Impact of regular enteral feeding via jejunostomy during neo-adjuvant chemotherapy on body composition in patients with oesophageal cancer
Imran M Mohamed,John Whiting,Benjamin HL Tan
Abstract
Key words: Body composition; Neo-adjuvant therapy; Oesophageal cancer; Enteral feeding
INTRODUCTION
Oesophageal cancer is the sixth most common cause of cancer death in the United States[1].Neo-adjuvant chemotherapy is now the mainstay of treatment prior to oesophagectomy and is associated with improved survival compared to surgery alone[2].However,use of such therapy places additional nutritional burden on patients and has an adverse impact on nutritional status and body composition as measured by the proportions of skeletal muscle and fat[3].Continued improvement in overall survival has led to increased focus on optimising patients' nutritional status throughout the whole continuum of their management.
阿里很久没见到母亲了,阿里只知道母亲在睡觉。他不明白,母亲为什么老是睡觉。他想不明白,也问不出来。虽然每天早上他去东湖放录音,听母亲的声音,但那到底不是母亲。没有母亲温热的手掌,也没有母亲的笑声,更没有母亲每天跟他说这说那,给他好吃的东西。这个世界跟以前不一样了。母亲一直在睡觉,阿里竟也一直都不进她的房间。阿里不能吵她。
Patients with oesophageal cancer have complex nutritional needs.Such patients often experience the onset of a catabolic state induced by the malignancy as well as tumour-associated dysphagia.The use of multiple strategies to manage disease progression including radiation,chemotherapy and surgical resection present an additional nutritional burden to these patients who are often malnourished at presentation.Adequate nutritional reserves are essential to allow patients to tolerate neo-adjuvant chemotherapy as well as subsequent surgery.Malnourishment and sarcopenia are well documented phenomena in oesophageal cancer.Local tumour effects,dysphagia,loss of appetite,physical inactivity as well as psychological factors all impair dietary intake and serve to compound cancer cachexia[2-4].Malnourishment reduces the potential response of the malignancy to chemoradiotherapy and impairs the patient's ability to tolerate the full course of treatment.Nutritional deficiencies may also contribute to increased perioperative morbidity and mortality.
There are various indicators of nutritional status that predict an individual's ability to tolerate neo-adjuvant chemotherapy and surgery for oesophageal cancer.Lower body mass index (BMI),skeletal muscle depletion,sarcopenia and sarcopenic obesity are associated with dose-limiting toxicity (DLT) during treatment with neo-adjuvant chemotherapy[4-6].Treatment with neo-adjuvant chemotherapy is also associated with changes in body composition.Indeed,Awadet al[7]showed a decrease in fat free mass and increased prevalence of sarcopenia in their observational study after treatment with neo-adjuvant chemotherapy.Tsujimotoet al[8]recorded statistically significant decreases in body weight and serum total protein in patients who received neoadjuvant chemotherapy.Toxicity from neo-adjuvant chemotherapy can also lead to further malnutrition and weight loss.Thus,further changes in body composition can affect tolerability of subsequent treatment,which in turn relates to poorer patient outcomes[3,9].
The period preceding surgery is the ideal time to ensure patients are in the best possible state for surgery.Optimisation of nutrition through nutritional screening and supplementation is an important consideration in the overall peri-operative management of oesophageal cancer,including during treatment with neo-adjuvant chemotherapy.
The use of enteral feedingviajejunostomy,particularly in oesophageal cancer,is a reliable method to optimise nutrition.The associated risks of insertion do not significantly outweigh the conferred benefits[8,10,11].Previous studies of feedingvialaparoscopically inserted jejunostomy prior to neo-adjuvant chemotherapy demonstrated an increase in weight ranging from 0.4 to 11.8 kg[2].The present study is the first to our knowledge to examine the changes of body composition from regular enteral feedingviajejunostomy during neo-adjuvant therapy for oesophageal cancer[2].
The aim of this study is to examine the effect of regular enteral feedingviajejunostomy on overall body composition in a cohort of patients with oesophageal cancer undergoing neo-adjuvant chemotherapy prior to oesophagectomy.The effect of regular jejunostomy feeding on the development of DLT during neo-adjuvant chemotherapy was also examined.
MATERIALS AND METHODS
Study population
Patients having potentially curative,locally advanced oesophageal and oesophagogastric junctional cancer without evidence of metastasis presenting to the Queen Elizabeth Hospital,Birmingham Upper Gastrointestinal Multidisciplinary Team between March 2014 and June 2017 were considered for this study.All patients were routinely staged with a combination of computed tomography (CT),endoscopic ultrasound and laparoscopy according to the International Union Against Cancer system[12].Those with locally advanced disease (T2 and greater and/or locoregional lymphadenopathy) and with sufficient physiological reserve to tolerate neo-adjuvant chemotherapy were further examined retrospectively for suitability for inclusion into the present study.
All patients included in this study were reviewed by a Consultant Oesophagogastric Surgeon after diagnosis and were assessed to have a dysphagia score of 3 (the ability to swallow liquids only) or 4 (complete dysphagia)[13].These patients had a feeding jejunostomy inserted at the time of staging laparoscopy prior to commencement of neo-adjuvant chemotherapy.
All patients were assessed by a dietician prior to starting regular jejunostomy feeding.After assessment by the dietitian,each patient had the feeding regimen tailored to their individual nutritional needs.The Henry equation[14]was used to estimate basal metabolic rate with 10% added as a stress factor during chemotherapy.Each individuals' activity factor was also considered.Based on these,the calorie intake required per day was calculated.The feeding regiment aims to provide 0.17-0.2 g nitrogen/kg/d.Each individuals' feeding regiment takes into account the amount of oral diet achieved.The feeding regimen aimed to provide 10-12 hr overnight continuous feeding if possible,to limit disruption of daytime activities.All patients were maintained on regular feeding daily throughout their period of receiving neoadjuvant chemotherapy.
Anthropometric measurements
Weight and height were recorded according to standard methods.Weight was measured with a medical balance beam scale,and height was measured with a stadiometer.BMI was calculated [weight (kg)/height (m2)].
Image analysis
CT has proven to be accurate for measuring human body composition[15,16].Regional muscle tissue was measured by CT from electronically stored images,which had been done previously for diagnostic purposes.CT scans were performed at two time points: The first at diagnosis prior to commencement of chemotherapy and the second after completion of neo-adjuvant chemotherapy prior to surgery (Figure 1).
The third lumbar vertebra (L3) was chosen as a landmark,and two consecutive slices were assessed to measure cross-sectional area of muscle and adipose tissue as described[17].The average value of two images was computed for each patient.Images were analysed using Slice-O-Matic software V4.3 (Tomovision).Cross sectional area for muscle and adipose tissue was normalized for stature (cm2/m2) and reported.Patients were classified as sarcopenic according to established cut offs: L3 muscle index < 41 cm2/m2for women and < 43 cm2/m2for men with a BMI < 25 or L3 muscle index < 53 cm2/m2for men with a BMI > 25[18].The mean Hounsfield unit measurement of all skeletal muscle within the L3 cross-section was recorded as a measure of myosteatosis,which was defined operationally as a mean skeletal muscle radiodensity of < 33 Hounsfield unit in those with a BMI > 25; and < 41 Hounsfield unit in those with a BMI < 25 across the axial orthogonal view[18].
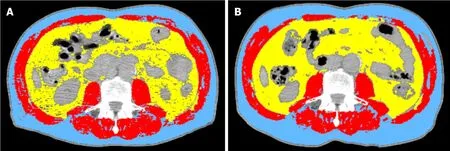
Figure 1 Radiologic image showing a comparison of body composition at diagnosis (A) and post neo-adjuvant (B) chemotherapy (two computed tomography scans of the third lumbar vertebra skeletal mass) for the same patient.
DLT
DLT was defined by intolerable toxicities requiring the postponement of treatment,a drug dose reduction or definitive interruption of drug administration.
Statistical analysis
Data were recorded as mean ± standard deviation unless otherwise stated.Comparison between values at diagnosis and post neo-adjuvant chemotherapy were calculated using the pairedt-test or McNemar's test.AP-value less than 0.05 was considered to be statistically significant.Statistical analysis was done using SPSS 15.0 statistical package (SPSS Inc.).
其中,Qxj为地类j中栅格x的生境质量;Hj为地类j的生境属性,当研究仅为宏观角度时,一般采用二分法定义该值,即土地利用类型j为生境时取1,否则取0[21];k为半饱和参数;Dxj为土地利用类型j中栅格x的生境退化水平。
RESULTS
During the study period,15 patients underwent regular jejunostomy feeding during neo-adjuvant chemotherapy prior to surgery.Demographic data of the 15 patients are shown in Table 1.The mean age was 61.3 ± 12.8 years,and 73% of patients were male.
The time interval between start of chemotherapy and surgery was 107 ± 21.6 d.During this period,there was no change in weight and BMI (Table 2).However,body composition analysis revealed a statistically significant loss of skeletal muscle despite regular feeding during neo-adjuvant chemotherapy.Lumbar skeletal muscle index decreased from 45.8 ± 8.0 cm2/m2to 43.5 ± 7.3 cm2/m2(P= 0.045).Adipose tissue index remained largely unchanged (Figure 2).The number of sarcopenic patients increased from five (33.3%) at diagnosis to nine (60%) after completion of neoadjuvant chemotherapy.There was no change in the number of myosteatotic patients before and after neo-adjuvant chemotherapy (Table 2).
Table 3 and Figure 3 show the individual values for changes in lumbar skeletal muscle index (cm2/m2) from diagnosis to after neo-adjuvant chemotherapy following regular enteral feedingviajejunostomy.Patients 4 and 8 showed an increase in lumbar skeletal muscle index between diagnosis and after neo-adjuvant chemotherapy.It is interesting to note that both patients were sarcopenic at diagnosis but were no longer sarcopenic after neo-adjuvant chemotherapy.
Table 4 and Figure 4 show individual values for changes in lumbar adipose tissue index (cm2/m2) from diagnosis to after neo-adjuvant chemotherapy following regular enteral feedingviajejunostomy.Patients 2,3,5,6,7 and 9 showed an increase in lumbar adipose tissue index from diagnosis to after neo-adjuvant chemotherapy.The greatest increases were seen in patients 2 and 9.None of these patients were sarcopenic at diagnosis,however patients 2,3,6 and 7 became sarcopenic after neoadjuvant chemotherapy.
Six patients (40%) experienced DLT during chemotherapy.Three patients required a dose reduction,two patients stopped chemotherapy early and one patient experienced a delay in treatment.

Table 1 Patient demographics
DISCUSSION
This observational study of 15 patients undergoing neo-adjuvant chemotherapy prior to oesophagectomy confirmed that formal nutritional intervention provided through the use of feeding jejunostomy had a positive effect on maintaining mean overall weight and BMI.
In concordance with previous studies,this study revealed a small amount of weight gain (0.35 ± 3.0 kg) after regular jejunostomy feeding,though this was negligible in the current study.Interestingly,adipose tissue was maintained but a significant loss in muscle mass remained.The incidence of sarcopenia increased from 33% of patients to 60% of patients after completion of neo-adjuvant chemotherapy.
The percentage of patients (40%) experiencing DLT whilst undergoing neoadjuvant chemotherapy was similar to a previous study despite regular jejunostomy feeding[5].This can perhaps be explained by the high prevalence of sarcopenia in oesophageal cancer patients despite regular feeding.Whilst the mechanism that links sarcopenia with increased chemotherapy toxicity in patients is unknown,this association is described in the literature[3,6,7].
Sarcopenia is prevalent in patients diagnosed with oesophageal cancer,and the incidence increases following neo-adjuvant chemotherapy.This is coupled with an overall decrease in body weight,BMI,fat mass and fat-free mass.BMI is often used as an objective marker to assess patients' nutritional status.However,patients can be acutely malnourished and still have a normal or elevated BMI,especially with the increasing prevalence of obesity.Therefore,assessment of specific changes in body composition is perhaps more important than overall changes in weight or BMI.
One of the strengths of this study was the use of the standardised technique of using CT imaging to measure muscle mass using the skeletal muscle index at the level of the L3 vertebra.This has been proven to be a well-established technique for this purpose.Although magnetic resonance imaging and dual energy x-ray absorptiometry are also considered extremely useful to evaluate body composition in clinical practice,CT is performed in all patients with oesophageal cancer.Therefore,analysis of these images is easily available with no additional cost or patient burden[9].
Sarcopenia,myosteatosis and loss of skeletal muscle during neo-adjuvant treatment have been shown to be associated with worse oncological outcomes in surgically treated oesophageal cancer patients[3,7,9].In sarcopenic patients,the doses of neoadjuvant therapy may have to be adjusted and even reduced in order to compensate for toxicity associated complications.If the development of sarcopenia can be minimised,patients may benefit not only from longer courses of neo-adjuvant therapy,but the documented poorer outcomes may also be averted.It would therefore seem logical to attempt to maintain or improve skeletal mass during neoadjuvant chemotherapy.
The use of feeding jejunostomy tubes in this cohort of patients has been shown to be safe.Although the current study did not specifically examine patient tolerance of feeding jejunostomy or complications associated with insertion,a systematic review examining nutritional optimisation in these patients reported no 30-d postprocedural complications in patients undergoing laparoscopically inserted feeding jejunostomy tubes.All such patients showed an increase in overall weight,and available evidence suggests that greater than 90% of patients completed their neo-adjuvant treatment[2].In light of the relatively few and minor complications associated with jejunostomy,it would make sense to employ this as a feeding technique over nasogastric tubes,which are prone to easily dislodge.The question of whether placement of jejunostomy would increase difficulty of the oesophagectomy is valid.However,with laparoscopic insertion of jejunostomy tubes,intra-abdominal adhesions should be kept to a minimum,and the benefit of the tube to the patient would lend further support to placement of these tubes.Indeed,the jejunostomy tube may aid with early nutrition post-oesophagectomy.
A randomised study has suggested that enteral feed enriched with eicosapentaenoic acid,a compound that modulates the immune system and reduces catabolism in advanced cancer,may contribute to maintaining fat-free mass[19].However,further research is required to determine the effect of such immunoenhanced nutrition on overall clinical outcomes in patients with oesophageal cancer.
There are several limitations in the current study.One being the relatively small sample size.There is also heterogeneity in the type of chemotherapy used,which may be a contributory factor towards the changes in body composition.Other factors that may affect changes in weight such as hypoalbuminaemia,oedema and other comorbidities were not specifically examined in this study population.However,by the time of diagnosis,patients with oesophageal cancer are often already suffering from malnutrition and involuntary weight loss with sarcopenia being a major marker of frailty.Thus,addressing malnutrition pre-operatively is a major area to target for pre-optimisation before surgery[4].
It would also be useful to examine data on patients who did not receive feedingviafeeding jejunostomy and especially those who were maintained on an oral diet.This would allow comparison with the current study group in order to measure any differences in outcome between the two groups and therefore would contribute to more detailed assessment of the effect of feeding in this group of patients.Nevertheless,this study has shown that regular jejunostomy feeding during neoadjuvant chemotherapy can attenuate the previously shown loss in overall weight and adipose tissue.It is clear,however,that feeding alone is not sufficient to maintain muscle mass in this group of patients.Further insight into the underlying processes causing reduced muscle mass in cancer patients may help to provide targeted interventions.
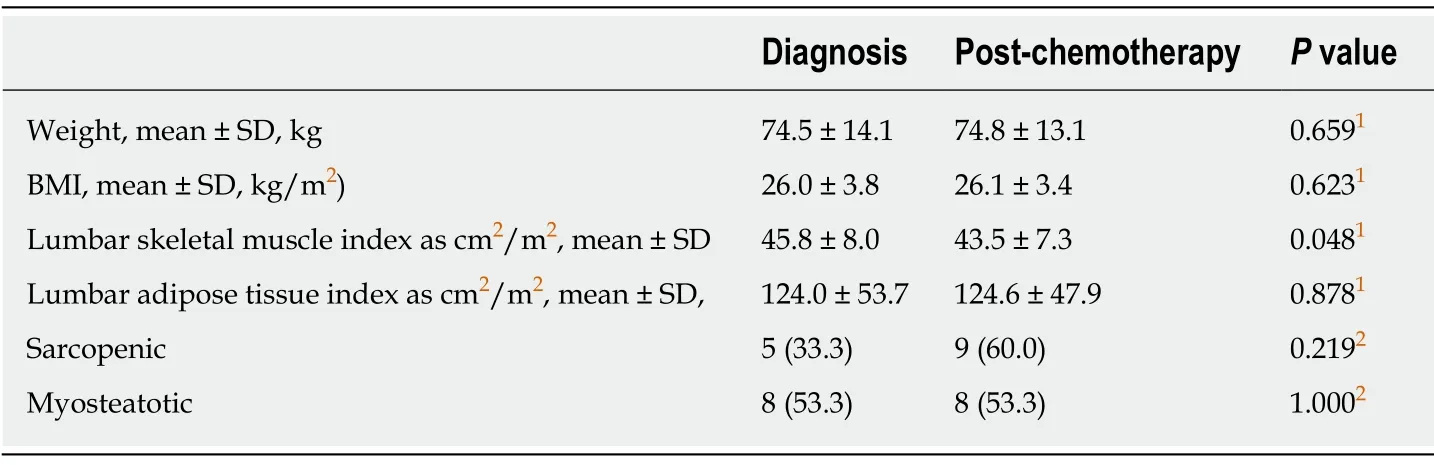
Table 2 Change in body composition from diagnosis to post neo-adjuvant chemotherapy following regular enteral feeding via jejunostomy,n = 15

Figure 2 Overall change in body composition from diagnosis to after neo-adjuvant chemotherapy.
In the meantime,there is evidence that physical exercise and resistance training has proven efficacy in catabolic conditions including sarcopenia and is advocated as a nonpharmacological intervention in cancer-related skeletal muscle wasting.Interestingly,resistance training during adjuvant breast cancer treatment has been reported to reverse sarcopenic status and lead to higher chemotherapy completion rates[20,21].Therefore,the potential for such interventions to attenuate DLT in oesophageal cancer warrants further investigation.
This study has shown that nutritional supplementation does serve to maintain overall weight and BMI.Looking more specifically at body composition,skeletal muscle mass decreased despite this nutritional support,and the proportion of sarcopenic patients increased.It is the authors' opinion that multimodal intervention incorporating a combination of regular nutritional support and exercise during the period of neo-adjuvant chemotherapy may lead to improvement in treatment tolerance and optimising surgical candidacy in patients with oesophageal cancer.This would be best investigated by means of a prospective multi-centre,multi-arm randomised controlled trial.

Table 3 Individual values for changes in lumbar skeletal muscle index (cm2/m2) from diagnosis to post neo-adjuvant chemotherapy following regular enteral feeding via jejunostomy

Table 4 Individual values for changes in lumbar adipose tissue index (cm2/m2) from diagnosis to after neo-adjuvant chemotherapy following regular enteral feeding via jejunostomy
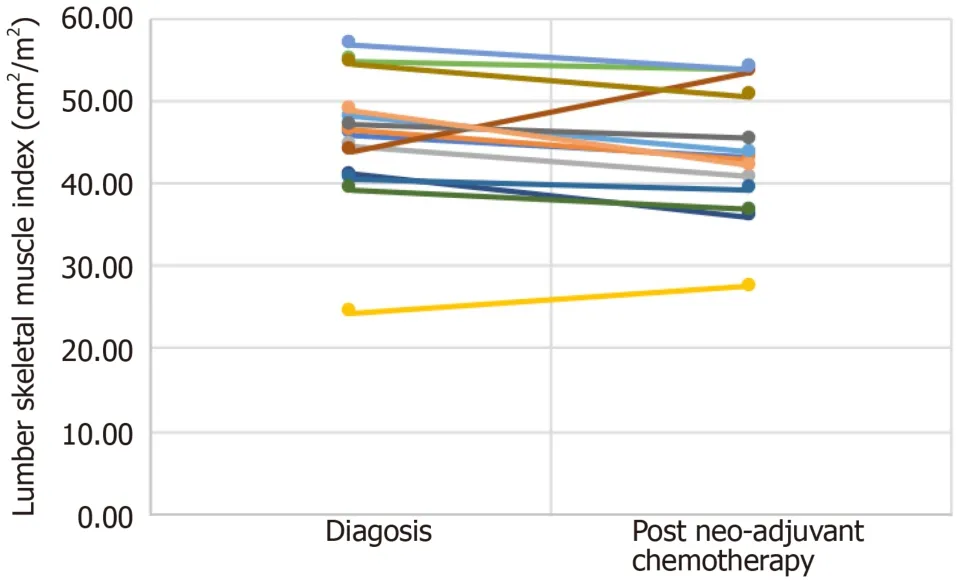
Figure 3 Individual changes in lumbar skeletal muscle index (cm2/m2) from diagnosis to after neo-adjuvant chemotherapy following regular enteral feeding via jejunostomy.

Figure 4 Individual changes in lumbar adipose tissue index (cm2/m2) from diagnosis to after neo-adjuvant chemotherapy following regular enteral feeding via jejunostomy.
ARTICLE HIGHLIGHTS
Research background
Malnourishment and sarcopenia are well documented phenomena in oesophageal cancer.Neoadjuvant chemotherapy is now the mainstay of treatment prior to oesophagectomy and is associated with improved survival compared to surgery alone.Patients undergoing neoadjuvant chemotherapy prior to oesophagectomy have complex nutritional needs.The use of multiple strategies to manage disease progression including radiation,chemotherapy and surgical resection present an additional nutritional burden to these patients who are often malnourished at presentation.
Research motivation
There are various indicators of nutritional status that predict an individual's ability to tolerate neo-adjuvant chemotherapy and surgery for oesophageal cancer.Lower body mass index (BMI),skeletal muscle depletion,sarcopenia and sarcopenic obesity are associated with dose-limiting toxicity during treatment with neo-adjuvant chemotherapy.The period preceding surgery is the ideal time to ensure patients are in the best possible state for surgery.The use of enteral feeding via jejunostomy,particularly in oesophageal cancer,is a reliable method to optimise nutrition.The associated risks of insertion do not significantly outweigh the conferred benefits.The present study is the first to our knowledge to examine the changes of body composition from regular enteral feeding via jejunostomy during neo-adjuvant therapy for oesophageal cancer.
Research objectives
The aim of this study is to examine the effect of regular enteral feeding via jejunostomy on overall body composition in a cohort of patients with oesophageal cancer undergoing neoadjuvant chemotherapy prior to oesophagectomy.The effect of regular jejunostomy feeding on the development of dose-limiting toxicity during neo-adjuvant chemotherapy was also examined.
Research methods
Patients having potentially curative,locally advanced oesophageal and oesophago-gastric junctional cancer without evidence of metastasis were considered for this study.All patients were routinely staged with a combination of computed tomography (CT),endoscopic ultrasound and laparoscopy according to the International Union Against Cancer system.All patients were assessed by a dietician prior to starting regular jejunostomy feeding.After assessment by the dietitian,each patient had the feeding regimen tailored to their individual nutritional needs.Weight and height were recorded according to standard methods.CT has proven to be accurate for measuring human body composition.Regional muscle tissue was measured by CT from electronically stored images,which had been done previously for diagnostic purposes.CT scans were performed at two time points: The first at diagnosis prior to commencement of chemotherapy and the second after completion of neo-adjuvant chemotherapy prior to surgery.The third lumbar vertebra was chosen as a landmark,and two consecutive slices were assessed to measure cross-sectional area of muscle and adipose tissue as described.The average value of two images was computed for each patient.
Research results
During the study period,15 patients underwent regular jejunostomy feeding during neoadjuvant chemotherapy prior to surgery.The time interval between the start of chemotherapy and surgery was 107 ± 21.6 d.During this period,there was no change in weight and BMI.However,body composition analysis revealed a statistically significant loss of skeletal muscle despite regular feeding during neo-adjuvant chemotherapy.Lumbar skeletal muscle index decreased.Adipose tissue index remained largely unchanged.
Research conclusions
This observational study of 15 patients undergoing neo-adjuvant chemotherapy prior to oesophagectomy confirmed that formal nutritional intervention provided through the use of feeding jejunostomy had a positive effect on maintaining mean overall weight and BMI.
Research perspectives
This study has shown that nutritional supplementation does serve to maintain overall weight and BMI.Looking more specifically at body composition,skeletal muscle mass decreased despite this nutritional support,and the proportion of sarcopenic patients increased.It is the authors'opinion that multimodal intervention incorporating a combination of regular nutritional support and exercise during the period of neo-adjuvant chemotherapy may lead to improvement in treatment tolerance and optimising surgical candidacy in patients with oesophageal cancer.This would be best investigated by means of a prospective multi-centre,multi-arm randomised controlled trial.
 World Journal of Gastrointestinal Oncology2019年12期
World Journal of Gastrointestinal Oncology2019年12期
- World Journal of Gastrointestinal Oncology的其它文章
- Current status of the genetic susceptibility in attenuated adenomatous polyposis
- Inflammatory pseudotumor-like follicular dendritic cell sarcoma: A brief report of two cases
- Deep learning with convolutional neural networks for identification of liver masses and hepatocellular carcinoma: A systematic review
- Analysis of factors potentially predicting prognosis of colorectal cancer
- Multi-parameter ultrasound based on the logistic regression model in the differential diagnosis of hepatocellular adenoma and focal nodular hyperplasia
- Difference in failure patterns of pT3-4N0-3M0 esophageal cancer treated by surgery vs surgery plus radiotherapy
