Growth kinetics of hydrate formation from water-hydrocarbon system☆
Youhong Sun,Shuhui Jiang,Shengli Li*,Guobiao Zhang,Wei Guo
Key Laboratory of Drilling and Exploitation Technology in Complex Conditions of Natural Resources,College of Construction Engineering,Jilin University,Changchun 130026,China
Keywords:Gas hydrate Formation Crystal Film Promotion Inhibition
ABSTRACT Gas hydrates have drawn global attentions in the past decades as potential energy resources.It should be noted that there are a variety of possible applications of hydrate-based technologies,including natural gas storage,gas transportation,separation of gas mixture,and seawater desalination.These applications have been critically challenged by insufficient understanding of hydrate formation kinetics.In this work,the literatures on growth kinetic behaviors of hydrate formation from water-hydrocarbon were systematically reviewed.The hydrate crystal growth,hydrate film growth and macroscopic hydrate formation in water system were reviewed,respectively.Firstly,the hydrate crystal growth was analyzed with respect to different positions,such as gas/liquid interface,liquid-liquid interface and gas-liquid-liquid system.Secondly,experimental and modeling studies on the growth of hydrate film at the interfaces between guest phase and water phase were categorized into two groups of lateral growth and thickening growth considering the differences in growth rates.Thirdly,we summarized the promoters and inhibitors reported(biological or chemical,liquid or solid and hydrophobic or hydrophilic)and analyzed the mechanisms affecting hydrate formation in bulk water system.Knowledge gaps and suggestions for further studies on hydrate formation kinetic behaviors are presented.
1.Introduction
Gas hydrates are nonstoichiometric crystalline solids,which consisted of water and guest molecules.The guest molecules are entrapped in host frameworks of water molecules bound by hydrogen bonds.Three primary gas hydrate structures were found in nature:structure I(sI),structure II(sII)and structure H(sH).If all hydrate cages are filled with guest molecules,there are~85 mol% water molecules and~15 mol%guest molecules in the three hydrate structures [1]1 vol gas hydrate can contain about 160 vol of gas guests (STP),which indicates gas molecules can be effectively trapped in hydrate structures.The distinct properties of gas hydrates have given rise to numerous new technologies.Gas hydrates are applied not only to natural gas transportation and storage for energy utilization [2,3],but also to gas separation [4,5],water desalination[6,7]and refrigeration system[8].These potential applications are mainly confined in laboratories by technological challenges related to hydrate formation,including the control of hydrate formation rate,the control of hydrate conversion ratio,the regulation of hydrate volume and so on.In order to solve the technical problems,the gas hydrate growth kinetics should be better understood to develop next-generation hydrate-based technologies.In most hydrate-based technologies,the rate of gas hydrate formation is required to be enhanced.In some other areas such as flow assurance,however,the hydrate formation rate needed to be depressed.
Hydrate growth starts from crystals at the interface.Then hydrate crystals aggregate to form polycrystalline hydrate film at the guest/water interfaces.While hydrate film grows laterally along the guest/water interface,it also grows in thickness normal to the interface.After the complete formation of hydrate film at the interface,the two phases of hydrate former and water are separated,which prevented further hydrate formation.In bulk water system,on one hand,measures such as stirring can break up the hydrate film covering at the interface and recover the direct contact of guest and water phase;on the other hand,hydrate formation is promoted or inhibited potentially by additives.Studies of the morphology of hydrate crystals,the growth kinetics of hydrate film and macroscopic hydrate formation kinetics are very important for the overall understanding of the kinetics of hydrate formation and for the application of above-mentioned hydrate-based technologies.Therefore,we will review the recent progresses achieved in gas hydrate formation kinetics from three aspects in this work:hydrate crystal growth,hydrate film growth at the interface of guest/water phases,and hydrate formation in bulk water system.
2.Hydrate Crystal Growth
The study of hydrate crystal growth kinetics is commonly based on morphological observations.Crystal morphology measurement such as the size or shape of crystals can provide direct and valuable information on hydrate crystal growth behaviors[9].The various crystal morphologies were attributed to factors such as the variation in hydrate guests,the supercooling,the pressure and the concentration of solutions.A number of studies on the morphologies of the hydrate crystals at gas/water interface,at liquid/liquid interface and in gas-liquid-liquid system were reported.
2.1.Gas/liquid interface
Methane,ethane and propane are the common hydrate-forming gases.The morphologies of gas hydrate crystals formed at the gas/water interface are mainly influenced by subcooling,gas composition,gas concentration in water and additives.The formation and growth of pure methane,ethane and propane hydrates morphology crystals at the surface of a water droplet were visually observed [10,11].The hydrate crystals aggregated and constituted the polycrystalline hydrate layer at the gas/water interface.It was found that morphology of the hydrate crystals formed at the interface can be classified using subcooling as a common criterion.The subcooling is the differences between the system temperature and the hydrate phase equilibrium temperature corresponding to the system pressure.There was a general trend that the shape of hydrate crystals was typically sword-like or triangular at lower subcoolings and the shape changed to polygons at higher subcoolings [10].There were numerous fine needle-like hydrate crystals at the surface of hydrate film when the high driving force was high enough [11](see Fig.1).The morphology of hydrate crystals formed from gas mixture of methane-ethane-propane was different from that of methane hydrate (sI).With the increasing subcoolings,the shape of mixed hydrate crystals changed from polygonal to swordlike.The faces of the mixed hydrate crystals were deduced to be[111]planes of the cubic sII hydrate.The hydrate films formed at the surface of water droplets were rougher at lower subcoolings as the size of the individual crystal increased with the decreasing subcooling.In addition,the size of the individual crystals of mixed hydrates decreased with the increasing concentration of methane in the gas mixture[9],which was attributed to the difference between the crystallographic structures of sI and sII hydrates.There was a tendency that the size of sII crystals was smaller than sI hydrate.For binary mixed hydrates at planar interfaces of gas/water[12,13]shown in Fig.2,floating crystals were also observed near the bottom of the hydrate film.They interacted with hydrate crystals originated from the hydrate film.The further hydrate growth in water below the hydrate/liquid interface should be driven by the high gas concentration gradient near the hydrate/liquid interface.Methane hydrate crystals were also observed to grow in liquid water saturated with or in contact with methane[14].The results indicated that the concentration of gas species in water and mass transfer from the bulk water to the hydrate/water interface had a significant impact on the growth behavior of hydrate crystals.Flow water was an important factor affecting the gas mass transfer for hydrate formation.Similar to quiescent system,the shape of methane hydrate crystals growing in a liquid water stream changed from the polygonal to dendrite with the increasing subcooling [15,16](see Fig.3).However,the growth of hydrate crystals lasted for a longer period in the liquid water flow as gas was continuously supplied by flowing water saturated with gas.The improvement of the gas mass transfer promoted the growth of hydrate crystal in water phase.

Fig.1.Methane hydrate growth at the surface of water droplets under high driving forces[11].

Fig.2.Floating crystals with equiaxed orthogonal shape(a)and dendrites equiaxed skewed dendrite shape(b)[12].

Fig.3.Hydrate crystal growth in a gas saturated water stream[16].
It is known that the addition of active agents or inhibitors will affect not only the rate of gas consumption during hydrate crystallization but also the morphological characteristics of crystal growth without affecting the hydrate equilibrium conditions.Yoslim et al.[17]found that the shape of methane-propane hydrate crystals changed from dendrite to porous fiber with the addition of surfactants.In addition,hydrate crystals grew extensively into porous hydrate layer on the crystallizer walls besides at the gas/water interface,which resulted in a promotion of the hydrate growth by approximately 14 times compared to pure water.In aqueous SDS solution[18],smoke-like crystals of methanepropane hydrate appeared at the top of gas bubble and the hydrate film growth was not strictly limited to the gas/water interface when SDS concentration increased.The hydrate film growth was blocked by the SDS molecules adsorbed at the interface.At the surface of water droplet [19],the crystal morphology and growth behavior of the methane hydrate depended on the concentration of surfactant and the subcooling(see Fig.4).It was deduced that the crystal growth in the system was controlled by the increased wettability and the strong capillary action induced by SDS.On one hand,the amount of hydrate crystals formed increased as a result of the detachment of hydrate crystals from the interface because of the increased wettability.On the other hand,water consumption was enhanced with larger capillary force due to the smaller pores generated between hydrate crystals.In Wang's study [20],the capillary force induced by SDS could even affect the growth direction of hydrate crystals with the modification to hydrate growth surface.Besides surfactant,amino acid acted as a promoter in hydrate growth at gas/water interface[21].When the concentration of leucine was higher than 0.3 wt%,there was a rapid mushy hydrate crystal formation in the bulk solution.The environment-friendly amino acid was regarded as a promising promoter for hydrate formation on a large scale.Morphological observations to study the effect of kinetic inhibitors were also reported in previous publications.Synthetic polymers of polyvinylpyrrolidone(PVP)and natural antifreeze proteins(AFPs)were common kinetic inhibitors evaluated in hydrate growth at gas/water interface.Kumar et al.[22]reported the influence of PVP on the formation of methane-propane hydrate in liquid water exposed to gas.It was found that hydrate layer formed only at the gas/liquid interface at lower PVP concentrations,covered at both the interface and wall above the interface with the increasing PVP concentration,and grew catastrophically on the wall of the hydrate formation vessel from the liquid water drawn up through a porous hydrate layer due to the capillary effect at higher PVP concentrations(see Fig.5).In the presence of AFPs [23],methane hydrate films were thinner and showed a single growth mechanism rather than multiple crystal growth mechanisms.The change in crystal growth mechanism resulted in variations of morphology and thickness of hydrate layer with kinetic inhibitors(VP/VC,PVP and AFP)[24].Under high driving forces,no dendritic hydrate crystals formed at the surface of droplets with inhibitors while dendrites would form on pure water droplets.The hydrate layer at the surface of water droplets without the inhibitors was found to be the thickest while hydrate layer on the droplet with inhibitors varied in thickness.

Fig.4.The variation of hydrate morphology with the increase of SDS concentration[19].

Fig.5.Catastrophic growth of methane/propane hydrate at higher PVP concentrations[22].
2.2.Liquid-liquid interface
Observations have been reported on the hydrate growth at the interface between a hydrophobic hydrate former(e.g.cyclopentane)and liquid water.The effects of subcooling,salinity,surfactant and inhibitor on hydrate crystals growth were analyzed,which may provide insights into hydrates inhibition in gas and oil delivery lines.Both Sakemoto et al.[25]and Kishimoto et al.[26]visually observed hydrate crystals growth at cyclopentane (CP)/seawater interface.It was found that hydrate crystals grew along the liquid-liquid interface to form hydrate layer covering the entire interface.The hydrate crystals formed from water or seawater changed from polygons to triangular or sword-like shape with the increasing subcooling,indicating that salt had little impact on the hydrate crystal morphology.Mitarai et al.[27]studied the effects of surfactants of sorbitan monooleate(Span 80),naphthenic acid and polypropylene glycol on hydrate crystal growth at cyclopentane/water interface.The observations showed the crystal growth behavior of cyclopentane hydrate varied distinctly with the chemical species of the surfactants,concentration of surfactants and the subcooling(see Fig.6).The surfactants may make the hydrate crystal more wettable.There was no compact hydrate film formation as the growing hydrate crystals detached from the interface and fell into the water,giving rise to the crystal size and growth rate.The mechanism was similar to the promotion effects of SDS on methane hydrate growth at the surface of water droplet[19].The simultaneous anti-agglomeration and promotion of surfactants in hydrate growth were further identified[27].In addition,the effects of a quaternary ammonium surfactant(DA 50)on hydrate growth were studied at cyclopentane/brine interface[28].The hydrate growth pattern was deduced to be controlled by the amount of surfactant adsorbed on water/CP interface that increased significantly with increasing concentration of NaCl.At higher concentrations of NaCl and surfactant,small pyramid-like hydrate crystals with their vertex pointing to the CP phase were observed at the interface(see Fig.7).The conjecture was that hydrate crystals formed became oil-wettable with the adsorption of surfactant at the surface of hydrate crystals and grew into the CP phase.In this way,hydrate growth was not confined merely at the interface of CP and seawater.At liquid/liquid interface,hydrate growth can also be inhibited.Sakaguchi et al.[25]reported the inhibition effects of PVP and PVCap on sII hydrates at HCFC-141b(CH3CCl2F)/water interface.The crystal morphology of sII hydrate at the liquid/liquid interface was observed to be substantially changed by the inhibitors.It was revealed that PVCap had a stronger suppression effect on hydrate film growth along the interface than PVP while PVP even promoted the crystal growth into the aqueous phase whether the phase was saturated with hydrate formers.Presumably,the adsorption of inhibitor at the interface from the aqueous phase would result in an enhanced inhibition effects at the liquid-liquid interface.Cha et al.[29]analyzed the inhibition effects of hydrophobic silica nanoparticles introduced into the interface of aqueous phase and cyclopentane(CP)on hydrate crystal growth after seeding the hydrate slurry.It was found that hydrate crystal growth merely occurred around the seeding positions of CP hydrate slurry,and the further growth of the hydrate crystal was retarded.The reduction of hydrate growth sites and restriction of hydrate crystal growth would delay the conversion of water to hydrate,which demonstrated that employing the hydrophobic silica nanoparticles at the water-oil interface could be another effective way to prevent hydrate formation.

Fig.6.The detachment of hydrate crystals from cyclopentane/water interface with Span 80[27].
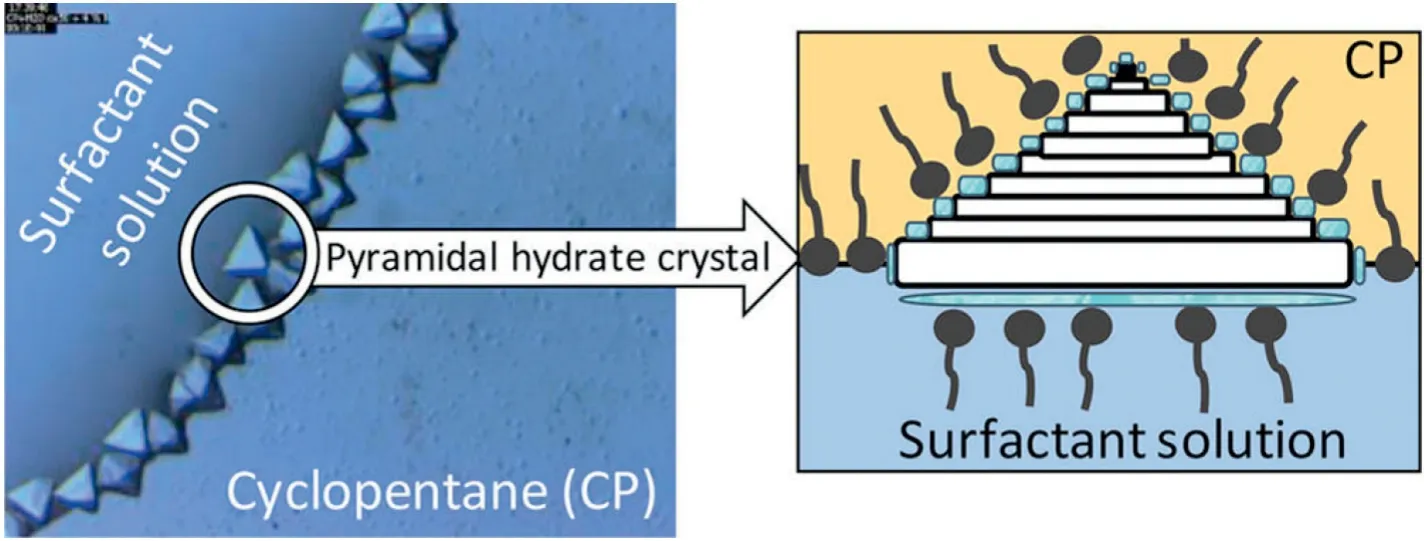
Fig.7.The formation of pyramidal hydrate crystals at the interface of CP and brine in the presence of a water-soluble surfactant[28].
2.3.Gas-liquid-liquid system
Gas hydrates formed in gas-liquid-liquid system are mostly sII hydrates with double guests or sH hydrates.Ishida et al.[30,31]reported the universality of the hydrate crystal growth behavior in gas-liquidliquid systems.In their visual observations,the formation of sII hydrate occurred at the surface of a water droplet partially immersed in liquid cyclopentane and partially exposed to difluoromethane gas [30]or krypton gas[31].It was found that the behavior of sII hydrate crystal growth in these three-phase three-component systems can be classified into three modes with respect to the pressure and subcooling:cover,expansion and line (see Fig.8).With the increase of pressure and subcooling,hydrate crystal growth varied from cover mode to expansion mode and then to line mode.These three modes were described as follows:Hydrate crystals formed at the surface of a water droplet,then grew into hydrate film covering the whole surface(Cover mode);or grew both along the surface and toward the gas phase(Expansion mode).Line mode:hydrate crystals grew along the three-phase line and spread radially from the line.For sH hydrates in gas-liquid-liquid system,Servio and Englezos[32]visually studied the growth behavior of sH hydrate crystals on a water droplet immersed in liquid neohexane exposed to methane gas.In the experiments,no pure methane hydrate formed as methane dissolved into the neohexene phase and then transferred through it to the liquid/liquid interface of water and neohexene to form sH hydrate.It was found that there were visible effects of driving forces on sH hydrate crystal morphology.The crystal growth was deduced to be controlled by the mass transfer of methane molecules through neohexene.Ohmura's method[33]was a little different from Servio's[32].The water droplet was partially immersed in the phase of a hydrate former of large molecule and partially exposed to methane gas.Experimental conditions were set to avoid possible formation of methane hydrate.It was found that there was no preferential formation of hydrate crystals along the three-phase line.The hydrate crystals grew laterally at the interface of water and methane and extended into gas phase.This growth behavior of sH hydrate was similar to the expansion mode of sII hydrate[31],which indicated that the occupation of methane molecules in hydrate small cavities may serve as an important factor in the formation of sH hydrates.Jin et al.[34]studied the crystal morphology of sH hydrate in a methane-liquid hydrocarbon-water system.It was found that the crystalline morphology of sH hydrate changed from two dimensional planar hexagonal to three-dimensional six-branched shape with increasing subcooling.The growth behavior was attributed to the fact that high thermal diffusion occurred at the edge of the hexagonal crystal.
3.Hydrate Film Growth at Guest/Water Interface

Fig.8.Variations in crystal growth behavior of sII hydrates in three-component three-phase system with pressure and subcooling[31].
It is generally acknowledged that gas hydrate crystals grow in the form of a thin film or layer covering the interface after the preferential formation at guest/water interfaces.The growth of hydrate film at the interface of guest and water includes lateral growth along the guest/water interface and thickening growth normal to the interface.A series of experimental data have been reported on the lateral growth rates of hydrate film [35-41]while the reports on thickening of the hydrate film are limited [42-46].This may be because the two-dimensional observation of lateral growth is easier than the three-dimensional study of hydrate film growth in thickness.
3.1.Lateral growth
The lateral growth rates of hydrate film at the interface of guest and water were mostly measured by using microscope with CCD camera or VCR.Hirai et al.[47]first measured the lateral growth rate of CO2hydrate film at the CO2/water interface under different subcoolings.The results indicated that the main process controlling crystal propagation rate was the heat diffusion from reaction sites of guest and water.After that,a number of studies reported the measurements of lateral growth rate of pure gas or natural gas hydrate films.Freer et al.[41]investigated methane hydrate film growth over the pressure and temperature ranges of 3.55-9.06 MPa and 1.0-4.0°C,respectively.It was found that the lateral growth rate of methane hydrate film was proportional to the subcooling.The transition from single crystal growth to continuous film growth occurred at a subcooling of about 0.4°C.The research group of Chen[39,48-50]measured the lateral growth rate of gas hydrates film at the surface of gas bubble suspended in water.The“hydrating bubble”method was as follows:[51]at first,a methane hydrate layer was formed at the planar interface of gas and water in a high-pressure cell,then a gas bubble was injected slowly into the water through a needle;the hydrate film lateral growth at the surface of the suspended bubble would start once the bubble right contacted to the pre-formed hydrate layer,and the subsequent growth of hydrate film was observed by using a microscope and recorded by a CCD camera for measurements.The effects of factors such as temperature,subcooling,salinity and surfactants on lateral growth of hydrate film were systematically analyzed based on experimental measurements.Kitamura and Mori[38]measured the lateral growth rate of methane hydrate at planar interfaces.It was found that the transition point from crystal growth mode to hydrate film growth mode was at subcoolings of 2-2.5 °C,which was different from the results of Li[51]and Freer[41].Liu et al.[35]measured the spreading rate of methane hydrate film at the surface of gas bubbles in a quiescent and saturated water.Their analysis showed that the lateral growth rate of hydrate film at the surface of gas bubble was universally larger than that along the planar gas/water interface.Tanaka et al.[10]and Saito et al.[9]studied the hydrate film growth at the surface of water droplet exposed to methane,ethane,propane or their mixture,respectively.Wu et al.[52]measured methane-propane hydrate film growth at the surface of water droplet and analyzed the effects of inhibitors on the lateral growth rate.Li et al.[50]studied the growth kinetics of methane-ethane hydrate film growth by using hydrating bubble method(see Fig.9).The lateral growth rate was found to be related to the composition of gas mixture.In addition,there are only a few studies on the hydrate film growth at liquid-liquid interface.Based on the“hydrating bubble”method,Li et al.[37]developed a method for the study of methane hydrate film growth at the surface of water droplet suspended in oil phase.The lateral growth rate of hydrate film formed from methane dissolved in oil phase was experimentally measured for the first time.Then Morrissy et al.[53]quantified cyclopentane hydrate film growth rate at moderate subcoolings by using a micromechanical force(MMF)apparatus.The results showed that the hydrate film growth rate decreased in the presence of natural surface-active species of asphaltenes or free resins.

Fig.9.The lateral growth of methane hydrate film at the surface of gas bubble suspended in water[39,51].
Based on experimental measurements,some efforts have been made to theoretically understand the lateral growth kinetics of gas hydrate film.Mass or/and heat transfer to/from the growing front of hydrate film were the main factors considered in modeling the lateral growth kinetics.Freer et al.[41]proposed a model for hydrate formation.The kinetic model accounted for both the heat transfer effects and the intrinsic kinetics of gas hydrate formation.It was assumed that the kinetic dependence followed an Arrhenius behavior and the heat transfer coefficient was constant.Three parameters were regressed from the experimental results,which were in reasonable agreement with values for crystallization.Mori [54]presented a simple model considering convective heat transfer.The model predicted that the product of lateral growth rate and film thickness was proportional to the 1.5 power of the subcooling.The heat of hydrate formation was assumed to be removed from the growing film front to the surrounding fluid phases by steady convective heat transfer.For the first time,the initial thickness of hydrate film was formulated into the lateral growth kinetics equation,which was the advantage of this heat transfer control model [54].Then the model was further modified by Mochizuki and Mori considering the geometry of hydrate film front.A new model was developed with the assumption that the heat transferred conductively from the growing film front to the surrounding three phases.However,the new model was computationally complicated to use[39].In Peng's experimental and modeling study,the convective heat transfer model developed by Mori[54]was used to correlate the hydrate film growth rate and the subcooling.It was assumed that the initial film thickness varied inversely with the subcooling.The prediction of the new relationship constructed was in good agreement with the experimental data.Liu et al.[35]developed another model for the lateral growth of hydrate film at a planar gas/liquid interface considering naturally convective heat transfer.The subcooling and temperature were both regarded as important factors affecting the lateral growth of hydrate film.Saito et al.and Kishimoto et al.[55,56]demonstrated that the hydrate film growth rates under various system pressures could be also well correlated with the mass transfer of dissolved methane from bulk water phase to the water layer adjacent to hydrate film front.It was claimed that the mass transfer played significant role in the lateral growth of hydrate film.Based on this idea,Li et al.[37]developed a mathematical model describing the lateral growth of methane hydrate film at the interface of water/oil.The initial film thickness was also assumed to change inversely with the lateral growth rate.Recently,Mochizuki and Mori[36]presented a new physical model for the lateral growth of hydrate film at water/guest interface considering simultaneous transfer of mass and heat to/from the growing film front.The predictions of hydrate film growth were compared with the existing observations reported.The results indicated that methane hydrate film growth was mainly controlled by the mass transfer of guest to the growing film front rather than the heat transfer from the front.
3.2.Thickening growth
Gas hydrate films grew in thickness though the films formed at guest/water interfaces provided a substantial mass-transfer barrier to further gas hydrate formation.Reports on the growth of hydrate film in thickness included the initial film thickness measurements,the observations of mass transfer of gas/water molecules in hydrate film and the measurements and modeling of thickening growth rate.
There were two methods for the determination of the initial thickness of hydrate film.One was experimental measurements and the other was estimation with lateral growth kinetic model.Direct measurements of initial film thickness are rarely reported.Ohmura et al.[57]measured hydrate film thickness using laser interferometry.The initial thickness was about 10 μm.The initial film thickness obtained by Taylor et al.[45]was 5-12 μm for methane hydrate and about 12 μm for cyclopentene hydrate in the measurements of hydrate film growth rate in thickness.Li et al.[51]measured the initial film thickness of methane hydrate using the hydrating bubble method under different pressures and temperatures.It was found that the initial film thickness decreased from dozens of microns to about a few microns with the increase of subcooling.In addition,the initial film thickness data were also calculated from the kinetic models for lateral growth,which were in a broad range according to different kinetic models.The initial thickness estimated ranged from 0.13 to 1.54 μm[40,47,49,51,54]for carbon dioxide hydrate and from 2 to 60 μm[35,39,41]for methane hydrate.
There is a controversy whether the mass transfer of the guest[58-60]or water molecules[61-65]through hydrate film accounts for the growth of hydrate film in thickness[37].Turner et al.[66]proposed an inward growing shell model for hydrate formation in water-in-oil emulsion.In the model,it was assumed that methane molecules transferred across the hydrate shell for further hydrate formation of hydrated water droplet.Davies et al.[65]studied the mass transfer mechanism in hydrate films by using high-resolution confocal Raman spectroscopy.Water molecules were found to be more mobile than methane molecules within hydrate films.Liang et al.[64]studied the migration behavior of water molecules among hydrate cages.The results showed that water molecules had good mobility in transferring through gas hydrate phase.Lee et al.[67]deduced that the mass transfer of both water and gas molecules in hydrate film with porous feature were responsible for hydrate thickening growth.Shi et al.[68]applied this deduction to present an inward and outward growth model for hydrate formation in water-oil system.With morphological observation by CCD camera,Li et al.[37]demonstrated that water molecules transferred through hydrate film for hydrate formation at the surface of hydrated water droplet suspended in oil phase(see Fig.10).
Mori[46]reviewed the modeling of hydrate film growth in thickness comprehensively in 1998.After that,it seemed that limited progresses were made on this topic[69].Mori et al.[70]developed a mass transfer model for the vertical growth of hydrate film to build a simple mathematical relation between the film thickness,the film-internal geometry and the water-phase-side mass transfer coefficient of the guest species.In the model,it was assumed that hydrate film was a solid plate preventing the direct contact of guest phase and water phase.Liquid water could permeate into hydrate film by filling the capillaries in it.The guest molecules dissolved in water and transferred through the film by the migration of water.Water migration was driven by the capillary pressure in hydrate capillaries.The model showed that the thickness of hydrate film varied inversely with the mass transfer coefficient of guest molecules in water layer at the surface of hydrate film and was affected by water flows along hydrate phase.More recently,Abe et al.[46]measured hydrate film thickness with respect to different flow velocities and temperatures using the interferometry method(see Fig.11).The results showed the hydrate film thickness increased over time without water flow and was constant with water flow.A model considering mass transfer was presented to estimate the thickness of hydrate film after lateral growth.In this model,it was also assumed that only water molecules could pass through the hydrate film.It was deduced that the dynamic thickness of hydrate film was the result of the balance between hydrate formation and dissociation.The hydrate film thickness would change if this dynamic balance was disrupted.The formation and dissociation of hydrate should be controlled by mass transfer caused by specific concentration distributions of water and guest molecules.The hydrate film thickness predicted by the model correlated well with the experimental data,which indicated that the model may accurately describe the effects of flow velocity and temperature on the growth in thickness of hydrate film.
4.Hydrate Formation in Bulk Water Syste m
4.1.Promoters
Slow hydrate formation and limited storage capacity impede the commercial application of hydrate-based technologies.A number of kinetic hydrate promoters such as amino acids,cyclodextrins (CD),sodium lauryl sulfate(SDS),tetrahydrofuran(THF)and nanoparticles are tested for the promotion of gas hydrate formation.

Fig.10.Observation of hydrate formation at the surface of hydrated water droplet suspended in oil phase[37].

Fig.11.Schematic of the concentration distributions of water and CO2[46].
4.1.1.Biological promoter
Some amino acids can promote the formation of methane hydrate due to their hydrophobicity[71-74](see Fig.12).Veluswamy and Lee et al.[73]found that tryptophan,histidine and arginine could accelerate the formation of hydrates in stirring or quiescent tank reactors.At a leucine concentration of 0.3 wt%,a functional methane bubble appeared in the liquid phase,which helped more methane molecules enter hydrate phase and resulted in the formation of highly porous and flexible hydrates.The best concentration of each amino acid for the promotion of methane hydrate growth was different.Nonpolar tryptophan presented a better promotion effect than the other two polar amino acids.In addtion,experimental and numerical results [75]showed that the formation of methane hydrate was faster in the presence of L-histidine.1 wt% L-histidine and 1 wt% SDS had the same effect on gas consumption.
Lignosulfonates[76],which are byproducts of the pulp and paper industry,could obviously shorten the induction time and induce a quick consumption of methane for hydrate formation.When the concentration of sodium lignosulfonate (Na-LS)was 0.50 wt%,the maximum volumetric capacity of 167 v/v was obtained.The promotion effect first increased with the concentration then decreased with the concentration.The concentration of 0.50 wt%was the transition point.
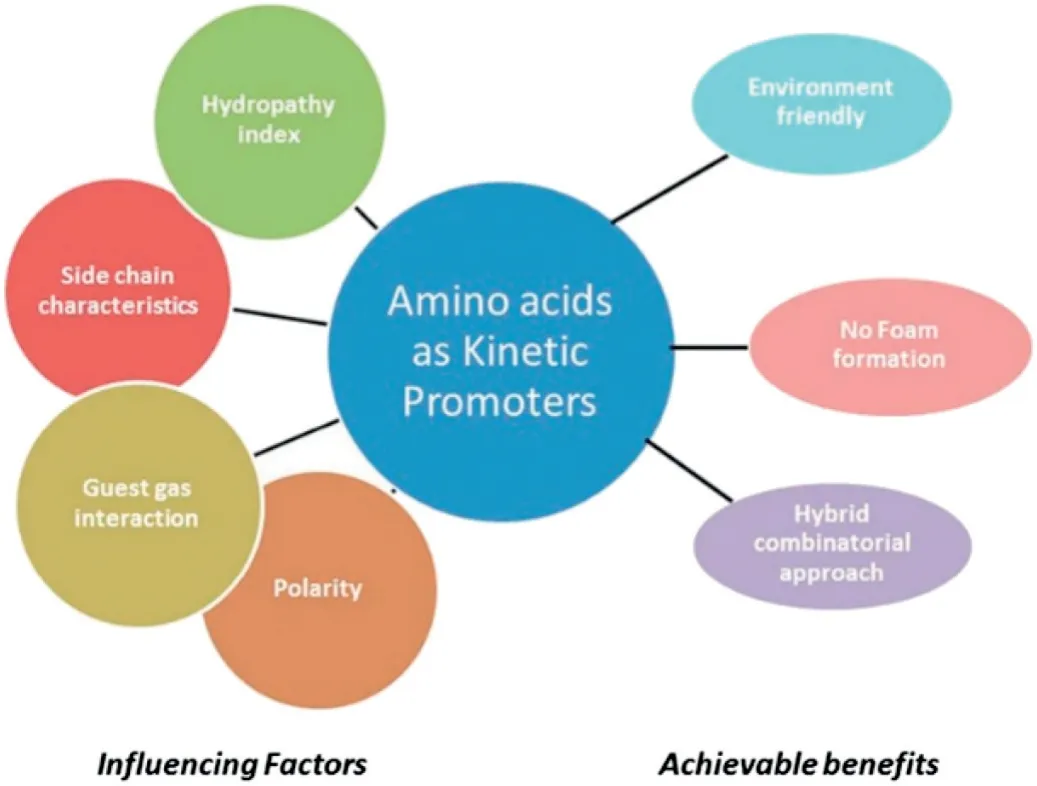
Fig.12.The biofriendly amino acids were kinetic promoters for hydrate formation[73].
Starch also has a good promotion effect on hydrate formation.Babakhani et al.[77]carried out experiments and revealed that maize starch accelerated hydrate formation when the concentration was higher than 400 ppm.The most effective concentration was 800 ppm when the methane storage capacity was 2.5 times than that of pure water system.In addition,the solubility measurements of methane showed that mung starch had solubilization effects which shortened the induction time and increased the methane hydration rate[78].The mechanism was shown in Fig.13.Cyclodextrin (CD)obtained from starch can also significantly promote hydrate formation.Kuji et al.[79]studied the effects of different cyclodextrins on xenon hydrate formation including β-CD polymers,modified β-CD,β-CD,and α-CD.The induction time in all experiments was reduced.α-CD,β-CD and β-CD polymers could increase the rate of hydrate growth.When the β-CD polymer was added,the growth rate of hydrate increased 5 times.The molecular simulation results showed that the addition of β-CD increased the curvature of gas-water interface and promoted gas hydrate formation[80].In addition,β-CD could also promote hydrate formation in methane-tetrahydrofuran system[81].
Rhamnolipid and surfactin are effective promoters [82].In rhamnolipid solution with concentration of 200 ppm,the induction time decreased from 5.77 h to 0.23 h.The test result indicated that rhamnolipid promoted methane hydrate formation with the reduction of the surface tension [83].The conversion ratio of methane gas to hydrate with surfactin was a little higher than that with SDS.
4.1.2.Chemical promoter
Zhong et al.[84]suggested that micellar solution could accelerate natural gas hydrate formation and change the formation mechanism.When the concentration of surfactant was higher than the critical micelle concentration(CMC),the hydrate formation rate was 700 times higher in stationary system.When hydrate nucleation occurred in micellar solution,particles agglomerated and migrated quickly to the surface of solid near the water/gas interface,forming concentric hydrate layers on the walls until the cell was fully filled with hydrates.It was widely observed that SDS accelerated hydrate growth under stirring conditions[85-87].SDS could both increase hydrate nucleation rate and promote hydrate growth by reducing the interfacial tension between hydrate phase and liquid phase and increasing gas-liquid interface area and the total surface area of hydrate particles [88].It was observed[89]that hydrates grew in the form of very small particles in the presence of SDS without stirring.The molecular simulation results[90]showed that SDS was adsorbed on the surface of hydrate with its hydrophobic tail anchoring into the open cages of growing hydrate crystals and the polar head groups exposed to the solution phase.The adsorption of SDS could reduce the surface free energy of hydrate crystals and accelerate hydrate growth.In addition,in a long-time scale,the adsorbed SDS molecules increased the porosity of hydrate particles,which led to a distinct morphology of hydrate phase.Porous morphology can significantly improve the mass transfer of methane and water in hydrate formation.Luzinova et al.[91]investigated propane hydrate formation in the presence of SDS by using infrared spectroscopic monitor.As was shown in Fig.14,firstly,SDS molecules formed associated complexes with propane molecules through strong hydrophobic interaction.Then,the high affinity of surfactants to metal(oxide)surfaces induced the migration of associated complexes to the surface.Finally,the reduction of the surface tension resulted in the release of gas molecules in the vicinity of the surface for gas hydrate formation.
To investigate the effect of the alkyl chain length of surfactant on hydrate formation,Okutani et al.[92]studied methane hydrate growth behavior with SDS,sodium tetradecyl sulfate(STS)and sodium hexadecyl sulfate(SHS)in static system.It was observed that SDS with the shortest alkyl chain and the highest water solubility had the best promotion effect while SHS with the longest alkyl chain and the lowest water solubility had the lowest promotion effect.Furthermore,Kwon et al.[93]investigated the effect of novel anionic multichain disulfonate surfactant with different hydrophobic carbon chain lengths on methane hydrate formation.The results indicated that the surfactant with the shortest chain length had the greatest promotion effects on the formation of methane hydrate,which was in accordance with the conclusion of Okutani[92].Analogously,Dicharry[94]studied the effects of carbon chain length of homologous surfactants on the promotion effects in stationary system.The promotion effects of the surfactant with different lengths of carbon chain were different.It was concluded that only surfactants containing 12 carbon atoms could accelerate the growth of all gas hydrates.Surfactants with the same carbon chain but different head groups differed in promotion effects on gas hydrate growth.Wang[95]found that the rate of hydrate growth on the reactor wall in the presence of SDS or SDSN was higher than that with SDBS.

Fig.13.The effects of CD on gas hydrate formation[79].

Fig.14.Schematic illustration of gas hydrate formation in the presence of SDS[91].
Surfactants could reduce the interfacial tension between gas phase and water phase,which facilitated the diffusion of gas into water phase[96].Lots of researchers studied the effects of different surfactants on hydrate growth.Ganji et al.[97]found that all of non-ionic surfactant ethoxylated nonylphenol (ENP),cationic surfactant cetyl trimethyl ammonium bromide (CTAB)and linear alkyl benzene sulfonate(LABS)accelerated gas hydrate growth at high concentrations and inhibited gas hydrate growth at low concentrations.Fazlali[98]found that SDS,polyoxy ethylene(20)cetyl ether(Brij-58)and hexa decyl trimethyl ammonium bromide(HTABr)could shorten the induction time of methane hydrate.Moreover,the mixtures of SDS and HTABr had a better promotion effect on reducing induction time.Karimi[99]found both sodium dodecyl benzenesulfonate(SDBS)and TritonX-100(TX-100)had promotion effects on the growth of ethane hydrate in stirring system.And the effect of SDBS was stronger than TX-100.In addition,Polyoxyethylene(20)sorbitan monopalmitate(Tween(R)40)could also promote the formation of ethane hydrate in stirring system under different initial pressure conditions[100].The surface of gas hydrate phase was more orderly and compact in the presence of tetrabutylammonium bromide(TBAB)[101].TBAB was also proved to be an effective promoter for gas hydrate formation[102,103].
Tetrahydrofuran(THF)is both a hydrate former and a hydrate promoter[104].Veluswamy et al.[105]observed the rapid formation of methane/THF hydrate near room temperature.Synergistic effects of THF and SDS on CH4,C2H6and C3H8hydrate formation were also confirmed[105,106].Ma et al.[107]developed a kinetic model to predict gas hydrate growth in THF solution.Long et al.[108]found that super absorbent polymer(SAP)could significantly reduce the induction time of THF hydrate.Similar to THF,cyclopentane(CP)is another promoter as well as a hydrate former.Baek et al.[109]concluded that a small amount of cyclopentane hydrate seeds could significantly accelerate gas hydrate formation and result in high storage capacities of methane hydrate without mechanical agitations.
4.1.3.Nanoparticle promoter
4.1.3.1.Metals and metal oxides.Metal and metal oxide nanoparticles such as silver,copper,copper oxide (CuO)[113],aluminum oxide(Al2O3)[114]and zinc oxide(ZnO)[115]play an important role in promoting hydrate growth and gas consumption.Arjang et al.[110]investigated the effect of silver nanoparticles on methane hydrate growth in static system.The increase of methane consumption and decrease of induction time were both observed.Other reports confirmed that the addition of silver or copper nanoparticles significantly increased the conversion ratio of water to hydrate and gas consumption rate[111,112].Nesterov et al.[116]studied the effects of oxide powders on hydrate formation.They deduced that the promotion effect of oxide powder originated from its surface properties,including the surface functional groups and the presence of surface contaminants.The results also revealed that the lower surface charge and smaller size of nanoparticles should account for the promotion of hydrate formation.
4.1.3.2.Mimic micelles.In surfactant solution,hydrate crystals grew along the reactor wall,resulting in a large number of pores in the hydrates,which limited the volume of hydrate formed.However,few hydrates grow upward in the presence of mimic micelles [117].The schematic mechanism of mimic micelles was shown in Fig.15.Wang et al.[118]fixed SDS onto the surface of polystyrene nanospheres(named as SDS@PSNS)and found that the contact angle increased with the increase of SDS@PSNS content,resulting in the formation of hydrates at the bottom of the reactor.To promote hydrate formation,Wang[119]grafted Ag nanoparticles on the surface of SDS@PSNS.At a certain concentration of Ag&SDS@PSNS,the complete time for hydrate formation could be decreased by ten times.Subsequently,SDS was replaced by--fi xed on the surface of polystyrene nanospheres(named as--@PSNS)[120]for the promotion of methane hydrate formation.The arrangement of--on the nanospheres greatly improved the promotion ability.--@PSNS had better promotion effects than SDS,nanofluids and activated carbon on gas consumption.Grafting Ag nanoparticles on the surface of--@PSNS also effectively improved the promotion effects on hydrate formation[121].Similarly,the sol of SDS@Fe3O4could shorten the induction time.SDS@Fe3O4with smaller size could better promote methane hydrate formation[122].In addition,the complex(CTAB@RR195)of red-195 dye molecule(RR195)and cetyltrimethylammonium bromide(CTAB)could increase methane consumption by providing a larger area of solid/liquid interface[123].
4.1.3.3.Activated carbon,graphite and carbon nanotube.Activated carbon particles could shorten the induction time of methane hydrate.The gas consumption increased with the concentration of activated carbon.However,once activated carbon was deactivated,the growth methane hydrate would be inhibited[124].It was found that the induction time of gas hydrate decreased noticeably in graphene suspension or graphene nano-fluid[125,126].The induction time was the shortest in graphene suspension with a concentration of 150 ppm.The addition of both SDBS and graphene markedly increased the conversion ratio of water to hydrate[127].In another study[128],carbon nanotube was used in combination with SDS to promote gas hydrate formation.The results indicated that the continuous Brownian motion of nanoparticles could accelerate the nucleation of gas hydrate.However,the aggregation of nanotube suspension with high concentrations reduced the promotion ability.The aggregation may be avoided with amphiphilic copolymers wrapped on carbon nanotubes.With the addition of multi-welled carbon nanotube,gas consumption in hydrate formation increased by 3 times[129].

Fig.15.Hydrate morphology with or without mimic micelles[117].
4.1.3.4.Hydrophilic or hydrophobic particles.Hydrophilic and hydrophobic particles are dynamic promoters for gas hydrate formation.Chari[130]found that nano-silica could speed up hydrate formation without stirring.Nanoparticles suspended in water provided multiple sites for hydrate nucleation.However,the promotion effects of nano-silica suspension on methane hydrate formation were weaker than those of activated carbon[124].Wang et al.[131]found methane hydrate formed easier on hydrophobic surface.It was attributed to the increasing tendency of water molecules near the hydrophobic surface forming partial clathrate structures.Li et al.[132]also found that the sturcture of water molecules near the hydrophobic surface were more ice-like.The results indicated that the order degree of hydrogen bond before hydrate nucleation was inversely proportional to the average induction time.Nguyen et al.[133]studied the microscopic effect of hydrophobic solid surface on gas hydrate formation (see Fig.16).It was observed that a large amount of gas was adsorbed on the hydrophobic surface while no gas existed on the hydrophilic surface.Meanwhile,water molecules near the hydrophobic surface formed cages locally but tended to concave near the hydrophilic surface.It was concluded that[134]the primary factors promoting hydrate formation on the surface of SiO2and graphite were the strong hydrogen bonds of silanol groups and the nature of adsorbing CH4molecules,respectively.
4.2.Inhibitors
The kinetic inhibitors(KIs),such as polymers,antifreeze proteins,ionic liquids and amino acids have attracted wide attentions due to economic and environmental considerations.Low dosage hydrate inhibitors(LDHIs)have been studied over the last 25 years[135].Unlike the thermodynamic inhibitors,the effective concentrations of LDHIs are less than 1 wt%.Besides the most common LDHIs of PVP and PVCap,there are biological inhibitors such as amino acids with low hydrophobicity and antifreeze proteins(AFPs)and chemicals inhibitors such as ionic liquids(ILs)in terms of delaying hydrate nucleation and growth.

Fig.16.Hydrate formability of hydrophobic and hydrophilic surfaces[133].
4.2.1.PVP and PVCap
Poly(N-vinylpyrrolidone)(PVP)and poly(N-vinylcaprolactam)(PVCap)are two of the most common and effective inhibitors.Sakaguchi et al.found that the effect of PVP on hydrate crystal morphology was more obvious while the inhibition effect of PVCap on hydrate growth was greater[25].The ether and hydroxy group of diethylene glycol monobutyl ether contributed to the inhibition effect of PVP[138].PVP had good inhibition effects on hydrate growth but not on hydrate nucleation.During methane hydrate growth,the concentration of PVP in bulk liquid phase decreased over time.It indicated that the decrease of PVP concentration was associated with the formation of hydrate particles.The mechanism of adsorption-inhibition should be further annalyzed [139].PVCap can significantly delay hydrate nucleation and prevent further formation of gas hydrates.In-situ Raman analysis results showed that the existence of PVCap decelerated the encapsulation of large cages in the early stage of hydrate formation[136].Seo et al.found that the inhibition performance of PVCap increased with the decrease of the molecular weight[137].
4.2.2.Biological inhibitors
Biological inhibitors are considered as a new type of gas hydrate inhibitors due to their good degradation and inhibition performance.The amine(-NH2)and carboxylic acid(-COOH)groups of amino acids tended to form hydrogen bonds with water molecules [71,140].Sa et al.directly observed the disturbance of amino acids to the water clusters and analyzed the selective inhibition of amino acids on hydrate cage formation by Raman spectroscopy[140].It was proposed that amino acids with lower hydrophobicity played a better role in delaying hydrate nucleation and growth due to the destruction effects on hydrogen bond network of water molecules[71,72,74].Conversely,amino acids with higher hydrophobicity could stabilize the local cage structure of water molecules and promote hydrate formation.Glycine with lower hydrophobicity has a stronger inhibition effect on hydrate formation than L-leucine[72].The measurements of induction time also indicated that hydrate nucleation was inhibited in the presence of all kinds of amino acids with low hydrophobicity except L-glutamine[74].Amino acids (glycine and L-alanine)with lower hydrophobicity disrupted hydrogen bonds among water molecules to inhibit hydrate formation while amino acids (L-valine,Leucine,and L-isoleucine)with higher hydrophobicity strengthened the structure of water cluster to promote hydrate formation,as shown in Fig.17[71].Talaghat et al.[141]found that the inhibition effect of L-tyrosine on hydrate formation was even higher than that of PVP.Amino acids can be used in combination with PVP or PVCap.Xu et al.[142]found that the induction time increased 16 times and the hydrate growth rate decreased in the presence of the mixture of glycine and PVCap.Similarly,Kakati et al.[143]also found that the inhibition effect of PVP increased with the addition of L-tyrosine.
Antifreeze proteins(AFPs)are a class of protein compounds which can improve biological antifreeze ability.Five species,including antifreeze glycoproteins(AFGPs),Type-I Antifreeze Proteins,Type-II Antifreeze Proteins,Type-III Antifreeze Proteins and Type-IV Antifreeze Proteins,have been reported to inhibit hydrate formation.Cruz-Torres et al.[144]investigated the inhibition effects of AFGPs on hydrate formation.Glycoproteins were expected to fix on the surface of the hydrate structures,blocking the entry of new water molecules and preventing the growth of new crystals.Al-Adel et al.[135]found that AFPs inhibited hydrate growth to the same extent as poly(VP/VC)at similar experimental conditions.Daraboina et al.[145]tested two chemical kinetic inhibitors of PVP and H1W85281,and two AFPs of Type I and Type III.H1W85281 was found to be the most effective one in prolonging the induction time and inhibiting hydrate growth.It was also found that Type I AFP had a better inhibition effect than PVP and Type III AFP.Xu et al.[146]found that chitosan was another good biological inhibitor for gas hydrate formation.With the increase of deacetylation degree of chitosan (less than 80%),the induction time was significantly prolonged.At an optimum concentration of 0.6%,chitosan could also decelerate hydrate growth.In addition,Talaghat[147]found that gas hydrate nucleation could be inhibited by oxidized starch whose efficiency was higher than that of PVP.
4.2.3.Ionic liquids

Fig.17.Schematic illustration of the effects of hydrophilic and hydrophobic amino acids on gas hydrate formation[71].

Fig.18.Schematic illustration of the dual effects of sodium halides on gas hydrate formation[171].
Ionic liquids (ILs)have strong inhibition effects due to its ionic solubility and strong electrostatic interaction with water molecules.Xiao et al.[148]first identified ILs as novel hydrate inhibitors which had both thermodynamic and kinetic functions.Therefore,ILs were named as dual-function inhibitors.Richard et al.[149]found that the inhibition capacity of 1-ethyl-3-methylimidazolium chloride(EMIM-Cl)mono-component solution gradually increased with the concentration.The inhibition effects of EMIM-Cl may even exceed that of MEG at high concentrations.Park et al.[150]found that the inhibition effect of morpholine on hydrate formation was superior to that of 1-ethyl-3-methyl imidazolium tetrafluoroborate and PVP due to the nucleophilicity of the ring compound forming hydrogen bonds with surrounding water molecules.Nashed et al.[151]studied the inhibition effects of ILs based on induction time measurements.It was found that the inhibition effects of some ILs on hydrate nucleation were even better than that of PVP.Lee et al.[152,153]also found that ILs prolonged the induction time of gas hydrate.The mixture of some ILs and PVCap could further decelerated gas hydrate nucleation and formation.Nazari et al.[154]proposed that ion clustering and salting-out effects were the source of ILs'inhibition effects.Ionization of these organic salts in aqueous media involved strong preferential ion dipole interactions that were stronger than the hydrogen bonds between water molecules.The strong electrostatic interactions led to the hydration of IL ions,which lowered the activity of water molecules and inhibited gas hydrate formation.
4.2.4.Other inhibitors
As one of the most widely used thermodynamic hydrate inhibitors(THIs),methanol has two effects on gas hydrate crystallization.On one hand,the hydroxyl group of methanol molecule formed hydrogen bonds with water molecules,which inhibited the formation of hydrate structure.On the other hand,methanol can greatly reduce the surface tension of methanol-water mixture[155].The test results of methanol and monoethylene alcohol[156]showed that hydrate formation was prevented at a concentration of 10%.However,at ultralow concentration,methanol had dual effects of both inhibition and promotion on gas hydrate formation[157].Ethylene glycol(MEG)is another reliable inhibitor for hydrate formation.MEG with concentrations of 30 wt%-35 wt%could completely prevent gas hydrate formation[158,159].However,its performance was limited in the range of 10 wt% [160].Long et al.[161]added glycine to ethylene glycol and found that the mixture had better inhibition effects.AlHarooni et al.[162]investigated the inhibition behavior of thermally degraded MEG.It was found that thermal treatment caused a depression in hydrate inhibition ability of MEG.Mech et al.[163]found that polyethylene glycol(PEG)with higher molecular weight had stronger inhibition effects.Polyethylene oxide(PEO)was also found to have remarkable kinetic inhibition behaviors at a concentration of 0.5 wt%[164].Polyethylene oxide(PEO)and polypropylene oxide(PPO),two common inhibitors,prolonged the induction time of gas hydrate with the addition of PVP and L-tyrosine[165,166].
SDS is widely regarded as a kinetic promoter.However,Nguyen et al.[167]present a converse finding that SDS with sub-millimolar concentration inhibited gas hydrate formation.Water molecules near SDS showed a strong alignment,which was not conducive to the formation of gas hydrate.The addition of an appropriate surfactant with opposite charge (e.g.TBAB)would eliminate the strong alignment of water molecules and the inhibition effect of SDS on gas hydrate formation.
There are a few cations(including K+,Na+,Mg2+,Ca2+,etc.)and anions(including Cl-,,etc.)in sea water.It was known that NaCl had a strong inhibition effect on gas hydrate formation[168].The inhibition effect of MgCl2on the growth of ethane hydrate was stronger than that of NaCl [169].KCl had a weaker inhibition effect than NaCl [170].Sodium halides also had dual effects on hydrate formation.As was illustrated in Fig.18,these ions formed a hydrophobic crust surrounded by water molecules,which was similar to gas molecules[171].At lower concentrations,the structured shells of sodium halides facilitated the nucleation of gas hydrate.However,at higher concentrations,sodium halides inhibited gas hydrate formation due to the favorable competition between halide ions and gas molecules in the hydration process[172,173].In addition,the effects of calcium carbonate on hydrate formation were also dependent of the concentration[174].
5.Conclusions
Over the past two decades,a large number of studies are reported focusing on different perspectives of gas hydrate formation behavior.The growth behavior of hydrate crystals was mainly affected by subcooling,gas concentration and surfactants at interfaces of different phases.The lateral growth of hydrate film was deduced to be controlled by heat or/and mass transfer while the thickening growth of hydrate film should be controlled by the mass transfer of water or/and guest through hydrate films.The additives in the macroscopic hydrate formation were divided into two classes:promoter and inhibitor.The formation of hydrate can be promoted by increasing the gas concentration in water,reducing the guest/water interfacial tension or changing the nucleation pathway.The inhibitors mostly prevent the construction of hydrate structure by interacting with water molecules to affect its movement.The growth law of hydrate film is very important to the study of hydrate formation kinetics as the macroscopic hydrate formation rate depends on both the growth rate of hydrate film and the renewing rate of hydrate former/water interface.However,hydrate film growth law is rarely applied to develop new additives or methods for the promotion or inhibition of hydrate formation.The overall understanding of hydrate formation kinetics requires a combination of multiscale study of hydrate crystal growth,hydrate film growth and macroscopic hydrate growth.
 Chinese Journal of Chemical Engineering2019年9期
Chinese Journal of Chemical Engineering2019年9期
- Chinese Journal of Chemical Engineering的其它文章
- Decomposition behaviors of methane hydrate in porous media below the ice melting point by depressurization☆
- Advances of experimental study on gas production from synthetic hydrate reservoir in China☆
- Experimental characterization of guest molecular occupancy in clathrate hydrate cages:A review☆
- Hybrid versus global thermostatting in molecular-dynamics simulation of methane-hydrate crystallisation
- Investigation of the hydrate formation process in fine sediments by a binary CO2/N2gas mixture☆
- Investigation on the effect of oxalic acid,succinic acid and aspartic acid on the gas hydrate formation kinetics
