.Some limitations have to be underlined also for organoid technology:the complex culture medium contains a cocktail of growth factors and molecular inhibitors which might affect the drug responses of organoids; the extracellular matrix Matrigel, used to grow the organoids, might hamper drug penetration and limit their potential in drug screening; genetic and epigenetic aberrations might occur during long-term culture making organoids unsuitable for transplantation/regenerative medicine.Therefore,further efforts are needed to overcome these limits and improve this promising technology for clinical applications.
CONCLUSION
ADRs are one of the major clinical problems, especially in populations at risk, such as pediatric patients.One of the most important tools to improve the comprehension,and thus the prevention, of ADRs, is represented by the possibility to set up groundbreaking patient-specific assays.This goal has been powerfully achieved using iPSCs, being characterized by genetic and physiological patient-specific differences and being able to be differentiated ideally into all the tissues of human body.The knowledge gained so far has demonstrated that iPSCs can be fruitfully used in multiple clinical applications, including not only toxicity screening in the frame of ADRs characterization, but also disease modelling, personalized and regenerative medicines.In addition, the recent development of related models, such as 3D organoids derived from pluripotent or adult stem cells, resembling the 3D architecture of different organs in a more physiological way, has paved the way to better characterize ADRs in a personalized way.
It should be considered that the number of studies on this topic is still limited,especially concerning the pediatric field.In this case, particular care should be taken,considering that children are a population at risk for ADRs and because of the difficulties to obtain biological materials necessary to establish iPSCs models or stem cells-based organoids.However, based also on studies carried out on these models derived from adult patients or by commercial suppliers, a major advancement has been made to develop and establish differentiation procedures to obtain more efficient differentiation protocols and toxicity screening methods.It is therefore conceivable that in the near future, thanks to this progress, also the number of pediatric studies will increase, enabling the possibility to efficiently study and predict ADRs also in pediatric patients.
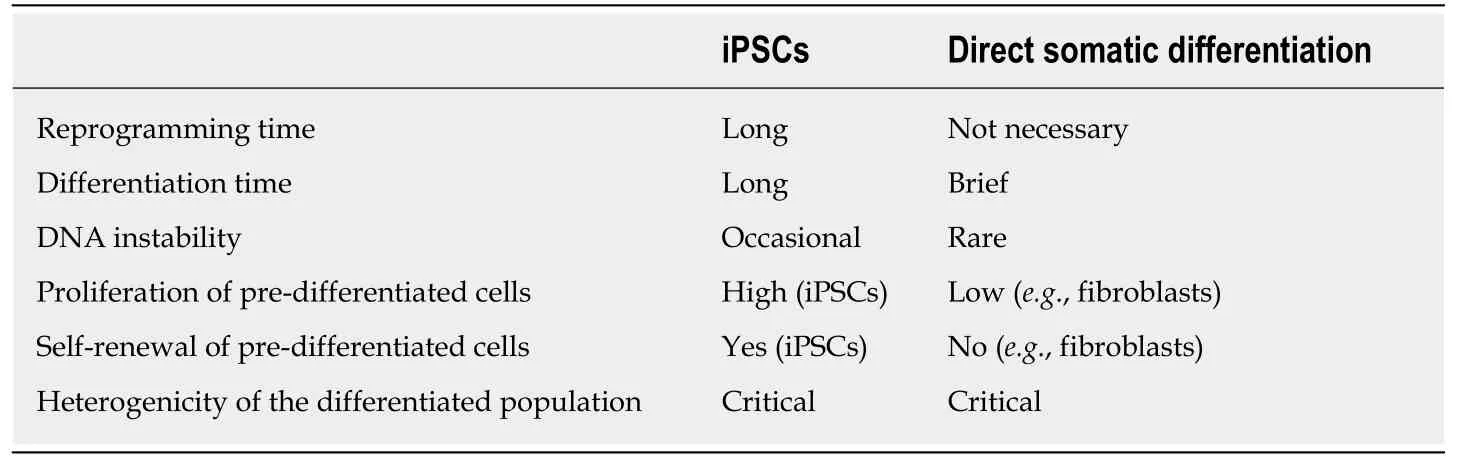
Table 1 Main differences of differentiation techniques based on iPSCs and direct somatic differentiation technologies
ACKNOWLEDGEMENTS
Figures were modified by smart server medical art.
 World Journal of Stem Cells2019年12期
World Journal of Stem Cells2019年12期
- World Journal of Stem Cells的其它文章
- MiR-301a promotes embryonic stem cell differentiation to cardiomyocytes
- Anti-osteoarthritis effect of a combination treatment with human adipose tissue-derived mesenchymal stem cells and thrombospondin 2 in rabbits
- Mechanoresponse of stem cells for vascular repair
- Small molecules for mesenchymal stem cell fate determination
- Three-dimensional cell culture systems as an in vitro platform for cancer and stem cell modeling
- Influence of olive oil and its components on mesenchymal stem cell biology
