Severe liver injury due to herbal and dietary supplements and the role of liver transplantation
Priya Grewal, Jawad Ahmad
Abstract Herbal and dietary supplements (HDS) are increasingly used worldwide for numerous, mainly unproven health benefits. The HDS industry is poorly regulated compared to prescription medicines and most products are easily obtainable. Drug induced liver injury (DILI) is a well-recognized entity associated with prescription and over the counter medications and many reports have emerged of potential HDS-related DILI. There is considerable geographic variability in the risk and severity of DILI associated with HDS but the presentation of severe liver injury is similar with a hepatocellular pattern accompanied by jaundice. This type of injury can lead to acute liver failure and the need for liver transplantation. Patients will often fail to mention their use of HDS, considering it natural and therefore harmless. Hence physicians should understand that these products can be associated with DILI and explicitly ask about HDS use in any patient with otherwise unexplained acute liver injury.
Key words: Herbal and dietary supplements; Drug induced liver injury; Liver transplantation; Acute liver failure; Geographic variability
INTRODUCTION
The appeal and use of herbal and dietary supplements (HDS) are increasing worldwide. These supplements come with a promise to improve and maintain many intangible aspects of the overall quality of life including energy, memory, longevity,mood, and self-image. While the proposed health benefits of such products are not supported by scientific data, their harmful effects specifically on the liver are well reported. For the purpose of this review, HDS refers to all products which have previously been named as traditional, alternative or complementary in the West and Unani, Ayurveda, Kampo, and Traditional Chinese Medicine in Africa and Asia. This review will provide an overview of landmark studies on severe drug induced liver injury (DILI) caused by HDS use in various regions of the world. Major trends will be summarized, including issues in interpretation and comparison of global data, liver transplantation (LT) in this population, and the need for physicians to be aware of the possibility of HDS use in patients with unexplained acute liver injury.
HDS USE
In the United States, the sale of HDS is a lucrative multibillion-dollar industry with nearly 80000 different supplements sold[1]. The 2-year National Health and Nutrition Examination Survey (2007-2008) revealed that 50% of adult Americans were using HDS[2]. In recent years, HDS use has increased to 64%-69% of the population[3]. In Europe, HDS use was highest in the United Kingdom (41%)[4]but other European countries including Finland, Germany, Romania, Italy, and Spain estimated an overall use of 18.8% with the highest rate in Italy (22.7%) and the lowest in Finland (9.6%)[5](Table 1). In densely populated regions of the world, such as Africa, Asia and Latin America, nearly 80% of the population uses HDS for their medical ailments[6]. Patients do not disclose HDS use to their physicians, as they assume that they are harmless. As people move across the world, they carry with them their cultural belief in HDS,contributing to the rising prevalence of HDS use in Western countries. These products are very appealing because they are easily accessible through the internet, often cheap and require no prescription or monitoring by a physician.
WORLDWIDE MAGNITUDE OF SEVERE LIVER INJURY CAUSED BY HDS USE
While the estimated incidence of severe DILI from HDS use in prospective studies ranges up to 3 individuals per 100000/year in the West the rates are higher in Asia[7]although information on the proportion of patients developing DILI from HDS use is difficult to determine because the actual number of the users is not always clear.
However, data from the Spanish DILI registry over the past 22 years have revealed that the hepatocellular pattern associated with DILI due to HDS use is marked by very high alanine aminotransferase values, predicting severe outcomes including death or need for LT[8]. By the same token, a United States registry study reported higher transplantation rates and lower survival among patients with DILI caused by HDS relative to prescription drugs[9].
THE EUROPEAN EXPERIENCE
Spain
A decade ago, 2% of DILI cases were attributed to HDS use[10]. More recently, the Spanish DILI registry (1994-2016) reported that HDS use was responsible for 4% of DILI cases, while only 2% were caused by anabolic androgenic steroids. Patients with HDS use were mainly female and usually less than 50 years of age. The pattern of liver injury was predominantly hepatocellular with 78% of the patients presenting with jaundice and 6% progressed to acute liver failure (ALF) (Table 2). Most HDS products were used for weight loss (47%). The most commonly used products wereHerbalife(multi-ingredient) and green tea extract (GTE). Alarmingly, 9% of the patients inadvertently took the HDS product again because the first instance of hepatoxicity was incorrectly attributed to an innocent bystander drug; consequently,most of these patients were readmitted after the rechallenge[8].

Table 1 Use of herbal and dietary supplements in the United States and Europe (data from references 3-5)
Iceland
In a prospective study, the annual incidence of DILI over the 2-year study period(2010-2011) was reported as 19.1 cases per 100000 inhabitants and 16% of these were attributed to HDS use. Similar to Spain, the most common HDS associated with DILI wereHerbalifeproducts (33%) and GTE (camellia sinensis) in 27%. The pattern of DILI from HDS was again predominantly hepatocellular. While most patients were symptomatic, only 26% were jaundiced[11].
France
Perhaps the most robust data on the incidence of DILI was reported in a French study that used a population based approach where 34 cases of DILI were noted in more than 80000 individuals over a 3 year period, giving a crude annual incidence of 14 per 100000[12]. However, in reviewing the 34 cases, all were from prescription or over the counter medications and there were no cases from HDS. This study was from the late 1990s when DILI from HDS was not as well described. A recent study of 808 patients listed for LT due to ALF noted acetaminophen as the cause in 22% but toxins and other drugs comprised 14% and 28% of all cases were undetermined, undoubtedly some from HDS related DILI[13]. Multiple cases of acute liver injury have been described with the use of various HDS in France[14,15], including green tea extract[16]and germander (Teucrium chamaedrys)[17].
THE ASIAN EXPERIENCE
South Korea
A prospective study over 2 years (2005-2007) across 17 university hospitals in Korea reported a total of 371 cases of DILI (based on the Roussel Uclaf Causality Assessment Method criteria), with an annual incidence of 12 cases/100000 persons. While prescription or non-prescription medications accounted for 27% of cases, the rest were attributed to HDS, including herbal medications, folk remedies and health foods or supplements. The median age of this cohort was 49 years, and the majority of patients were female. The pattern of liver injury was 76.3% hepatocellular, 14.8% mixed, and 8.9% cholestatic. Among the 5 patients (1.8%) who died or underwent LT, 4 were due to HDS. Twenty five cases (21 HDS and 4 medications) were excluded from the study due to missing information on previous HDS and the exact timing of use likely underestimating the actual incidence of DILI from HDS. Hence, the authors proposed that in patients with DILI from HDS a more specific and reproducible tool for causality assessment is necessary[7].
Japan
Among the 1676 cases of DILI in Japan from 1997-2006 reviewed retrospectively,17.1% were related to dietary supplements and Chinese herbal drugs. The diagnostic scale used to adjudicate cases was postulated by the Digestive Disease Week-Japan(DDW-J) 2004 workshop[18]. Most of the patients were middle-aged females with a predominantly hepatocellular pattern of injury. Interestingly, severe liver injury leading to death or LT was rare among HDS users (1.2%), relative to prescription drug users (4.2%)[19].
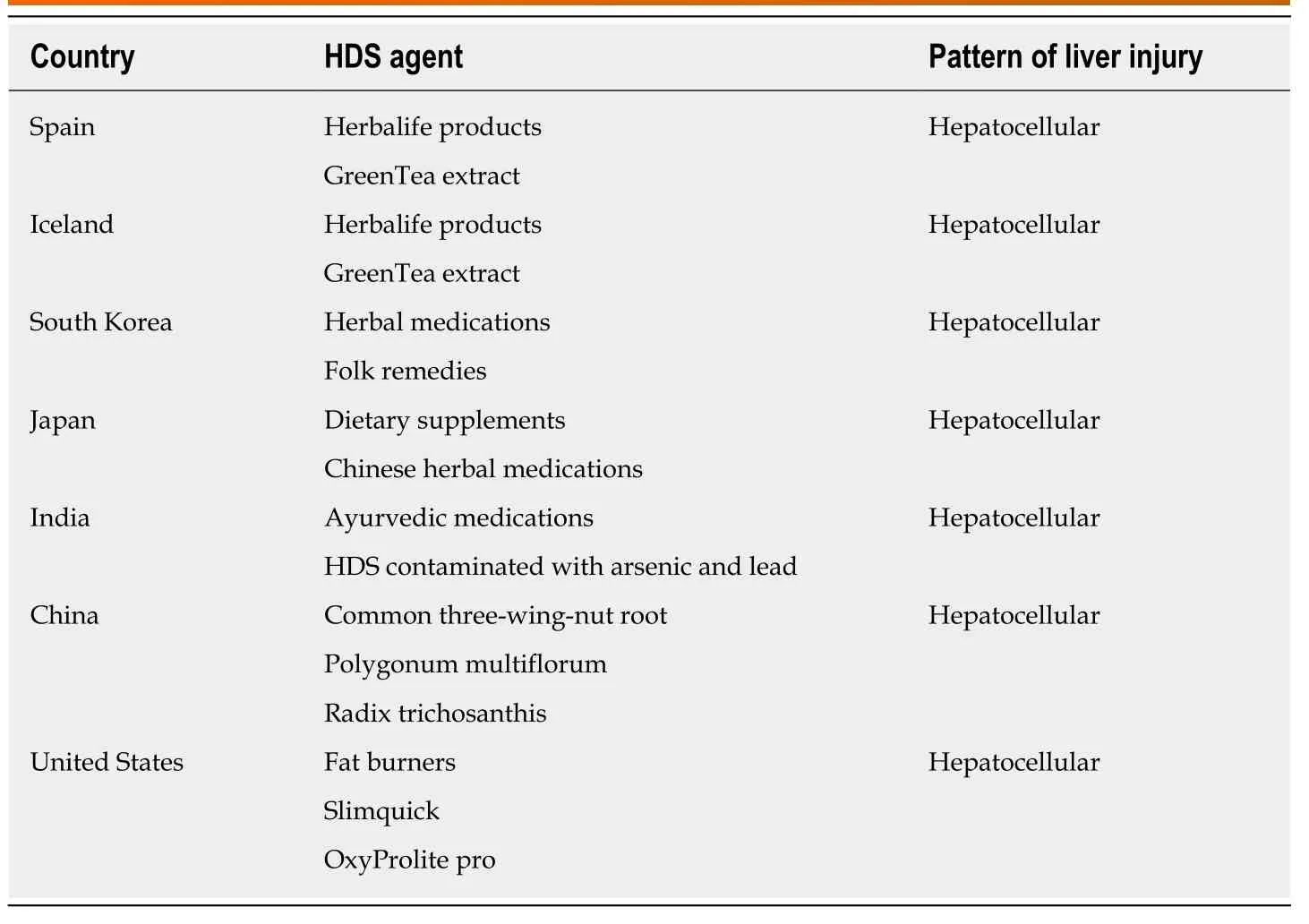
Table 2 Main herbal and dietary supplements used and their pattern of liver injury across the world
India
A large single center retrospective analysis of 313 DILI cases over a 12-year period(1997-2008) reported an overall mortality of 17.3%, primarily related to use of antituberculosis medications. Only, 1.3% cases were attributed to HDS, mainly Ayurvedic medications. Notwithstanding, half of the patients with HDS related DILI died. The authors postulated that despite the rising popularity of HDS in India, they are increasingly taken after the onset of jaundice from viral hepatitis which may contribute to the increased mortality noted. Also, more so than hepatitis, patients present with constitutional and neurological symptoms due to heavy metal contamination of HDS with lead and arsenic[20].
China
A recent review of nearly 26000 confirmed DILI cases; hospitalized from 2012-2014 at approximately 300 medical centers in mainland China were analyzed. Most cases of DILI presented with hepatocellular injury (51%). The major classes of implicated drugs were traditional Chinese medicines (27%) followed by medications for tuberculosis (22%). Only 1% of DILI cases associated with HDS progressed to hepatic failure. However, among the 72 who died only from DILI, HDS was the leading culprit (35%)[21].
A retrospective analysis of 279 studies on DILI involving 24000 patients over a 17-year period (1994-2011) reported that HDS were the second leading cause of DILI(18%) after anti-tuberculosis drugs. Most cases were male (54%), older than 40 years,and 58% had a hepatocellular pattern of injury. The overall mortality in the group was 2.9%. The most frequently implicated drugs wereXiao He Pian, Common three-wingnut root (Lei Gong Teng), Polygonum multiflorum (He Shou Wu) Radix Trichosanthis(Tian Hua Fen) andZhuang Gu Guan Jie Wan[22].
A study from 7 tertiary hospitals in China over 5 years (2007-2012) reported on the etiology of ALF in China with particular focus on cases attributed to traditional Chinese medications. Of the 177 patients with DILI, 112 (63.28%) eventually died. The most common cause of ALF was drug toxicity (43.50%), with traditional Chinese herbs responsible for approximately 26% of cases. No patients in this study underwent LT. The authors concluded that traditional Chinese medicine was an important and under recogonized cause of ALF in China, accompanied by a high mortality[23]. Among the 30 patients with ALF from traditional Chinese medications,most were women (80%) in their 30's, with hepatocellular pattern of injury and rapid development of hepatic encephalopathy. Nine patients (30%) were using HDS for their skin disorders. More than half the patients eventually died in the absence of LT which was not performed at their treating hospitals[24]. In a review of 145 cases with DILI from Polygonum multiflorum, 23% had underlying liver disease, mainly from alcohol. When compared to patients without preexisting liver disease, these patients had much higher risk of mortality (9.1%vs0.9%) and likelihood of developing chronicity[25].
THE UNITED STATES EXPERIENCE
In earlier studies (1998-2001) from the Acute Liver Failure Study Group (ALFSG),which analyzed 308 ALF cases, 17% had an indeterminate cause of liver failure that the authors speculated was likely related to a drug, possibly HDS not revealed during history, or a hepatotropic virus. This group had a less than 20% transplant free survival rate[26].
A subsequent analysis by the ALFSG group of 1198 cases over a 10.5 year period classified 11.1% as idiosyncratic DILI. Of these, 70% of patients with idiosyncratic DILI were women, of which 27% belonged to minorities. Amongst the DILI cases,10.6% were determined to be HDS users and continued to show a poor transplant free survival (21.4%)[27]. As the awareness of DILI from HDS has increased in recent years,the AFLSG now reports an increasing contribution of HDS as the etiology of ALF, up from 12.4% in the earlier cohort (1998-2007) to 21.1% in the more recent cohort (2007-2015). The predominant HDS were “muscle builders” and “fat burners” hence the patients were mostly male. While the hepatocellular pattern of injury was comparable in all respects to ALF from prescription medications the HDS group had a lower alkaline phosphatase. However significantly more patients in the HDS group needed LT (56%vs32%) and had a lower transplant free survival (25%vs38%). The authors concluded that while the use of HDS is severely underreported, the minority of patients who present with ALF due to HDS tend to have a more severe presentation and higher mortality in the absence of LT and should be referred to a transplant center promptly. Thus, in the United States, patients with HDS use make up about 10%-20% cases of DILI related ALF, do poorly without transplant and end up utilizing this scarce resource[9].
In the United States DILI Network (DILIN), 85 patients with DILI from HDS,excluding body building supplements, were mostly middle-aged females with predominantly hepatocellular pattern of liver injury and marked ALT elevations.There was a higher percentage of HDS patients that underwent LT (13%) compared to 3% in the group that used conventional medications[28].
A review of the Scientific Registry of Transplant Recipients (2003-2015) examining patients transplanted for acute hepatic necrosis of the liver revealed that 21/625 (3%)of DILI was caused by HDS. Most patients were young women transplanted after 2007 with excellent survival. However, 25% of all LT cases (2408) lacked a specific cause for liver failure and 20% of all drug-induced liver failure did not specify the incriminating drug, suggesting that HDS maybe responsible for many more ALF cases needing transplant[29].
LT FOR HDS RELATED LIVER INJURY
The role of LT for DILI related to HDS is very similar to other causes of fulminant hepatic failure (FHF) since the presentation is almost always acute and occurs in patients without underlying liver disease. There are scarce reports of chronic DILI due to HDS that can evolve into an injury that ultimately requires LT such as vanishing bile duct syndrome[30]. Although the data is limited, it does appear that severe liver injury due to HDS requiring LT is on the increase.
A recent report examined the characteristics of patients listed for LT in the United States using the status-1 designation which is mainly used for patients with FHF and how it has evolved over time[31]. Interestingly they found that the proportion of LTs performed for status-1 decreased in the period 2009-2015 compared to 2003-2009 although the proportion of DILI related FHF cases did not change. However, there was a more than 2-fold increase in the cases of FHF due to HDS that were listed as status-1 in the later period 2009-2015.
An earlier study looked at FHF due to DILI from a population perspective and noted that it is an uncommon event but it was not generally due to prescription medicines. Of the 32 cases of FHF they found, 18 (56%) were due to acetaminophen, 8 due to prescription medicines (25%) and 6 were due to HDS (19%), but the nonacetaminophen cases were more likely to be fatal or require LT[32].
Several case series have demonstrated the risk of FHF from HDS used for weight loss. The 2 most prominent examples are GTE[33,34]and OxyElite Pro[35,36]which in the latter case led to legal ramifications with 5 individuals and 2 companies pleading guilty to felony charges with the FDA in the United States emphasizing they would continue to aggressively investigate and prosecute companies involved in the sale of mislabeled and potentially unsafe dietary supplements.
The DILIN in the United States described 6 cases of acute liver injury due to“SLIMQUICK”, a line of proprietary weight loss products, comprised of multiple ingredients[33]. All the patients were female and taking the products for weight loss.They all presented with an acute hepatitis and 3 patients were hospitalized with 1 requiring LT. Reviewing the SLIMQUICK preparations, GTE and/or its component catechins were the common ingredient, along with caffeine and extracts of several fruit, vegetable and plant products. The typical presentation of GTE related DILI was also observed in the 19 cases reported by Mazzantiet al[34]with the majority women taking the products for weight loss with an acute hepatitis after short or long latency and 4 patients requiring LT.
The case of OxyElite Pro led to investigation by the federal government in the United States and guilty pleas from the manufacturers who admitted to several charges including fraudulent labeling and the finding of 1,3-dimethylamylamine(DMAA) in the product- an additive that was banned by the FDA in 2013[37]. This product was sold as a weight loss and exercise supplement and was associated with multiple cases of FHF all occurring about the same time with multiple fatalities.Independently, the United States military described 7 cases, 2 of which required LT[35];8 cases reported in Hawaii with 2 undergoing LT[36], and the DILIN reported 7 cases with 2 requiring LT[38]. All the cases clustered in 2013 and OxyElite Pro was withdrawn from the market (but not before a total of 43 cases of DILI were observed in Hawaii). The actual ingredient that likely caused the liver injury was unclear and may not have been DMAA but rather aegeline, a compound extracted from a plant used in Ayurvedic medicine, Aegle marmelos (bael), with reported beneficial effects on blood sugar and lipids.
The development of FHF has been described with multiple HDS but the need for LT has recently been shown for patients that develop a chronic biliary type injury due to drugs or HDS. The DILIN described 26 patients who developed a vanishing bile duct syndrome associated with DILI with 5 fatalities and another 2 patients requiring LT. The majority of the 26 cases were due to prescription medications but 3 cases were associated with HDS[30].
KEY TAKEAWAYS
HDS use is rising across different regions of the world. HDS use in the Far East is upwards of 70% while usage in the West is not far behind. While the HDS contributing to DILI are different, the pattern of injury is usually hepatocellular affecting mostly young women. Approximately 1% of patients will develop severe liver injury leading to death or the need for LT. Acute liver failure study groups across the globe report that 20%-40% of ALF from DILI is a result of HDS use.Information on the proportion of patients developing DILI from HDS use is difficult to determine because the actual number of the users is not always clear.
BARRIERS TO MAKING GLOBAL COMPARISONS
Firstly, the regulation of HDS products varies considerably across the world. In the United States, herbs are defined as dietary supplements and undergo less strict regulation relative to prescription drugs. The LiverTox website is a good reference source of reported hepatoxicity related to HDS[39]. Moreover, under the 1994 Dietary Supplement Health and Education Act (DSHEA), 21 herbs are defined as dietary supplements. Unlike conventional drugs, which are required by the Food and Drug Administration to undergo well-designed clinical studies to prove efficacy and safety before marketing, HDS products can be marketed without these requirements.
On the other hand, in Europe, HDS may be labelled either as a dietary supplement or a medicinal product. While the traditional herbal medicinal products are regulated in the European Union, the lack of regulation for “natural” dietary supplemental products, along with the limited awareness among physicians and patients has led to a rise in severe liver injury cases. Additionally, statistics on DILI cases attributable to HDS do no always tell the whole story. For instance, HDS use is much higher in the East, but the reported incidence of severe DILI is not correspondingly that much higher than the rest of the world. This is likely due to the issue of under-reporting and the fact that clinicians are not the sole providers of healthcare. Traditional healers may be an avenue through which patients are receiving not only their HDS but also their medical care. Furthermore, the higher mortality rate in Asia is not necessarily a reflection of greater severity. Rather, it probably reflects lower access to hospitalization and LT.
CONCLUSION
HDS use is rising across different regions of the world. HDS use in the Far East is upwards of 70% while usage in the West is not far behind. While the HDS contributing to DILI are different, the pattern of injury is usually hepatocellular affecting mostly young women. Approximately 1% of patients will develop severe liver injury leading to death or the need for LT. Acute liver failure study groups across the globe report that 20%-40% of ALF from DILI is a result of HDS use.Information on the proportion of patients developing DILI from HDS use is difficult to determine because the actual number of the users is not always clear.
Physicians should be aware of the typical clinical presentation of severe DILI from HDS use. It is characterized by a female predominance and an acute hepatocellular injury with markedly elevated serum aminotransferases and bilirubin. Most patients are otherwise healthy, hence HDS use should be suspected particularly in this group.Severe HDS induced liver injury is prone to unintentional re-challenge, and portends a greater risk of death or need for LT. An accurate history of HDS use can be difficult to obtain, in particular in patients that are taking multiple HDS products simultaneously or intermittently. Interactions between common drugs and HDS can also occur, increasing the possibility of toxicity. HDS are also susceptible to adulteration, contamination, rebranding or mislabeling which can further delay the diagnosis and prompt discontinuation of the incriminating HDS product in patients presenting with acute liver injury. Heightened awareness among physicians is crucial in reducing severe liver injury and liver failure from HDS.
 World Journal of Gastroenterology2019年46期
World Journal of Gastroenterology2019年46期
- World Journal of Gastroenterology的其它文章
- Long non-coding RNA HULC as a diagnostic and prognostic marker of pancreatic cancer
- Tailored eradication vs empirical bismuth-containing quadruple therapy for first-line Helicobacter pylori eradication: A comparative,open trial
- Mesenterico-portal vein invasion should be an important factor in TNM staging for pancreatic ductal adenocarcinoma: Proposed modification of the 8th edition of the American Joint Committee on Cancer staging system
- Clinical relevance of fluorodeoxyglucose positron emission tomography/computed tomography and magnifying endoscopy with narrow band imaging in decision-making regarding the treatment strategy for esophageal squamous cell carcinoma
- Transumbilical enterostomy for Hirschsprung's disease with a twostage laparoscopy-assisted pull-through procedure
- Two-week bismuth-containing quadruple therapy and concomitant therapy are effective first-line treatments for Helicobacter pylori eradication: A prospective open-label randomized trial
