Advances of experimental study on gas production from synthetic hydrate reservoir in China☆
Jingchun Feng,Longtao Sun,Yi Wang,Xiaosen Li,*
1 Institute of Environmental&Ecological Engineering,Guangdong University of Technology,Guangzhou 510006,China
2 South China Sea Institute of Oceanology,Chinese Academy of Sciences,Guangzhou 510301,China
3 CAS Key Laboratory of Marginal Sea Geology,South China Sea Institute of Oceanology,Chinese Academy of Sciences,Guangzhou 510301,China
4 Key Laboratory of Gas Hydrate,Guangzhou Institute of Energy Conversion,Chinese Academy of Sciences,Guangzhou 510640,China
5 Guangzhou Center for Gas Hydrate Research,Chinese Academy of Sciences,Guangzhou 510640,China
6 Guangdong Key Laboratory of New and Renewable Energy Research and Development,Guangzhou 510640,China
Keywords:Natural gas hydrate Experimental investigation Gas production Exploitation technology Sediment deformation
ABSTRACT China has entered the area of new normal economy which requires the harmonious development of energy consumption,environmental protection and economic development.Natural gas hydrate is a potential clean energy with tremendous reserve in China.The successful field test of marine hydrate exploitation in South China Sea created a new record of the longest continuous gas production from natural gas hydrate.However,the corresponding fundamental research is still urgently needed in order to narrow the gap between field test and commercial production.This paper reviewed the latest advances of experimental study on gas production from hydrate reservoir in China.The experimental apparatus for investigating the performance of hydrate dissociation in China has developed from one dimensional to two dimensional and three dimensional.In addition,well configuration developed from one tube to complicated multi-well networks to satisfy the demand of different production models.Besides,diverse testing methods have been established.The reviewed papers preliminary discussed the mechanical properties and the sediment deformation situation during the process of hydrate dissociation.However,most reported articles only consider the physical factor,the coupled mechanism of physical and chemical factor for the mechanical properties of the sediment and the sand production problem should be studied further.
1.Introduction
With the consuming and decrease of oil and conventional natural gas resources,researchers around the world commit themselves to seek new alternative energy sources.According to the BP Energy Outlook[1],natural gas will be the fastest-growing type of energy demand with the annual growth rate of 1.6%from 2015 to 2040,especially in the emerging economics such as China and India.Given the growing effects of energy crisis and environmental deterioration in China,as well as the vast amount of natural gas trapped in the solid form of natural gas hydrate(NGH),NGH has been paid great attention for the sake of sustainable development[2,3].
NGH is an ice-like solid compound which is stabilized when water and guest molecules contact in the conditions of high pressure and low temperature,such as the marine deposit and the permafrost regions[4].Methane,ethane,propane,nitrogen,hydrogen,etc.,are regarded as the guest molecules trapped in the cage constituted by water molecules.Methane is the most common guest gas in nature.It is commonly recognized that 1 m3of hydrate can accommodate 170 m3of natural gas.This huge storage capacity makes hydrate as a promising energy in the future[5].
Unlike the conventional gas and oil reserves,recovering natural gas from the NGH refers to hydrate dissociation process,as well as gas and water flow.This is a complex process which combined multi-phase change,heat transfer and mass transfer.Hydrate dissociation from insitu situation should break the stable pressure-temperature condition which NGH exists,and gas release from the NGH must overcome the van der Waals force between the water molecule and guest molecule[6].
During the past four decades,over 230 natural gas hydrate deposits have been found in our planet.If only 17%to 20%of this resource can be exploited,NGH can be a sufficient supply of energy source for at least 200 years[7].Field test of NGH exploitation plays an important role not only in improving production technology but also in assessing environmental and safety effects.Seven field tests of gas production from hydrate deposits have been conducted around the world since 2002.Four filed tests have been carried out in the permafrost region,and three tests have been performed in the marine environment.The main target test areas are(1)Alaska North Slope of the United States:hydrate dissociation by depressurization method and CO2replacement method have been applied in this area under the collaborations of the US government,ConocoPhillips,Japan Oil,Gas and Metals National Corporation and other research institutes[8,9].(2)Mallik Area in the Mac-Kenzie Delta of Canada [10,11]:hydrate dissociation by depressurization and heat stimulation methods have been carried out in this region with the cooperation of Japan,Canada,the United States,India and Germany.The highest gas production rate up to 3000 m3·d-1was obtained in the field tests of this area.(3)Nankai Trough of Japan [12]:the first marine hydrate exploitation test has been successfully conducted in this area by depressurization and about 120 thousand m3natural gas was extracted.However,this test was terminated by sand production in 2013.Additionally,another test was carried out in this region in 2017.About 35 thousand m3natural gas was produced from the first production well during 12 days,and 200 thousand m3natural gas was extracted from the second production well during 24 days[13].(4)Shenhu Area of South China Sea:60 days of continuous gas production from hydrate reservoir was successfully performed during May to July in 2017,and 309 thousand m3of natural gas was extracted,making a huge breakthrough of the exploitation technology in the sandy-clay hydrate reservoir.In addition,this successful gas production test makes it a new record of the longest stable gas production from the hydrate reservoir in the world[14].
Field tests play a crucial role in the development path from fundamental research to commercial exploitation for NGH.However,considering the drawbacks of huge cost,long preparation period,and risks of environmental damage,laboratory-scale simulation is indispensable for the in-depth study of exploitation technology.During the past decades,laboratory-scale simulation for hydrate dissociation evolved from one dimensional to three dimensional [15-19],and the corresponding experimental apparatus developed from several hundred milliliters [20]to more than 1000 L [21].Furthermore,parameter measuring method during hydrate dissociation process was changed from single-parameter monitoring[22]to joint monitoring of multi-parameters[23],which can satisfy the monitoring requirements of different dissociation methods.
In China,the research group at the University of Petroleum (UP)established the first experimental study plan at the early 1990s[24].Later on,Guangzhou Institute of Energy Conversion(GIEC)of Chinese Academy of Sciences(CAS)strengthened gas hydrate application and exploitation technology from the middle of the 1990s.Currently,gas hydrate exploitation research in China has entered a new stage under the encouragements and aids of the national policy.Strong supports were achieved under the funding of the national research projects such as the National Natural Science Foundation,the National Science and Technology Major Projects,the National Key Basic Research and Development Program(973),and the National High Technology Research and Development Program(863).Nowadays,there are more than twenty research groups studying the laboratory-scale gas hydrate exploitation issues.The main research institutes are the Institute of Oceanology,Chinese Academy of Sciences(IOCAS),the CNOOC Research Center,the China University of Petroleum-Beijing (CUPB),Dalian University of Technology(DUT),GIEC,Zhejiang University(ZJU),Qingdao Institute of Marine Geology,China Geological Survey(GIMG-CGS),et al.[25].
In this paper,the recent advances in the experimental study on gas production from hydrate reservoir in China were reviewed,including the outline of gas hydrate resources in China,the experimental devices in China,the dissociation methods,and the safety research.
2.Natural Gas Hydrate Resources in China
NGH in China mainly distributed in the Qilian Mountain Permafrost of the Northwest China,and the Maine sediment of the South China Sea according to current explorations.
2.1.Gas hydrate resources in permafrost areas
China has the world's third largest permafrost region.Based on the conditions of the ground temperature,temperature gradient,permafrost thickness,and measured gas components in Muri Coalfield,Qilian Mountain,the thermodynamic conditions of gas hydrate formation were calculated [26].Later on,the scientific drilling project of NGH was implemented by China Geological Survey in 2008-2009[27].As shown in Fig.1,this scientific drilling project is located in Muri Town,Tianjun Country,Qinghai province.Four scientific drilling wells(DK-1,DK-2,DK-3,and DK-4)were completed,and gas hydrate samples were achieved from wells DK-1,DK-2,and DK-3.This is the first discovery of NGH in the global low-middle latitude permafrost zone and in the China permafrost regions.Fig.2 displays that the samples are white crystal and easy burning.Measuring data shows that NGH in the Qilian Mountain Area has the feature of complicated gas component.Except high concentration of methane,the contents of ethane,propane,and other heavy hydrocarbon components are high as well.As shown in Table 1,comparing to NGH in the Mackenzie Delta,NGH in the Qilian Mountain has the features of complicated gas components,thin thickness of permafrost,and shallow buried depth[28].The characteristics of hydrate reservoir in Qilian Mountain are special,which is of great scientific value.
2.2.Gas hydrate resources in marine environment
Gas hydrate investigations have been conducted in the northern slope of South China Sea since 1990.Bottom simulating reflectors(BSRS)have been discovered in the Pearl River Mouth Basin,the deep slope of the east of Pearl River Mouth Basin,the Qiongdongnan Basin,and the Xiasha Trough[29,30].Later on,geochemical and geological surveys confirmed the existence of NGH in the northern slope of South China Sea[31].Since 2007,China's Geological Survey and Guangzhou Marine Geological Survey(CMG and GMS)have carried out four gas hydrate drilling expeditions in the South China Sea.The drilling tests of GMGS1,GMGS3,and GMGS 4 are located in the Shenhu Area of the Northern South China Sea.And GMGS 2 is situated in the Pearl River Mouth Basin,which is about 1 km northeast of GMGS1,GMGS3,and GMGS4.Large concentrations of disseminated gas hydrate in extremely fine-grained sediments were found in the GMGS1 expedition in 2007.The morphology of the discovered gas hydrate sample is pore-filling,which is unique from other known marine hydrate deposits[32].For the GMGS2 expedition,gas hydrate samples with 99%methane were obtained in 5 drilling sites.In addition,the drilling project in GMGS2 displays overlying authigenic carbonates,which confirms that the geologic condition in the drilling area is favorable for the occurrence of gas hydrate.The surprising findings in the GMGS2 drilling expedition is a milestone in the NGH researches and developments offshore China.
3.Experimental Reactors
Experimental reactor plays a core role in the experimental simulation of gas hydrate dissociation.Generally,hydrate simulators mainly include a high-pressure reaction system,low-temperature controlling system,measuring system,and data collecting system.By and large,a high-pressure reaction system consists of a high-pressure reactor,gas supply module,water supply module,and pressurizing module.A low-temperature controlling system is composed of a thermostatic chamber and temperature control module.A measuring system aims at monitoring the variations of pressure,temperature,gas production system,water production system,and other thermos-physical parameters during the process of hydrate dissociation[33].

Fig.1.Drilling cores containing gas hydrate and burning from the Scientific Drilling Project of Gas Hydrate in Qilian Mountain Permafrost.
A one-dimensional hydrate simulator was manufactured in the GIEC[34].Fig.2 shows that the inner diameter of this simulator is 30 mm,and the inner length is 534 mm.The whole device is immersed in an air bath to maintain temperature controllable.Four resistance thermometers and two pressure sensors are placed in the simulator to monitor temperature and pressure changes.As displaced in Fig.3,another one-dimensional visual experimental cell was established in the GIEC [37].The effective volume of this simulator is 3 L.
A two-dimensional hydrate simulator was set up in the China University of Petroleum(CUP).Fig.4 shows that this hydrate simulator is square.The length and width of this simulator are 350 mm,and the thickness is only 60 mm.16 groups of electrode and temperature probes are symmetrically distributed in the simulator to measure the changes of temperature and electrical resistivity during the process of hydrate formation and dissociation.Two pressure transducers are placed at the top and bottom of the simulator to monitor the pressure evolution.This two dimensional hydrate simulator can withstand working pressure as high as 16 MPa.
A middle-scale hydrate simulator was developed by Yang et al.[39]in CUPB.As shown in Fig.5,the core component of the experimental reactor is a cylindrical reactor with an inner diameter of 300 mm,and an inner height of 100 mm.The effective volume of this simulator is 7.065 L.In order to simulate the oceanic environment of hydrate-cap gas reservoir,the reactor was separated into the porous sediment region and the free gas region by a porous stainless steel board.Sixteen thermal couples are placed at different depths and radii to measure the three dimensional temperature distribution of the hydrate reservoir.
Another hydrate simulator was fabricated in Dalian University of Technology[40].As shown in Fig.6,the reaction tank of this simulator is also cylindrical with an internal diameter of 300 mm and a height of 70 mm.The effective volume of this simulator is 4.95 L.16 thermal resistances and 3 pressure transducers which can monitor the variations of pressure and temperature in time are placed in this apparatus.

Fig.2.Schematic of the one-dimensional hydrate simulator in GIEC[35].
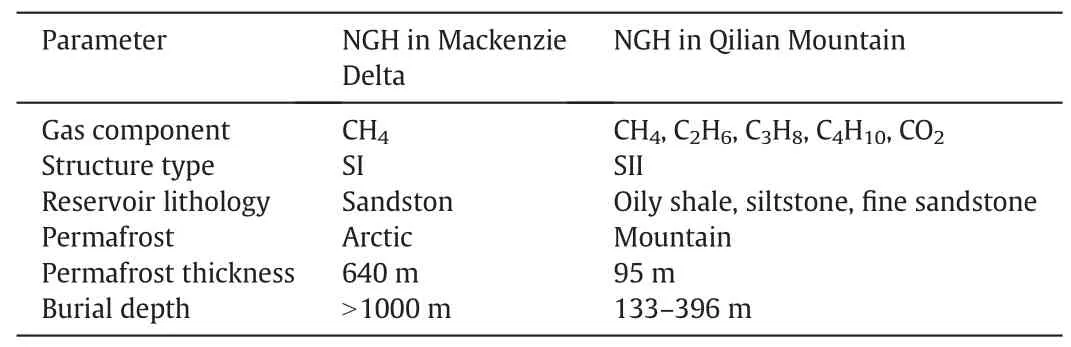
Table 1 Comparisons of NGH properties in the Mackenzie Delta and Qilian Mountain
Most of the reported hydrate simulators are one-dimensional or two-dimensional.However,the real hydrate reservoir in nature is three dimensional.For the sake of mimicking and studying hydrate dissociation behaviors in a three-dimensional hydrate reservoir,simulating the hydrate dissociation situation in a three-dimensional experimental apparatus is of significant importance.Therefore,Li et al.established a novel three dimensional hydrate simulator [41].As depicted in Fig.7,the shape of this hydrate simulator is cubic,with a length of 180 mm.The effective volume of this cubic hydrate simulator(CHS)is 5.8 L.The maximum working pressure of the CHS is 25 MPa,and it is immersed in a water bath to keep stable temperature.In order to capture the temperature distribution during hydrate dissociation,the inner space of the CHS is divided into 4 parts by 3 equidistant horizontal layers.There are 25 temperature measuring points and 12 resistance measuring points evenly distributed on each layer.
These above-mentioned hydrate simulators are small scale.However,the control mechanisms of hydrate dissociation in the laboratory-scale and field-scale hydrate reservoirs are different.Hydrate dissociation kinetics is the dominant factor influencing hydrate dissociation,while fluid flow mechanism mainly affects hydrate dissociation in the field-scale hydrate reservoir[42,43].Therefore,within the small scale hydrate simulator,it is difficult to predict the hydrate exploitation situation in the field scale or test the validity of the numerical simulation results in the field scale.In order to eliminate the boundary effect and get close to the real production environment,a large-scale hydrate simulator is indispensable.Li et al.manufactured the first large-scale hydrate simulator in China which is named as a pilot-scale hydrate simulator(PHS)[18].As shown in Fig.8,the PHS is cylindrical with an effective inner volume of 117.8 L.The maximum working pressure of the PHS is up to 30 MPa.In order to maintain the low temperature condition for hydrate existence,a dual temperature-control system of the circular water jacket and the walk-in cold room was designed.The inner space of the PHS is divided into four isometric parts by three horizontal layers.For the sake of monitoring the variations of temperature and electrical resistivity as accurate as possible,49 thermal couples and 49 electric probes are distributed evenly on each layer.9 horizontal wells and 9 vertical wells are placed in the PHS which makes it possible to investigate the different production performance of the vertical well and horizontal well for hydrate dissociation[44].In addition,a multiwell network in this system allows the experimental simulations of hydrate dissociation with the model of multi-well injection or production[45-47].
Despite these traditional hydrate simulators,some new hydrate simulators equipped with microcosmic measures have been reported.Zhang et al.[48]established a hydrate simulator with a nuclear magnetic resonance imager system.As displayed in Fig.9,this simulator is composed of a polyimide reactor with a maximum working pressure of 12 MPa.The diameter and height of the reactor are 15 mm and 200 mm,respectively.Quartz glass beads are tightly packed in the reactor to be performed as porous media.To observe and measure the process of methane hydrate formation and dissociation,1H was selected to be measured.And the nuclear magnetic resonance imager(NMR)system works at the resonance of 400 MHz and 9.4 T.
In general,the experimental apparatus for investigating the performance of hydrate dissociation in China has developed from one dimensional to two dimensional and three dimensional.In order to get close to the real environmental condition as much as possible,the volumes of the hydrate simulators were enlarged from few hundred milliliters to several hundred liters.In addition,well configuration in these hydrate simulators developed from one tube to complicated multi-well networks to satisfy the demand of different production models[49].Besides,diverse testing methods have been reported gradually.This is helpful for measuring more parameters and characterizing the dissociation behavior from different angles.However,with the development of hydrate research,the experimental research should be focused on the hydrate dissociation in a real condition.The anisotropic and heterogeneous conditions in the hydrate reservoir should be the bottleneck of the hydrate production research in the future.
4.Hydrate Exploitation Technology with Experimental Investigation
Hydrate exists as a solid form in the sediment under certain thermodynamic condition.Hence,the known methods of dissociating hydrate are based on shifting the thermodynamic equilibrium state of gas hydrate[50],which can be realized by depressurization,heat stimulation,inhibitor stimulation,CO2replacement,and the combined methods.
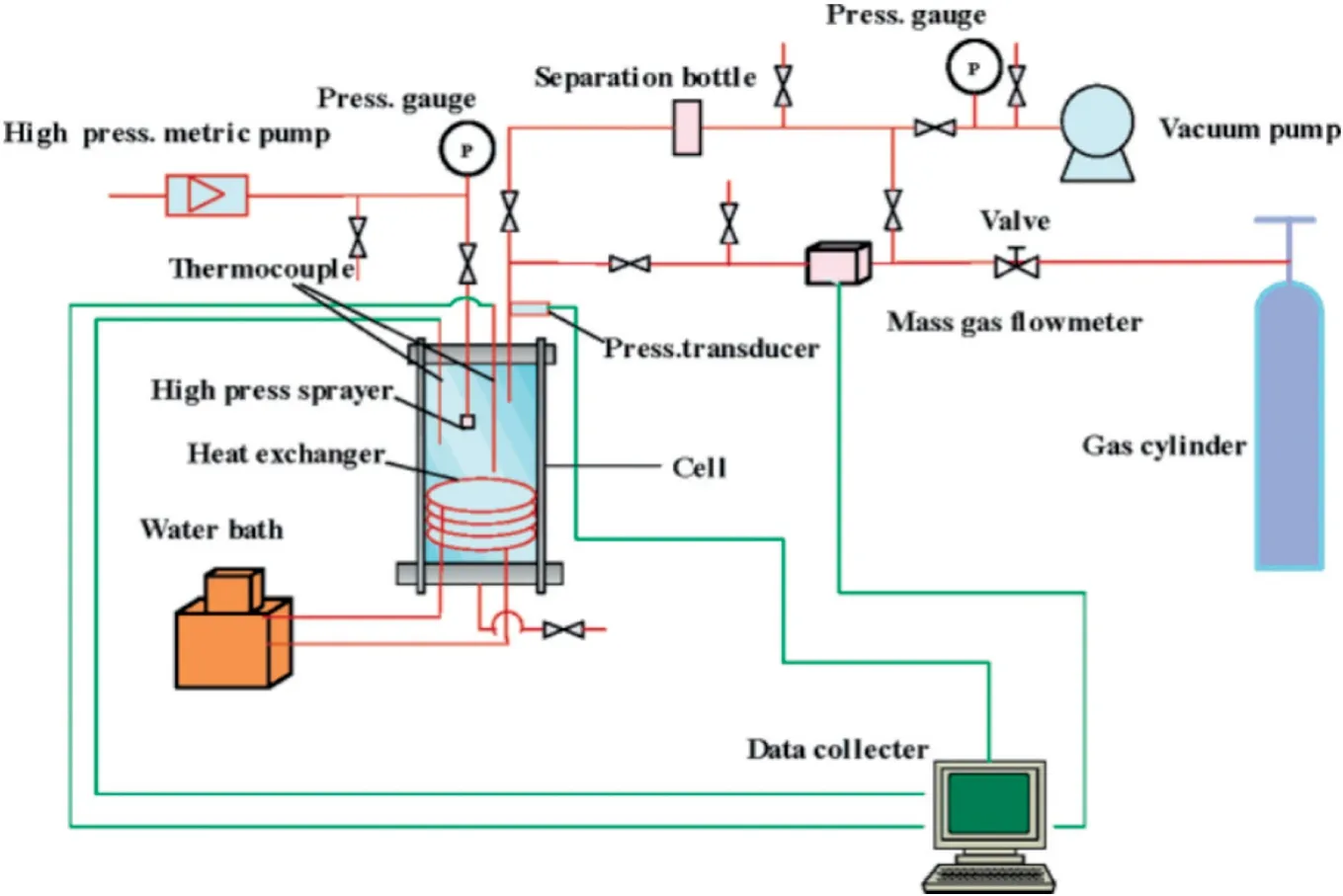
Fig.3.Schematic of the visual experimental cell for hydrate formation and dissociation in GIEC[36].
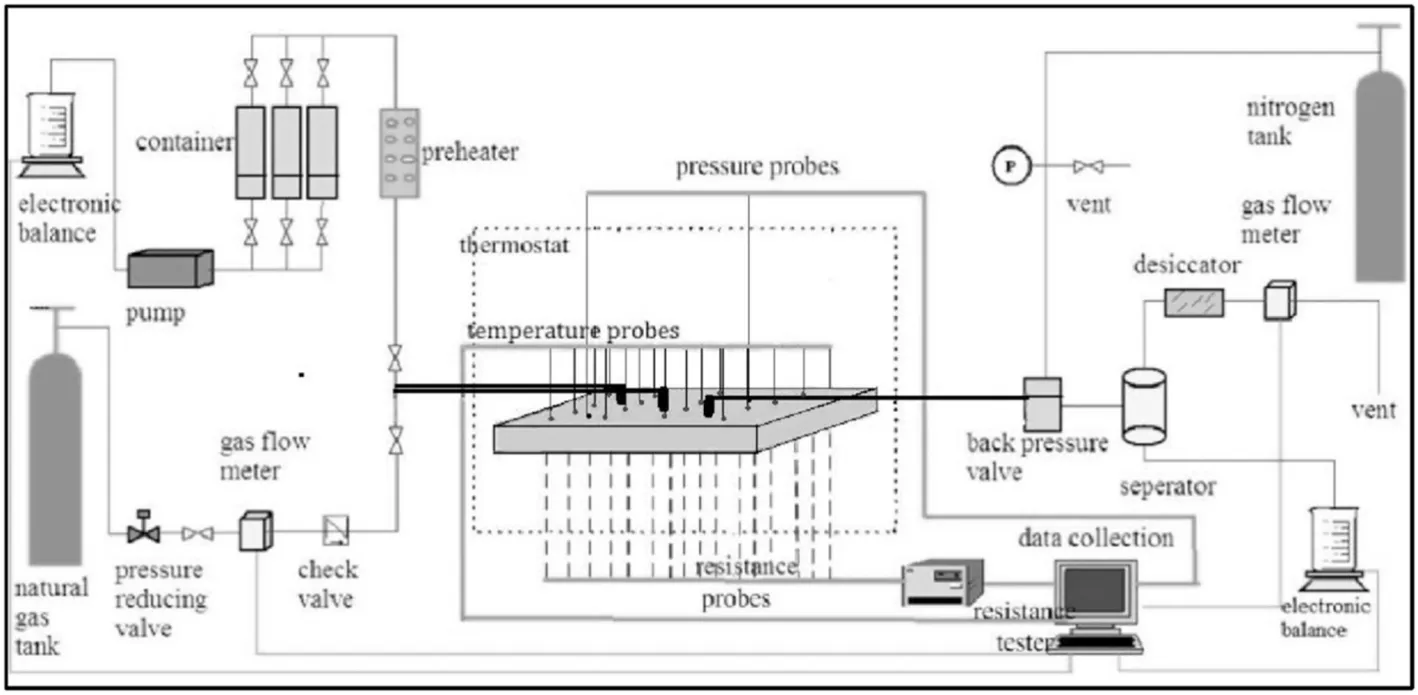
Fig.4.Schematic of the two-dimensional hydrate simulator in CUP[38].
4.1.Depressurization
Depressurization refers to declining the pressure in the sediment below the equilibrium hydration pressure at the prevailing temperature[51].Depressurization has the merits of low cost,easy operation,and no other additives import.Depressurization has been widely applied for hydrate dissociation in the laboratory simulation and field test.In China,hydrate dissociation by depressurization has been investigated in different scales of experimental reactors[52,53].
Hydrate dissociation was successfully conducted in a one-dimensional experimental apparatus by depressurization.Xiong et al.[54]divided the experimental process into free gas release stage,rapid dissociation stage,and gradual dissociation stage.In addition,they concluded that reducing the dissociation pressure can increase the hydrate dissociation rate and may negatively influence hydrate reformation.Furthermore,increasing hydrate saturation decreases the amount of hydrate dissociated during the rapid stage and allows more amount of hydrate to be dissociated in the slower stage.
Li et al.[55]also investigated the dynamic of hydrate dissociation via depressurization and its influencing factors in a one-dimensional reactor.Results showed that high permeability of the sand packs which hydrate formed is conductive for hydrate dissociation.However,it can also cause a fast decrease of pressure and temperature.
Wang et al.[56]analyzed the process of gas production from gas hydrate by depressurization in a hydrate simulator.Methane hydrate dissociation was carried out at three different working pressures and three different kinds of porous media.They founded that hydrate reformation and ice formation were always accompanied by hydrate dissociation.Sensible heat of the reservoir and heat transfer are both dependent on the working pressure,and these two factors play a dominant role in hydrate dissociation.
For hydrate dissociation by depressurization,the initial temperature and the sensible heat of the reservoir are of significant importance[57].The experimental investigations of Li et al.[41]in a CHS and Yang et al.[39]in a middle sized reactor indicate that depressurization is beneficial for hydrate dissociation when the sensible heat of the reservoir is sufficient for hydrate dissociation.However,during the later stage when the sensible heat of the reservoir is exhausted,gas production and hydrate dissociation with single depressurization method will be very slow.Other dissociation methods such as thermal stimulation may be required.
Initial free gas saturation is an important factor in determining hydrate dissociation rate.Both experimental and numerical simulations of hydrate dissociation in a low gas saturation system with depressurization were investigated by Li et al.[58].Not only the kinetic model but also the equilibrium model shows that gas hydrate dissociation is controlled by the heat and mass transfer in the PHS,and the kinetic limitations are very small.
Later on,Wang et al.[59]studied the behaviors of hydrate dissociation by depressurization below the quadruple point.It is interesting that ice formation occurs when methane hydrate dissociation is below the quadruple point and this in turn can strongly enhance hydrate dissociation rate.This is because hydrate dissociation can consume the latent heat released by ice formation.They further concluded that ice formation rate in the water saturated hydrate reservoir is slower than that in the gas saturated hydrate reservoir.

Fig.5.Schematic of the middle-size hydrate simulator in the CUPB[39].

Fig.6.Schematic of the hydrate simulator in DUT[40].
Wu et al.[60]studied the effect of rapidly depressurizing on methane hydrate dissociation below the ice point.Anomalous preservation was observed because the dissociation of methane hydrate at the temperature range from 261 to 266 K was much faster than that from 269 to 272 K.They also found that the size of the ice particles of methane hydrate formation may dominate the hydrate dissociation process.

Fig.7.Schematic of the three dimensional cubic hydrate simulator in GIEC[41].

Fig.8.(a)Schematic of the Pilot-scale hydrate simulator in GIEC,(b)Inner design and well distribution(c)Distributions of thermal couples and resistance ports[18].
Reservoir scale is a core element that governs hydrate dissociation behaviors.By comparing and studying the hydrate dissociation behaviors in three relative hydrate simulators which the inner volumes are scaling up,the model of scaling criterion for hydrate dissociation by depressurization has been developed[46].It is noteworthy that gas production rate in the depressurizing stage is in proportion to the inner volumes of the hydrate simulator.While in the constant depressurizing stage this model needs to be modified.This scaling model was verified and modified by the experimental data,and this model was further applied for predicting the gas production performance in a field scale hydrate reservoir.

Fig.9.Schematic of the hydrate simulator with magnetic resonance image.
To sum up,the reservoir conditions of gas-water-hydrate saturation[61],intrinsic permeability of hydrate reservoir,initial temperature and pressure of the reservoir[62]as well as the parameter condition of outlet pressure,depressurizing rate and depressurizing range[63]are important factors influencing the depressurization process and hydrate dissociation.The controlling factors of hydrate dissociation in different scales of hydrate simulator are different.Mechanism of secondary formation should also be further studied thoroughly[64].
4.2.Heat stimulation
The principles of heat stimulation are to heat the temperature of the hydrate bearing sediment above the hydrate equilibrium temperature and to provide sufficient heat for hydrate dissociation.Hot water injection and microwave stimulation are common heat stimulation methods for hydrate dissociation.The advantage of this method is that hydrate dissociation rate can be controlled by the heat supply model.
For hot water injection,studies of continuous injection and cyclic injection have been studied in detail by several groups.Yuan et al.[65]studied the hydrate dissociation behaviors with continuous injection model in a three-dimensional quiescent reactor.The authors evaluated the effects of injection temperature,injection rate,and the injected solution type on hydrate dissociation.The examined results show that an optimal injection temperature and an injection rate exist for hydrate dissociation.
Later on,Yang et al.[66]carried out the experimental simulation of hydrate dissociation by-hot water cyclic injection.Results showed that temperature in the reactor increases with hot water injection and decreases with gas production.Permeability and porosity of the hydratebearing samples affect temperature variation as well.Hydrate saturation and hydrate forming temperature have a positive effect on energy efficiency ratio.In contrast,the injection temperature and well pressure have negative effect.They also concluded that cyclic injection is beneficial for the field with the features of high hydrate saturation and low permeability.
Huff and puff method is another type of cyclic injection which is derived from the heavy oil industry.The single huff and puff process includes the injection stage,the soaking stage,and the production stage.The huff and puff method with two-spot well[67]and five-spot well[68]have been studied in detail.Results showed that under the experimental condition,heat conduction plays a more significant role than heat convection during the huff and puff process.Improving the injection rate of hot water can enhance the hydrate dissociation rate,shorten the production time,and decrease the water production volume,however it has little influence on the final gas production volume.The amount of the recovered gas can be improved by increasing the huff and puff cycle.
Parameter selection of thermal stimulation is important for hydrate dissociation.For example,increasing the injection temperature can improve the production rate but it is associated with more energy loss[69].In view of this problem,the principle of entropy production minimization based on the second law of thermodynamics was firstly introduced to the investigation of the optimal injected temperature for hydrate dissociation[70].As shown in Fig.10,the results of maximizing energy production and minimizing entropy production agree quite well.The injected temperatures for the maximum energy ratio and the minimize entropy production are 38.8°C and 37.9°C,respectively.Hence,under the experimental condition,the temperature range of approximately 38-39°C is suitable for hydrate dissociation,and the hot water injection beyond 39°C is uneconomical for hydrate dissociation.
Microwave stimulation is another heat supply kind for thermal stimulation.Experimental investigation of hydrate dissociation induced by microwave stimulation was conducted successfully by He et al.[71]in a laboratory scale.2.45 GHz microwave with the average radiation densities ranging from 3 to 19 kW·m-2was applied to heat the unconsolidated hydrate-bearing sediment from the South China Sea.Results demonstrated that although thickness of hydrate layer exceeds the microwave penetration depth,the far-field hydrate layer was dissociated at equilibrium.And they concluded that hydrate dissociation rate was controlled by the intrinsic kinetics when sufficient heat for hydrate dissociation can be supplied by the sediment itself.In addition,the model of Zhao et al.[40]further verified that microwave stimulation provided sufficient energy conversion for hydrate dissociation,and they also concluded that gas production rate and energy efficiency are affected by the initial water saturation,hydrate saturation and the thermal conductivity of the porous media.
4.3.CO2replacement
Gas recovery from hydrate bearing sediment by CO2replacement has attracted significant attention.Fig.11 gives the schematic diagram of hydrate dissociation by CO2replacement.As seen,this method can exploit clean energy and sequestrate and store CO2at the same time.Flue gas has been widely used for the replacement of gas hydrate.A micro-differential scanning calorimeter and the13C NMR method were employed for analyzing the replacement process[72].As shown in Fig.12,the preferential enclathration of N2molecules in small 512 cages of structure I hydrates improved the extent of the CH4recovery.Furthermore,reliable hydrate stability conditions and heat of dissociation values in the porous silica gels after the replacement were provided by a high pressure micro-differential scanning calorimeter.This verified that CH4in the flue gas was successfully replaced by CO2.

Fig.10.Evolutions of energy ration(a)and entropy production(b)in the system and the corresponding fitting function for runs 1-5 during the injection stage[70].
The experimental investigations of Wang et al.[73]and Li et al.[74]verified the formation of CO2hydrate and the dissociation of CH4hydrate by injecting gaseous CO2into the system containing CH4hydrate.Both of them found that the existence of free water in the system influences the replacement effect.In addition,the replacement rate in the early stage is fast,and declines remarkably overtime after several hours of CO2injection.The final replacement efficiencies are limited in these experiments which are lower than 15%.
In order to improve the production method of hydrate dissociation by CO2replacement,Ota et al.[75]studied the hydrate dissociation by introducing liquid CO2.The recovery ratio can reach about 37%after 307 h,which verifies that the replacement by liquid CO2is more efficient than that by gaseous CO2.They indicated that the replacement mechanism is that CO2molecular occupies the cages of the methane hydrate further.
Afterwards,Yuan et al.[76]studied the dynamics of the replacement of CH4in natural gas hydrate with liquid CO2in a three-dimensional reactor.They demonstrated that increasing water saturation is beneficial for increasing the replacement percent of methane hydrate;however,increasing hydrate saturation has a negative effect.The existence of underlying gas room is unbeneficial for the replacement process as well.
Not only liquid CO2,but also CO2emulsions were also introduced for the methane hydrate replacement[77].The authors performed that replacement experiment of methane from the hydrate in quartz sand with CO2emulsions and liquid CO2.The replacement results confirmed that the percentage of the displaced CH4by the CO2emulsions is 1.5 times larger than that by liquid CO2.This advantage of the emulsion is firstly caused by CO2hydrate formation,and secondly resulted from the CO2diffusion.
Li et al.carried out the investigation of the replacement of CH4from methane hydrate in NaCl solution by applying pressurized CO2solution[78].Results showed that high NaCl concentration had a negative effect for replacement,and high temperature was beneficial for the replacement.
In addition,gas production from fracture-filled hydrate in Muri Basin,Qilian Mountain by CO2replacement was investigated in a laboratory scale apparatus[79].The prepared hydrate samples are according to the reservoir condition of permafrost-associated gas hydrate in Muri Basin,Qilian Mountains.The ratio of CO2to N2was selected as 1:0,3:1,and 1:3,respectively.Results showed that rapid hydrate dissociation was observed when pressure was lower than the equilibrium pressure of CO2/N2binary hydrate and the equilibrium pressure of methane hydrate.The optimal ratio of CO2/N2is 3:1 to recovery fractured hydrate in Muri Basin,Qilian Mountain.
Researchers have applied a series of experimental investigations on methane hydrate dissociation by CO2replacement.The replacement rate,replacement efficiency,replacement mechanism and the corresponding influence factors are investigated.It is founded that the CO2emulsion is more efficient than liquid CO2,and liquid CO2has the advantage of higher replacement efficiency over gaseous CO2.
4.4.Inhibitor stimulation
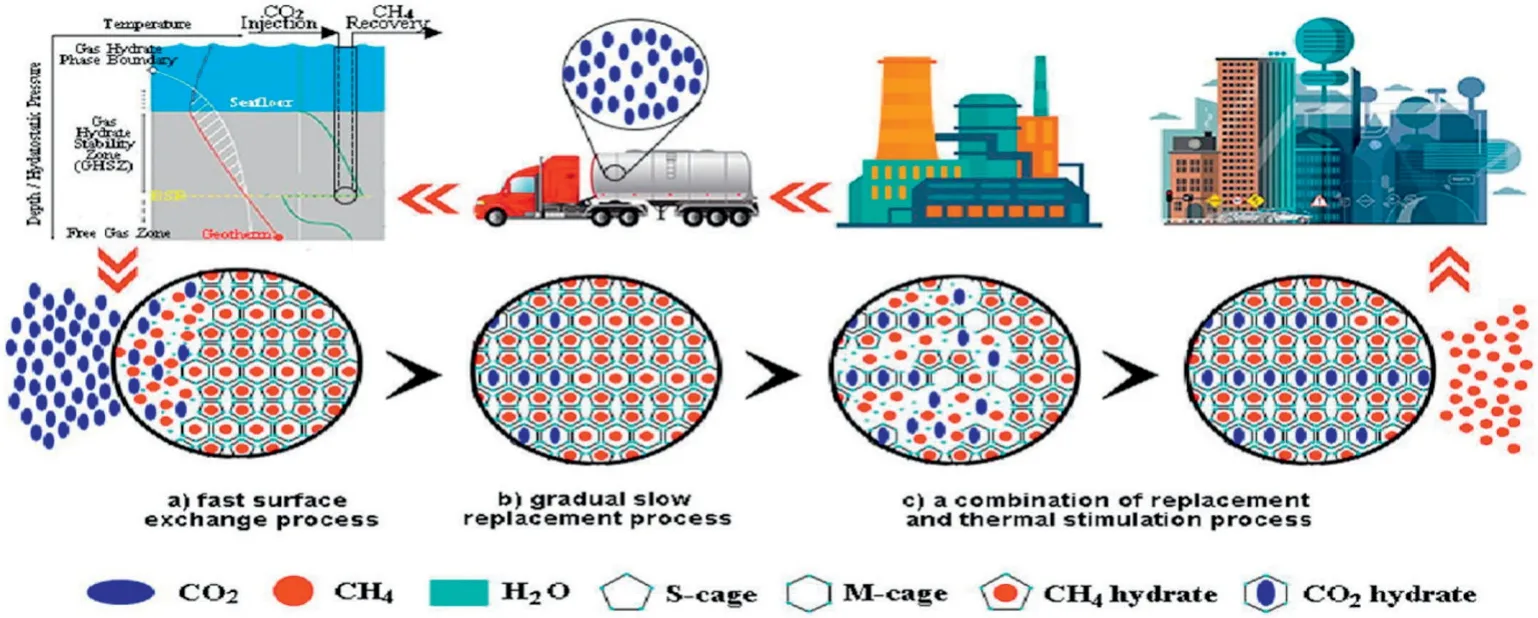
Fig.11.Schematic diagram of hydrate dissociation by CO2replacement.

Fig.12.(a)13C NMR spectra of the pure CH4hydrate before replacement and the CH4+CO2+N2hydrate after replacement.(b)Area ratio of CH4molecules in the large and small cages(AL/AS)after replacement[72].
Thermodynamic inhibitor stimulation induces hydrate dissociation by shifting hydrate equilibrium curve to the situation of higher temperature and lower pressure[80].Compared to hydrate dissociation by single depressurization,thermodynamic inhibitor stimulation may be more effective and it was founded that gas production can be increased by a factor of 4 with methanol injection[81].Ethylene glycol(EG)which has been widely applied in the gas and oil industries to prevent hydrate formation may be beneficial for hydrate dissociation.Production behavior of methane hydrate dissociation with EG injection in consolidated sediments was carried out by Li et al.in a one-dimensional hydrate simulator[35].They founded that gas production process can be divided into four stages of initial injection,EG dilution,hydrate dissociation,and remaining gas production.Gas production efficiency reached the maximum value when the EG concentration is 60 wt%.Fan et al.[36]further tested the behaviors of hydrate dissociation by EG injection in a visible cell.Results showed that hydrate dissociation rate depends on flow rate and concentration of EG.Later on,Yang investigated the gas production from hydrate-bearing sand by EG injection in a three-dimensional reactor [34],and they founded that gas production efficiency increases with increase of the EG concentration and the decline of the EG quantity.
Addition of chemical additives can shift equilibrium curve of gas hydrates to promote hydrate dissociation.For the additive type,on account of the low viscosity of NaCl solution and the higher surface charge density of Cl-,NaCl is more favorable of hydrate dissociation compared to EG and Na2SO4[65].
Production behavior of methane hydrate with brine injection in a one-dimensional was analyzed[82].The salinity of the injected brine ranges from 0 to 24 wt%,and the temperature range of the injected brine is-1 to 130°C.The authors founded that the flow of the injected brine can be regarded as the movement of a piston from the inlet to the outlet.The instantaneous hydrate dissociation rate,the energy ratio,and the thermal efficiency can be increased by raising the salinity of the injected brine.Nevertheless,this enhance effect is weak when the salinity is higher than a certain degree.
Subsequently,the effects of hydrate saturation on hydrate dissociation induced by brine injection with a two-dimensional reactor were investigated[38].Results showed that under the experimental condition,optimal production performance will be obtained when the hydrate saturation is 48%.When the hydrate saturation increases to 64%,the average gas production rate,movement of thermal front,and energy efficiency all decrease remarkably because of the high pressure which is caused by the Jamin effect and low permeability.
Reviewing these works,it can be found that hydrate dissociation performance by inhibitor stimulation is influenced by factors of the inhibitor type,the injected temperature,the injected rate,and the injected quantity.
4.5.Combined methods
Depressurization,thermal stimulation,and inhibitor stimulation each has its advantages and disadvantages.Several studies verified that the combination of these two or three methods can have a strong effect than the single method[83].
Depressurization in conjunction with thermal stimulation is the most common combined method.The experimental results of Wang et al.[84]indicated that thermal stimulation in conjunction with depressurization is superior to that by single depressurization or single thermal stimulation considering the aspects of gas production rate,gas recovery,thermal efficiency and energy efficiency.It is also be verified in the later experimental investigation that water injection can effectively shorten the hydrate dissociation rate although the injection temperature is equal to the reservoir temperature which means the sensible heat supplied by the injected fluid is limited[69].This is because water injection can enhance the heat convection rate in the reservoir and increase the hydrate dissociation rate further.
Additionally,hydrate dissociation experiments induced by depressurization in conjunction with warm brine stimulation have been carried out in a three-dimensional reactor[85].Different from the above studies,horizontal well is set as the well configuration in this work.The salinity in the reservoir decreases under the dilution effect caused by free water production and it increases gradually during the injection period.Hydrate dissociation rates with brine injection are larger than that with pure water injection.Moreover,hydrate dissociation rate and gas production rate increase when the injected brine increases from 3.5%to 10%.Energy analysis indicates that the promotion effect of heat stimulation on hydrate dissociation decreases when the injected brine is enhanced.Accordingly,the influence of depressurization on hydrate dissociation increases.However,these trends get reversed when the injected brine is higher than 10%.The authors confirmed that the optimal injected brine for hydrate dissociation is 10%under the experimental condition.
Depressurization combined with gas sweep can also be employed for hydrate dissociation[86].Pure N2was injected into the hydratebearing reactor to study the influence of gas sweep on methane hydrate dissociation.Results indicated that the driving force of hydrate dissociation increases with the increase of the N2mole fraction,which enhances hydrate dissociation rate.
In order to enhance CO2storage and CH4recovery,a combination model of CH4/CO2replacement and thermal stimulation was proposed[87].The authors analyzed the influences of the freezing point,the replacement zone,and the hydrate saturation level on the replacement behavior.Results suggested that the combined method can improve the CH4recovery effect,and the maximum CH4replacement percentage can reach a high level of 64.63%.They also found an interesting phenomenon that the total quantity of CO2stored is unequal to CH4recovered,which is due to the fact that the replacement process is sensitive to the free water in the pores of the hydrate sediments.
The combined method for hydrate dissociation not only incorporates the advantage of each single method,but also motivates a synergistic effect of heat and mass transfer in the reservoir,which can effectively improve the hydrate dissociation rate and gas production performance.
5.Safety Research for Hydrate Dissociation
Hydrate exists as a solid compound in the sediment.Inappropriate exploitation process will create huge environmental and safety problems,such as methane leak,sediment deformation,wellbore collapse,and seaquake.Hence understanding the safety of hydrate dissociation technology is of great importance.
Laboratory studies are useful for understanding the mechanical properties of gas hydrate reservoir.The mechanical properties of methane hydrate-ice mixtures are investigated in a laboratory test[88].The authors proposed a modified Mohr-Coulomb criterion considering the influence of methane hydrate content on shear strength.The experimental results show that increasing the confining pressure will significantly increase the failure strength,and the cohesion reduces with the increase of hydrate saturation.However,increasing the methane hydrate saturation plays a positive effect on the internal friction angle.
Then Zhang et al.studied the mechanical properties of hydrate-bearing silty clay containing tetrahydrofuran which is similar to that of the South China Sea[89].Results indicate that the shear strength increases linearly with the rise of confining pressure and hydrate saturation.In addition,the secant modulus increases when improving the hydrate saturation.Later on,Han et al.[90]observed the sediment deformation situation during hydrate dissociation in sandy and clay sediments.They found that due to the combined consequence of the cementation effect of hydrate and gas seepage,radial shrinkage effect of hydrate dissociation was observed in both the sandy sediment and the clay sediment.
Gas hydrates commonly existed in the shallow sedimentary zone where water depth is deeper than 500 m.Many problems such as gas blowout,open-hole enlargement and wellbore collapse may occur during the drilling process associated with hydrate dissociation and gas invasion into the wellbore.Lu et al.[91]quantify the effects of hydrate dissociation to drilling risks in a special experimental apparatus.Results show that the rates of hydrate dissociation and gas production are greatly influenced by the temperature of drilling fluid,hydrate saturation and pressure.While hydrate zones only have modest and controllable risks to drilling operation if careful control of various drilling parameters is implemented.
6.Conclusion and Prospect
Natural gas hydrates are considered as potential clean energy resources which has huge reserves.Exploiting natural gas hydrate safely and effectively is of great strategic significance for China.In this paper,the authors reviewed the latest advances of gas production from hydrate reservoir by experimental simulation in China.The resources of permafrost hydrate and marine hydrate are introduced.In addition,the development process of an experimental apparatus for hydrate dissociation was summarized.Moreover,the main production technologies for hydrate dissociation are reviewed in detail.The safety of hydrate dissociation by experimental investigation was introduced as well.The following conclusions can be drawn:
(1)There are abundant gas hydrate resources in China.The Qilian Mountain may be the most promising target area for gas hydrate in the permafrost region,which is of great scientific and economical value.Three gas hydrate drilling expeditions in the South China Sea confirmed that Shenhu Area in the South China Sea has huge potential for gas hydrate exploitation.
(2)The experimental apparatus for investigating the performance of hydrate dissociation in China has developed from one dimensional to two dimensional and three dimensional.In addition,well configuration developed from one tube to complicated multi-well networks to satisfy the demand of different production model.Besides,diverse testing methods have been established.
(3)Hydrate dissociation by depressurization has been widely studied in different scales of laboratory simulators.Hydrate dissociation performance was influenced by the reservoir characteristics,such as the initial gas-water-hydrate saturation,reservoir temperature,reservoir pressure,and reservoir permeability.The operational conditions such as the depressurizing rate,the outlet pressure,and the depressurizing range are important factors in controlling the production performance as well.Ice formation and secondary hydrate reformation may accompany the hydrate dissociation process;the mechanism of this phenomenon should be clearly understood.Hydrate dissociation by depressurization in the early stage is fast,while in the later stage is very slow when the sensible heat of the reservoir is exhausted.The controlling mechanism of hydrate dissociation in different scales of simulator using depressurization is different,which should be clearly elucidated in the future.
(4)Hydrate dissociation using different forms of thermal stimulation has been investigated in China.Hot water injection and microwave stimulation have been applied.Different models of hot water injection such as continuous injection and cyclic injection were used for experimental simulation.Single vertical well,single horizontal well and complicated multi-well networks were considered.The injection condition,for example the injection temperature and injection rate has an optimal range,which should be investigated in detail.The reviewed paper proposed the methods of energy ratio maximization and entropy production minimization to obtain the optimal value.
(5)Hydrate dissociation by CO2replacement has the win-win effect of recovering clean energy and reducing greenhouse effect.Experimental investigations show that the effect of injecting CO2emulsion is better than that of liquid CO2,and the effect of introducing liquid CO2is more beneficial than that of gaseous liquid CO2.In the future,the replacement efficiency should be developed to satisfy the requirement of commercial production.Moreover,the micromechanics of the replacement process need to be elucidated.
(6)The mechanism and effect of hydrate dissociation by inhibitor stimulation have been studied in the laboratory by several institutes in China.Experimental results confirmed that brine injection is prior to other types of inhibitor stimulation.Hydrate dissociation rate and gas production rate are affected by the inhibitor type,injection temperature,injection rate,and injection quantity.Furthermore,the advances of the combined method have been evaluated,and the combined method is more beneficial than each single method.
(7)Issues of safety of exploiting natural gas hydrate were proposed.The reviewed papers preliminarily discussed the mechanical properties and the sediment deformation situation during the process of hydrate dissociation.The coupled mechanism of physical and chemical factors for the mechanical properties of the sediment and the sand production problem can be studied further.
 Chinese Journal of Chemical Engineering2019年9期
Chinese Journal of Chemical Engineering2019年9期
- Chinese Journal of Chemical Engineering的其它文章
- Decomposition behaviors of methane hydrate in porous media below the ice melting point by depressurization☆
- Experimental characterization of guest molecular occupancy in clathrate hydrate cages:A review☆
- Hybrid versus global thermostatting in molecular-dynamics simulation of methane-hydrate crystallisation
- Growth kinetics of hydrate formation from water-hydrocarbon system☆
- Investigation of the hydrate formation process in fine sediments by a binary CO2/N2gas mixture☆
- Investigation on the effect of oxalic acid,succinic acid and aspartic acid on the gas hydrate formation kinetics
