Two-week bismuth-containing quadruple therapy and concomitant therapy are effective first-line treatments for Helicobacter pylori eradication: A prospective open-label randomized trial
So Jeong Kim, Jun-Won Chung, Hyun Sun Woo, Su Young Kim, Jung Ho Kim, Yoon Jae Kim, Kyoung Oh Kim,Kwang An Kwon, Dong Kyun Park
Abstract BACKGROUND Increasing levels of antibiotic resistance have reduced the Helicobacter pylori (H.pylori) eradication rates afforded by the standard triple therapy. Thus, 2-wk firstline four-drug regimens must be considered.AIM To analyze the eradication rates of modified bismuth-containing quadruple therapy (mBCQT) and concomitant therapy (CT), the associated adverse events,and compliance.METHODS Patients infected with H. pylori were prospectively randomized to receive mBCQT or CT for 2 wk. mBCQT featured a proton pump inhibitor (PPI),bismuth, metronidazole, and tetracycline, taken twice daily. CT included a PPI,clarithromycin, metronidazole, and amoxicillin, taken twice daily. The 13C-urea breath test was performed no earlier than 4 wk after therapy concluded to confirm eradication. If either the histological or rapid urease test was positive, H.pylori infection was diagnosed.RESULTS The demographic characteristics of 68 patients who received mBCQT and 68 who received CT did not differ significantly. On intention-to-treat analysis, the eradication rate was 88.2% (60/68) in the mBCQT group and 79.4% (54/68) in the CT group (P = 0.162). By per-protocol analysis, the respective eradication rates were 98.4% (60/61) and 93.1% (54/58) (P = 0.199). More CT than mBCQT patients experienced adverse events [33.8% (23/68) mBCQT vs 51.5% (35/58) CT patients,respectively, P = 0.037]. All patients showed good compliance [85.3% (58/68)mBCQT vs 82.4% (56/68) CT patients, P = 0.641].CONCLUSION The H. pylori eradication rates of the 2-wk mBCQT and CT regimens are high.Most patients show good compliance, and more CT than mBCQT patients experience adverse events.
Key words: Helicobacter pylori; Therapy; Bismuth-containing quadruple therapy;Concomitant therapy
INTRODUCTION
Helicobacter pylori(H. pylori) infection is the principal cause of peptic ulcer disease,mucosa-associated lymphoid tissue lymphoma, and gastric adenocarcinoma.H. pylorieradication cures or prevents these diseases[1]. However,H. pyloriis becoming resistant to previously efficacious antibiotic regimens[2]. Clarithromycin resistance is increasing worldwide[2-4]. The eradication rate afforded by legacy triple therapy (LTT)(the historical first-line treatment forH. pyloriinfection; LTT includes clarithromycin)has declined over the past decade, and has decreased to near 80% or below in some countries of Asia and Europe[2-4]. Dual clarithromycin-/metronidazole-resistance ofH.pyloriis also of concern worldwide[5]. In South Korea, the dual resistance rate is 9.6%,and it is 19% [95% confidence interval (CI): 0%-39%] in the Eastern Mediterranean Region[5,6].
Thus, many studies have explored the efficacy of first-line alternatives to LTT; these include bismuth quadruple therapy (BQT) and concomitant therapy (CT)[7-13], which feature in the current guidelines[2,14-16]. The Maastricht V consensus concluded that, in areas exhibiting high-level (> 15%) clarithromycin resistance but low-level dual clarithromycin/metronidazole resistance (< 15%), BQT or CT should be recommended. In areas exhibiting high-level dual clarithromycin/metronidazole resistance (> 15%), BQT is the recommended first-line treatment (level of evidence:Low; grade and recommendation: High)[2]. The revised guidelines for the diagnosis and treatment ofH. pyloriinfection in Korea recommend the use of bismuthcontaining quadruple therapy (BCQT) if clarithromycin resistance is suspected[16]. A meta-analysis found that the eradication rate afforded by CT was slightly higher than that of sequential therapy (ST) [pooled odds ratio (OR) = 1.382,P= 0.03, intention-totreat (ITT) analysis; 2.102,P= 0.001, per-protocol (PP) analysis][10]. However, only a few reports on the efficacy of BCQT and CT have appeared; direct comparisons are rare. Therefore, we performed a prospective, randomized controlled study comparing modified BCQT (mBCQT) and CT as potential first-line treatments forH. pyloriinfection.
MATERIALS AND METHODS
Study design and patients
We performed a prospective, open-label, randomized controlled trial in a single tertiary medical center. Patients who underwent endoscopy between February 2016 and August 2017 at Gachon University Gil Hospital, and who received first-of-life diagnoses of peptic ulcer disease, gastritis, gastric polyps, or early gastric cancer, and who then underwent endoscopic mucosal resection, were initially included.
Sample size calculations were performed before the trial began. We assumed, based on previous studies, that the eradication rate of CT was 90%. With little data on BCQT, we assumed an eradication rate of 85% and calculated that a sample size of 60 patients in each of the two groups was necessary (α = 0.05, power 80%, non-inferiority limit of -10%-10%). The actual sample size was increased to 136 patients to account for patient drop-out.
Of these patients, 136 with confirmedH. pyloriinfections [at least two positive results on either or both of a histological test (Giemsa staining) and the rapid urease test] were enrolled. The 136 patients were randomly divided into two 68-patient groups using a computer-generated block randomization table; the groups received 2-wk treatments with either mBCQT or CT.
The13C-urea breath test was performed at 6 wk to 8 wk after antibiotic suspension.
The primary outcome was the eradication rate ofH. pylori, as determined by ITT and PP analysis. The secondary outcomes were adverse events and compliance.
The study was approved by the Clinical Research Ethics Committee of Gachon University Gil Hospital (approval No. GCIRB2016-006). Written informed consent was obtained from all patients enrolled in the study.
Confirmation of H. pylori infection
When either Giemsa staining or rapid urease test (HP kit; Chong Kun Dang Bio,Seoul, South Korea) was positive upon upper gastrointestinal endoscopy,H. pyloriinfection was diagnosed.
Eradication of H. pylori
The mBCQT regimen featured lansoprazole (Lanston LFDT Tab.) 30 mg, tripotassium bismuth dicitrate 600 mg (Denol Tab.), tetracycline (Tetracycline Cap.) 1000 mg, and metronidazole (Flasinyl Tab.) 500 mg twice daily for 2 wk. CT featured lansoprazole(Lanston LFDT Tab.) 30 mg, clarithromycin (Kloma Tab.) 500 mg, amoxicillin(Amoxapen Cap.) 1000 mg, and metronidazole (Flasinyl®Tab.) 500 mg twice daily for 2 wk.
Adverse events and compliance
Inadequate drug compliance was defined as ingestion of less than 90% of the total recommended dose. Adverse effects were defined as unexpected symptoms that developed between the commencement and 4 wk after completion of treatment.
Confirmation of H. pylori eradication
The13C-urea breath test (UBiT-IR 300/13C-Urea breath test system; Otsuka, Tokyo,Japan) was performed no earlier than 4 wk after treatment concluded, at which time no patient had taken proton pump inhibitors for at least 2 wk. Eradication failure was defined as a positive (delta 2.5) breath test.
Statistical analysis
IBM SPSS statistical software (ver. 22.0; IBM Corp, Armonk, NY, United States) was used for all analyses. We employed theχ2test to compare discrete variables and the independentt-test to compare continuous variables. We ensured that our sample size had a sufficient non-inferiority margin.
RESULTS
Patients
A total of 136 patients were randomly divided into two groups of 68 patients each and comparedviaITT analysis. Seven mBCQT patients were excluded because of loss to follow-up. Nine CT patients were lost to follow-up and one patient withdrew. The remaining 61 patients in the mBCQT group and 58 in the CT group were comparedviaPP analysis (Figure 1).
The mean age of mBCQT and CT group patients was 57.37 ± 11.31 and 60.09 ± 9.93 years, respectively (P= 0.138). The demographic characteristics of the two groups did not differ significantly (Table 1).
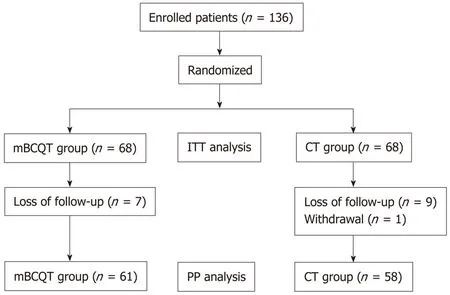
Figure 1 Study flow chart. mBCQT: Modified bismuth-containing quadruple therapy; CT: Concomitant therapy; ITT:Intention-to-treat; PP: Per-protocol.
H. pylori eradication rates
ITT analysis revealed an eradication rate of 88.2% (60/68) in the mBCQT group and 79.4% (54/68) in the CT group (P= 0.162). PP analysis revealed that the eradication rates in the mBCQT group and CT group were 98.4% (60/61) and 93.1% (54/58),respectively (P= 0.199) (Table 2). ITT analysis showed that the eradication rates in both the mBCQT and CT groups were close to 80%, but the PP values for both groups were over 90%.
Adverse events and compliance
Most of the adverse symptoms affected the digestive system. No serious side effect was noted in any patient. The adverse effect rates in the mBCQT group and CT group were 33.8% (23/68) and 51.5% (35/68), respectively,i.e., slightly higher in the CT group (P= 0.037). The compliance level was 85.3% in the mBCQT group and 82.4% in the CT group (Table 3).
DISCUSSION
Both mBCQT and CT proved to be useful empirical first-line treatment options forH.pylorieradication. In an area exhibiting high-level clarithromycin resistance (> 15%),both regimens were effective on ITT analysis (mBCQT, 88.2%; CT, 79.4%;P= 0.162)and PP analysis (mBCQT, 98.4%; CT, 93.1%;P= 0.199)[16,17]. mBCQT was somewhat,but not significantly, better than CT, by about 8% on ITT analysis and 5% on PP analysis. A large randomized trial performed in Taiwan reported similar results. The cited work used culture to identify clarithromycin-susceptible and -resistant strains,and predicted the efficacy of various drug regimens accordingly. The predicted efficacy of 10-d BQT was greater than that of 10-d CT and 14-d LTT in regions exhibiting high-level clarithromycin resistance, as revealed by multivariate analysis.Similarly, CT was more effective than LTT[8]. A prospective study performed in Spain yielded results similar to ours; 10-d BQT (using capsules) and 14-d CT, employed as first-line eradication treatments in an area of high-level clarithromycin resistance,were equally effective[12]. A South Korean study found that 10-d PAM-B therapy(rabeprazole 20 mg, amoxicillin 1 g, metronidazole 750 mg, and tripotassium dicitratobismuthate 600 mg twice daily) compared favorably with 14-d CT. The eradication rate of CT was similar to that of the present study, but the PAM-B eradication rate could not be directly compared to the CT data because the regimens differed markedly (ITT analysis, 88.1%vs83.0%,P =0.299; PP analysis, 96.6%vs95.5%,P= 0.743). The incidence of adverse events was lower than that in our study(23.0%vs25.2%,P= 0.776), perhaps because the duration of 10-d CT of the cited study was shorter than that of our CT[13]. The effects of treatment duration on eradication rates and side effects should be studied in future. Both of our regimens featured multiple drugs; compliance remains an issue, but the relatively high eradication rates may reflect good compliance[18]. Our mBCQT regimen featured twice-daily dosing,unlike previous studies. Doreet al[19]reported that daily two- or four-dose second-line regimens afforded similar eradication rates.
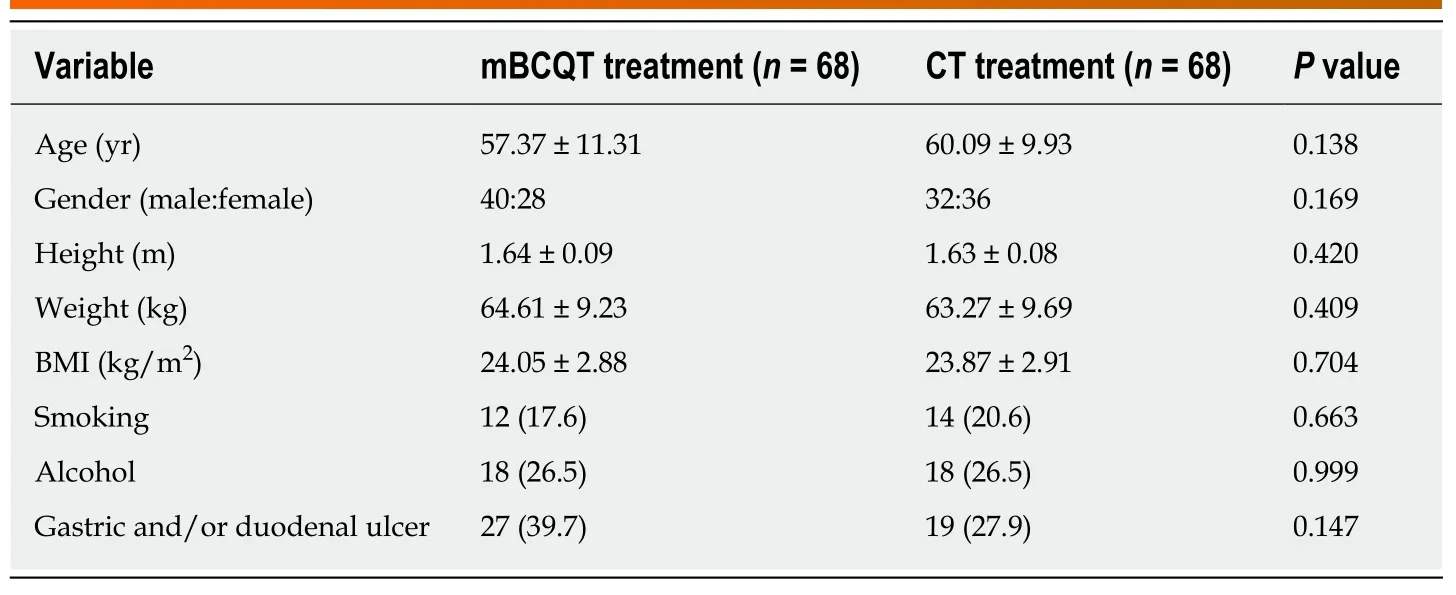
Table 1 Demographic and endoscopic data of the two patient groups
Also, our mBCQT regimen used a higher dose of tetracycline than other regimens(1000 mg twice daily). A previous Korean study revealed that the extent of tetracycline resistance was relatively low (minimal inhibitory concentration ≥ 2.0 mg/mL, 0%-3.3% of isolates), perhaps attributable to a reduction in tetracycline prescriptions when medical practice and pharmaceutical dispensing were separated in 2000[6,20,21]. The tetracycline resistance rate was 0.7% in Spain, 0.5% in the United Kingdom, and 0.5% in Hong Kong; in many countries, the rate was 0%[22]. Some studies have explored the efficacy of tetracycline-/amoxicillin-containing regimens. A Korean study comparing a 10-d ST and 14-d PBAT regimen (40 mg pantoprazole, 600 mg bismuth subcitrate, 1 g tetracycline, and 1 g amoxicillin, twice a day) found that ST seemed to be more effective; however, neither regimen afforded ‘excellent'eradication (over 90%)[23]. A Taiwanese study found that quadruple therapy featuring tetracycline and amoxicillin effectively rescued patients in whom triple therapy failed(78% rescue on ITT analysis and 89% on PP analysis). However, apart from our mBCQT regimen, ST featuring tetracycline was associated with relatively low-level eradication[24]. These variable results may reflect the fact that both tetracycline and amoxicillin are not very active againstH. pylori, or that the drugs may act antagonistically[23]. In most studies, tetracycline was taken four times daily, but twicedaily regimens are more appropriate and probably also improve compliance;tetracycline is a time-dependent (half-life, 8-10 h) antibiotic agent exhibiting a long post-antibiotic effect. Further studies are needed to explore the adequacy of currently used tetracycline dosages and durations[23].
Although neither of our regimens was associated with serious side effects, the overall incidence of side effects was relatively high. Further efforts to reduce side effects, perhapsviaaddition of probiotics, are essential[25]. Previous studies have found that certain probiotics, such asLactobacillus genusandBacillus clausii, reduce gastrointestinal problems and other side effects of eradication therapies[2,26]. It has been suggested that the increase inH. pylorieradication seen with use of these probiotics is not a direct effect of the probiotics, but a result of the associated reduction in side effects of therapy[2,26].
Although both regimens seemed to be effective first-line therapies, their efficacy in terms of salvage therapy have not been confirmed. The Maastricht V guidelines indicate that, after failure of non-BQT, levofloxacin triple therapy is appropriate[2].However, according to the World Health Organization, quinolone resistance has been reported in over 30% of all regions, and primary levofloxacin resistance in over 15%,except Europe[5,20,22]. Therefore, it is necessary to consider rescue regimens containing drugs associated with lowH. pyloriresistance rates.
One study of rifabutin-based therapy reported an eradication rate over 70% (72.7%,95%CI: 67.3%-77.7%, PP analysis; 71.5%, 95%CI: 66.1%-76.5%, ITT analysis)[27].Although rifabutin-resistantH. pylorihas been reported, rifabutin-based regimens may be useful as rescue therapies because rifabutin can be used without culture and antibiotic susceptibility testing[27]. Also, furazolidone-resistantH. pyloriis rare; the drug afforded satisfactory eradication rates (relative risk = 1.17, 95%CI: 1.05-1.31)[28].In a multivariate analysis, an increased eradication rate when using high-dose furazolidone was noted (OR = 1.5, 95%CI: 1.3-2.7;P< 0.001)[29]. According to a recentin vitroanalysis, there were no strains resistant to rifabutin or furazolidone among multi-drug resistantH. pylori(minimum inhibitory concentration < 0.008 and < 0.5 μg/mL, respectively). This suggests that rifabutin- and furazolidone-containing regimens have promise as rescue therapies[30]. However, there is controversy over the use of furazolidone-containing regimens because of the drug's potential carcinogenicity. Thus, furazolidone-containing regimens are recommended only in areas with high rates of resistance and for patients who have multiple prior treatment failures and have received patient counseling[29,31].
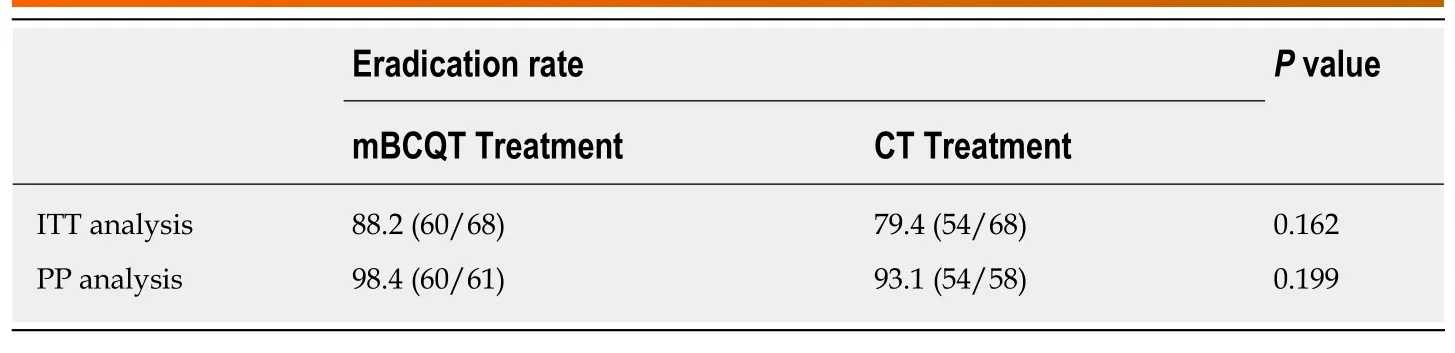
Table 2 Eradication rates afforded by modified bismuth-containing quadruple therapy and concomitant therapy
The limitations of this study included the small number of patients, due to the single-center design. Also, we did not cultureH. pylori. The strengths of our study include its randomized controlled design and the acceptable therapeutic efficacy in both the mBCQT and CT groups. In conclusion, both mBCQT and CT are promising empirical first-line regimens in an area exhibiting high-level clarithromycin resistance.However, mBCQT is somewhat preferable, considering both side effects and compliance. A large-scale prospective study is required to validate our findings.

Table 3 Adverse events and compliance
ARTICLE HIGHLIGHTS

 World Journal of Gastroenterology2019年46期
World Journal of Gastroenterology2019年46期
- World Journal of Gastroenterology的其它文章
- Transumbilical enterostomy for Hirschsprung's disease with a twostage laparoscopy-assisted pull-through procedure
- Clinical relevance of fluorodeoxyglucose positron emission tomography/computed tomography and magnifying endoscopy with narrow band imaging in decision-making regarding the treatment strategy for esophageal squamous cell carcinoma
- Mesenterico-portal vein invasion should be an important factor in TNM staging for pancreatic ductal adenocarcinoma: Proposed modification of the 8th edition of the American Joint Committee on Cancer staging system
- Tailored eradication vs empirical bismuth-containing quadruple therapy for first-line Helicobacter pylori eradication: A comparative,open trial
- Long non-coding RNA HULC as a diagnostic and prognostic marker of pancreatic cancer
- Severe liver injury due to herbal and dietary supplements and the role of liver transplantation
