Fundamental mechanisms and phenomena of clathrate hydrate nucleation☆
Jinlong Cui,Zhenfeng Sun,Xiaohui Wang,*,Bin Yu,Shudong Leng,Guangjin Chen,Changyu Sun,*
1 State Key Laboratory of Heavy Oil Processing,China University of Petroleum,Beijing 102249,China
2 China Ship Design&Research Center Co.,Ltd.,Dalian 116001,China
Keywords:Hydrate Nucleation Mechanism Induction time Memory effect
ABSTRACT Insights into the mechanism of hydrate nucleation are of great significance for the development of hydrate-based technologies,hydrate relevant flow assurance,and the exploration of in situ natural gas hydrates.Compared with the thermodynamics of hydrate formation,understanding the nucleation mechanism is challenging and has drawn substantial attention in recent decades.In this paper,we attempt to give a comprehensive review of the recent progress of studies of clathrate hydrate nucleation.First,the existing hypotheses on the hydrate nucleation mechanism are introduced and discussed.Then,we summarize recent experimental studies on induction time,a key parameter evaluating the velocity of the nucleation process.Subsequently,the memory effect is particularly discussed,followed by the suggestion of several promising research perspectives.
1.Introduction
Clathrate hydrates are non-stoichiometric crystalline inclusion compounds,where guest molecules are trapped in cages formed by host water molecules at relatively low temperature and high pressure.The cages are a class of polyhedral cavities with different sizes,which are connected by vertices or faces in a gas hydrate lattice.Various combinations of molecules are required to fill the three-dimensional cages.The stability of hydrate depends on the percentage of its cavities filled by the guest molecules,and a high percentage leads to a more stable hydrate lattice.It has been reported that there are more than 180 different types of molecules that can form hydrates [1].Although a great variety of guest molecules can form hydrate,only three types of crystal structures are identified for gas hydrates,denoted as structure I(sI)[2],structure II (sII)[3],and structure H (sH)[4],respectively.Table 1 summarizes the main properties of these three hydrate lattice cells,each comprising two or three kinds of small polyhedral cavities.For instance,two types of cavities are present in one unit cell of sI hydrate:two small pentagonal dodecahedrons(512),composed of 12 pentagonal edge-sharing rings and six tetrakaidecahedrons(51262),composed of two hexagonal and twelve pentagonal rings.Similarly,the unit cell of sII is composed of sixteen 512cages and eight 51264cages.In a sH unit,three 512cavities,two 435663cavities and one 51268cavity are needed.The structures of all these cavities are shown in Fig.1.
Gas hydrate was found as early as 1811 by Davy [5].However,it drew wide attention only when Hammerschmidt [6]found that the formation of hydrate may lead to the blockages in gas and oil pipelines in 1934.This nuisance may cause damage to the structural integrity of the facilities and ultimately lead to production disruptions.Subsequently,numerous investigations were applied to solve this issue.As a result,the phase equilibrium conditions[7-20]for gas hydrate and the influences of inhibitors[21-24]were extensively studied in this field.It is also notable that the in situ natural gas hydrate(NGH)has received continuous attention since it has been recognized as the largest alternative clean energy resource on the earth.Meanwhile,gas hydrate is accepted as a technology enabler for numerous applications in energy,water,and environment research domains.A series of notable applications,such as energy storage [25,26]and recovery [27-29],carbon sequestration,gas separation[30-32]and desalination of sea water,have become one of the most active research areas of this field.
In terms of clathrate hydrate science,the most important outstanding issues that need to be understood include hydrate nucleation,growth and the memory effect.Although the thermodynamics of hydrate formation and decomposition are relatively well investigated,there are still some major difficulties for understanding hydrate nucleation and growth kinetics.Among them,the primary difficulty is the stochastic nature of hydrate nucleation and the small length and time scales of the formation event.The objective of this work is to review the recent progress on the mechanism and phenomena of hydrate nucleation,including both the theoretical and experimental studies.It isexpected that all the information is helpful for the exploration of natural gas hydrate,gas separation and storage,prevention of hydrate plugging,seawater desalination,etc.

Table 1 Main properties of three hydrate lattice cells
2.Hypotheses on Nucleation
The hypotheses for the nucleation and formation of clathrate hydrate have received considerable attention since its discovery.There have been several hypotheses proposed to give explanations for the microscopic mechanisms of hydrate nucleation.It is notable that these hypotheses could be verified more easily by molecular simulations since the direct visualization of nucleation events,which may occur in nanoseconds and on the nanometer length scale,is still technically challenging.Here,we attempt to review and classify the studies on the basic hypotheses for hydrate nucleation that have been proposed since the early stage of hydrate studies.
2.1.Labile cluster nucleation hypothesis
The fundamental theory regarding the nucleation of clathrate hydrate was first proposed by Sloan and Fleyfel[33]from ice and by Muller-Bongartz et al.[34]from water.The theory was based on“the labile cluster nucleation hypothesis”,in which the labile cluster diffuses in water as a single entity and the nucleus having a critical size is formed by the agglomeration of these labile clusters.Within the framework of this theory,the nucleation of some simple gas hydrate,such as methane hydrate and argon hydrate,can be well described.
It is hypothesized that the nucleation of clathrate hydrate from ice is a series of four consecutive reaction stages[33].In the first stage,free water molecules on the ice surface agglomerate around an absorbed hydrate former (the guest molecule)to form unstable 512cavities.These unstable cavities may either decompose into the original water molecules or agglomerate to form sI or sII hydrate unit.Then,in the second stage,cavities that encage guest molecule may oscillate between the 512cavities of sI and those of sII,after which they eventually form either small units of sI through vertex sharing or small units of sII through face sharing in the third stage.Particles in the third stage are still metastable,and only a fraction of these particles can continue to grow until they eventually reach the critical nucleus size.In the four-stage mechanism,they postulate four first-order chemical kinetic equations for the time dependence of each intermediate set of species.
In terms of the aforementioned hypothesis,Christiansen and Sloan[35]made an extension of the labile cluster nucleation mode to the hydrate nucleation from liquid water,which is shown in Fig.2.
As is shown in Fig.2,hydrate formation process can be described by the following four steps:
(1)Initial condition.There is pure water without guest molecules,but with many transient and labile ring structures of pentamers and hexamers.
(2)Labile clusters.Water molecules rearrange around the dissolved guest molecules to form labile clusters.The unstable clusters are always present despite being able to decompose into water again.The cluster size with different coordinate number of water molecules depends on the size of the dissolved gas molecules.
(3)Agglomeration.The labile clusters act as building blocks in hydrate formation and agglomerate as a consequence of hydrophobic bonding.These agglomerations may either grow or shrink until they reach the critical size.
(4)Primary nucleation and growth.When the size of cluster agglomerations reaches a critical value,unit cells are formed and the growth period begins.To form structure I,two types of clusters with coordination numbers of 20 and 24 are required,while the formation of structure II requires clusters with coordination numbers of 20 and 28.The hydrate nucleation is facilitated if there are clusters with both types of coordination numbers in the phase for either structure I or structure II.If the clusters with required coordination numbers do not exist in the phase,the present clusters must be transformed by breaking or forming hydrogen bonds.The cluster transformation needs an activation energy barrier,which affects the rate of hydrate nucleation.
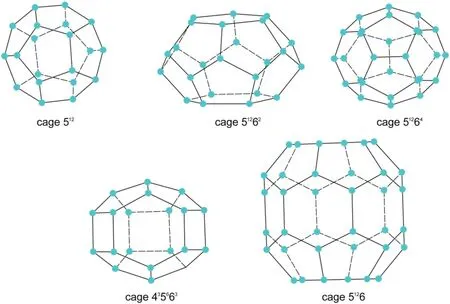
Fig.1.The cages and corresponding crystal structures of clathrate hydrate.
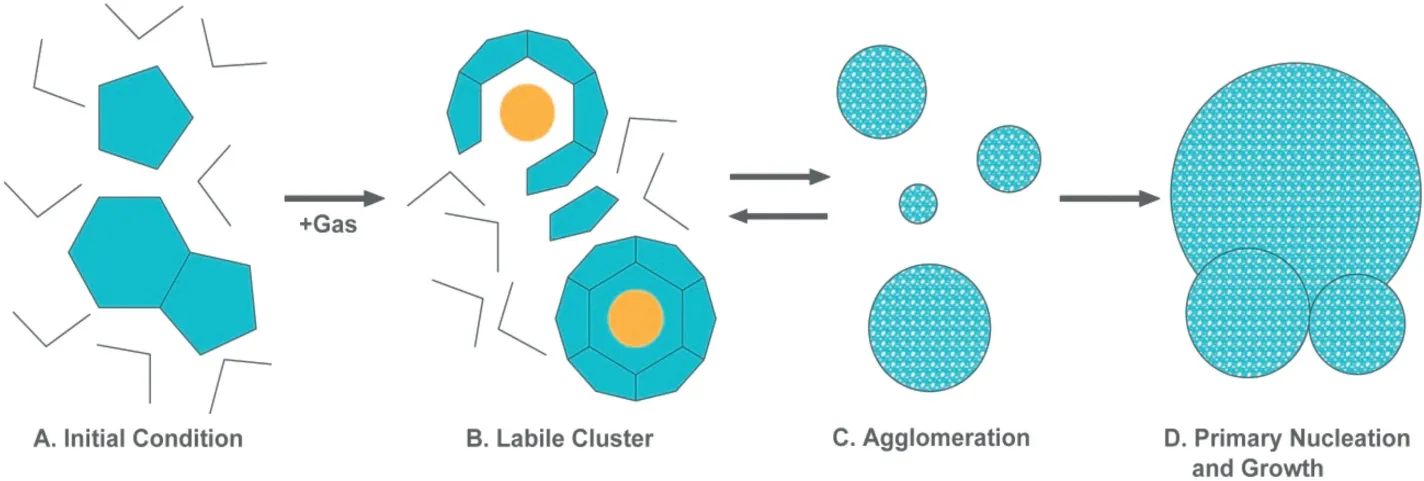
Fig.2.Proposed kinetic mechanism for hydrate formation from water[35].
Although it is difficult to verify the labile cluster hypothesis through experiments,evidence from microsecond molecular dynamic simulations sustains this hypothesis.For instance,the recent work shows the formation of hydrate cages,as the guest molecules interact with the labile water cages,supporting the labile cluster nucleation hypothesis[36,37].
2.2.Reaction nucleation hypothesis
Lekvam and Ruoff[38]proposed a reaction kinetic mechanism for the formation of methane hydrate in water based on their experimental studies.The hypothesized model of hydrate formation consists of three dynamic elements.First,methane gas molecules dissolve into the water.An oligomeric precursor of methane hydrate is then established,followed by the autocatalytic growth of methane hydrate.
This mechanism can be summarized as the following five processes,where CH4(g)and H2O are the reactants,hydrate species H is the product,and the dissolved CH4(aq)and hydrate precursor species N are the reaction intermediates:

where process 1 represents the physical dissolution procedure of gas methane into liquid water,while process 2 describes the formation reaction of hydrate precursor N.Process 3 is the uncatalyzed formation of product H from intermediate precursor N.Processes 4 and 5 describe the autocatalytic growth process of hydrate H from the preceding nuclei N and the dissolved gas with water,respectively.Process 5 shows the formation of methane hydrate directly from the reaction between the dissolved methane gas and water.
The aforementioned formation of methane hydrate in water can be divided into two main procedures,the induction period(processes 1 to 3)and the reactive phase(processes 4 to 5).During the induction period,processes 1 and 2 are in a steady state,while process 3 is the rate-determining step.During this period,processes 4 and 5 are negligible due to the low concentration of hydrate H.Although the model can be particularly good for describing the experimental results between the induction period and the reactive phase,it is still too simple to explain the complex formation event and needs further improvements.
2.3.Interface nucleation hypothesis
A hypothesis of nucleation at the interface was proposed by Long[39]and Kvamme [40].They suggested that the nucleation process occurs on the vapor side of the interface.
The heterogeneous nucleation on the interface can be described as the following steps.First,gas molecules transport to the interface.It is noted by Long [39]that the gas impingement rate is 1022mol·(cm2·s)-1at the equilibrium temperature and pressure of hydrate formation.Kvamme[40]pointed out that molecules transport through a stagnant boundary in this step.Gas absorption on the aqueous surface occurs then,followed by the migration of gas to a suitable location for absorption due to surface diffusion.At this location,water molecules rearrange around the absorbed species to form labile clusters.Finally,the labile clusters agglomerate and grow on the vapor side of the surface until they reach the critical size.
While stressing the role of the interface played in hydrate nucleation,the interface nucleation hypothesis,to a certain degree,contains the essential ingredients of the labile cluster hypothesis.
2.4.Two-step hydrate nucleation mechanism
Chen and Guo [41,42]proposed a two-step hydrate formation mechanism,as shown in Fig.3.In the first step,the dissolved gas molecules combine with surrounding water molecules to form a kind of labile clusters through a quasi-chemical reaction.The labile clusters are fully occupied by gas molecules,and the type of cluster structures is also determined by the sizes of the gas molecules.Then,the formed labile clusters will in turn connect with each other to form the socalled basic hydrate,while another new kind of empty cavity is created naturally.Compared with the basic hydrate,these new formed cavities are called linked cavities and left empty.The second step denotes the non-stoichiometric absorption kinetics of the process.During this step,the empty linked cavities of basic hydrate absorb gas molecules,resulting in the non-stoichiometric property of hydrates.Only small gas molecules,such as N2,O2,and CH4,can be absorbed into these empty linked cavities.The Langmuir absorption theory can be then applied to reasonably describe the filling of the linked cavities by gas molecules.
2.5.Local structuring nucleation hypothesis
Recently,there have been increasing efforts to obtain information regarding hydrate formation by molecular simulations.Among these,Radhakrishnan and Trout[43]proposed the local structuring nucleation theory based on their study on CO2hydrates.They argued that the nucleation proceeds via local structuring rather than by the labile clusters,as proposed by Sloan and Fleyfel[33].

Fig.3.Two-step hydrate nucleation mechanism[41,42].
In their work,the Landau free energy was calculated to evaluate the nucleation mechanism of CO2hydrate at the liquid carbon dioxide-liquid water interface.The result shows that the energy barrier for the agglomeration of labile clusters is much larger than that for disintegration,indicating that carbon dioxide hydrate is not likely to nucleate through the labile cluster mechanism.Radhakrishnan and Trout stated that the rate-determining step in the nucleation event is a concentration fluctuation that arranges a set of guest molecules in a configuration similar to that of the clathrate crystal[43].
Based on the analyses abovementioned,the authors proposed the local structuring hypothesis which is described as follows.The initial process is a thermodynamic perturbation of liquid phase and stochastic in nature.In this process,the CO2molecules are arranged in a configuration similar to that in the clathrate phase due to thermal fluctuation.The structure of the water molecules around these CO2molecules is also perturbed.When the number of the guest molecules in the local ordered arrangement exceeds that of a critical nucleus,the relaxation of the surrounding guest (CO2)and water molecules causes the free energy hypersurface to be locally stable,procuring the formation of the nucleus.
2.6.Blob nucleation hypothesis
As there are arguments between the labile cluster mechanism and local structuring mechanism,a combination of these two theories was proposed by Jacobsen et al.[44]named blob hypothesis.
The schematic diagram of blob hypothesis is presented in Fig.4[45].There are three steps in the formation of the clathrate hydrate in this theory.In the first step,the dissolved guest molecules concentrate in water to form amorphous clusters called“blobs”.The blobs,long-lived aggregates of guests in water-mediated configurations,are in a reversible equilibrium step between the formation and decomposition until they transform into the amorphous clathrate nucleus in the subsequent step,while the clathrate is in the metastable state.In the third step,the amorphous clathrate nucleus transforms into crystalline clathrate.The blob and amorphous clathrate are different,as water molecules are not yet locked into hydrogen-bonded polyhedral cages in a blob.
The blob nucleation hypothesis was proposed based on the combination of the labile cluster and local structuring hypothesis.In the subsequent work of Jacobsen et al.[45],they investigated hydrophobic and hydrophilic hydrate formers and demonstrated that blobs in hydrophobic guest hydrates are rather rare.Lauticella et al.[46]verified the blob hypothesis via the study of the Landau free energy surface.
Generally,these early hypotheses of nucleation are in a process of continuous improvement.With the development of experimental methods and computer technology,new improvements on the mechanism of hydrate nucleation have been made.For instance,a novel theory was proposed by Guo et al.[47]and Guo et al.[48],called the cage absorption nucleation hypothesis.Certainly,more studies are needed for deeper insights into the nucleation mechanism.
3.Induction Time of Hydrate Nucleation
Apart from the abovementioned theoretical studies,most of the experimental studies focused on the induction time of hydrate nucleation.There are several definitions on the induction time[49-51].Up to now,two approaches have been widely recognized for the definitions of induction time.Following Volmer[52],the first approach relies on the presumption that the appearance of the first nucleus indicates the system out of the state of metastable condition.The other approach is followed by the statistical rule regarding the appearance of a great quantity of nuclei and then growth to detectable size,indicating the termination of the metastable condition[51].

Fig.4.Blob hypothesis of hydrate nucleation[45].
Induction time is a very important parameter for clathrate hydrate crystallization which can be used to assess the hydrate nucleation rate.It represents the ability of a supersaturated system to remain in the metastable equilibrium state.Although it is not the fundamental physical property of a system,the induction time still contains valuable information about the nucleation and growth of clathrate hydrate.A long induction time is favorable for flow assurance of gas and oil pipelines,while a short induction time is usually necessary for the hydrate-based applications such as separation of gas mixture,desalination of sea water via forming hydrate,and storage and transportation of natural gas.
3.1.Determination method for induction time
It is quite difficult to quantify the rate of hydrate nucleation.First,the emergence of hydrate nuclei is difficult to be detected.Second,there must be a large number of nucleation events in order to obtain a statistical average value of the nucleation rate.Meanwhile,the experimental conditions must be taken into consideration,such as pressure,temperature and the compositions of the vapor and liquid phases.Nonetheless,great progress has been achieved in the determination of the reproducible hydrate induction time.The widely used methods in determining the induction time of hydrate formation are summarized as follows.
(1)Recording pressure and temperature profiles.This method seems to be the most popular one and has been adopted by many researchers [53-59].In this method,aqueous solution is first loaded,then hydrate forming gas is injected into an autoclave,where the temperature is controlled by a thermal bath.Once the autoclave is filled with gas,the temperature and pressure are recorded in a certain interval,as shown in Fig.5.Generally,the process can be divided into three steps.At first,the pressure gradually decreases as a result of the dissolution of gas into the liquid phase until the vapor-liquid equilibrium is established.The second step is the induction period,where the pressure and temperature tend to be stable.In this step,the liquid phase becomes supersaturated and hydrate nucleation occurs.Small hydrate nuclei continuously form and dissociate until reaching a critical size at induction time tind.In the third step,a distinct drop of pressure accompanied with fluctuation of temperature occurs,due to the growth of hydrate,an exothermic process.With the consumption of gas,the formation of hydrate gradually slows down,and the system pressure finally becomes stable again.Skovborg et al.[59]used the changes of pressure to measure the induction time of CH4,C2H6and their mixtures in a magnetically stirred batch reactor.Subsequently,this method was applied to determine the induction time of propane,carbon dioxide,and natural gas in different systems[56-58].
(2)Direct observation.The reactors with transparent windows and mixing devices have been applied by researchers [60-62]to determine the induction time.The nucleation events could be observed with the naked eye.One identified the beginning of hydrate nucleation when the solution became turbid.Nevertheless,the identification of hydrate formation by the naked eye may result in large uncertainty.
Some advanced and sophisticated instruments have been introduced to determine the hydrate nucleation.For instance,hydrate nucleation in heavy water was measured via neutron diffraction by Benmore and Soper[63].Another method for the determination of hydrate nucleation is the application of a laser granularity analyzer.With this method,Nerheim et al.[64,65]determined the critical radius of the formation of mixture gas(94%CH4+6%C3H8)hydrate.When the stirring rate was 50 r·min-1,the critical radius was estimated to be between 3 and 30 nm.The same method was also used by Yousif et al.[66]later.
It is notable that all of the abovementioned methods are used to identify the induction time in batch reactors.Moreover,there are two methods proposed by Sun et al.[67]to determine the induction time of hydrate nucleation in flow systems as follows.
(3)Shading ratio method.The shading ratio of the flow system can be measured by a laser granularity analyzer.When laser beam penetrates the measuring area,its intensity will be continuously weakened.The intensity of the transmitted light I is less than the incident light I0(I <I0),and the ratio I/I0is called the shading ratio.Finally,the nucleation and growth of hydrate can be confirmed when the shading ratio changes dramatically.

Fig.5.Representative temperature and pressure profiles of hydrate formation[53,54].
(4)Pressure drop method.Pressure drop refers to the flow resistance when the fluid flows in the test section of the pipe loop.It mainly depends on the flow rate and physical properties of the fluid.The pressure drop remains constant when the fluid flow is constant and no hydrates are generated.In contrast,the flow resistance will increase with the formation and growth of hydrate nuclei,causing the pressure drop increases correspondingly.Therefore,the induction time of the flow system can be determined by the pressure drop method.
3.2.The stochastic nature of induction time
Experiments on the induction time reveal that the interval of the induction period in the identical operating conditions is not a constant,which means there is a stochastic characteristic inherent to the nucleation period.Numerous experimental studies have verified this random,non-deterministic characteristic of induction time [68-71].For example,Parent and Bishnoi[71]studied the nucleation behavior of methane hydrate,and the results show that the nucleation process is stochastic.Ohmura et al.[70]and Wilson et al.[69]also demonstrated the statistical nature of the clathrate-hydrate nucleation process in different systems.When studying the nucleation kinetics of propane hydrate,Jensen et al.[58]found that the induction time exhibits a stochastic nature and poor reproducibility at low stirring rate operating conditions.Despite the stochastic nature,the induction time of the hydrate nucleation period can also exhibit high repeatability and reproducibility under certain conditions,such as,high operating pressure[61,72].Servio and Englezos[73]carried out a macroscopic morphological study of methane and CO2hydrate crystals formed on nearly spherical water droplets.The experiments were performed with two droplets or three droplets in different particle diameters on a Teflon covered chamber.Dramatically,all water droplets in a given experiment nucleated simultaneously,which was denominated“bridge effect”by the authors,as shown in Fig.6.After that,this phenomenon was also reported by Lee et al.[74],indicating that the water droplets can nucleate simultaneously even with distinct thermal histories.Englezos and co-workers attributed this effect to the presence of numerous microscopic water droplets on the surface,which would transmit the nucleation information to each droplet,similar to a bridge.

Fig.6.The evolution of two water droplet morphologies under low driving force:(a)the initial state of water droplets at the beginning of the experiment;(b)the water droplets were covered with hydrate when the elapsed time was 10 h[72].
3.3.Factors affecting induction time
In fact,the intrinsic factor determining the nucleation rate or induction time is the thermodynamic driving force,which is the difference between a hydrate forming system and its thermodynamic equilibrium state.The driving force depends on the composition of the hydrate forming system,as well as temperature and pressure conditions.Higher pressure or lower temperature usually corresponds to higher driving force when the system composition is known,which naturally leads to a higher nucleation rate or shorter induction time for a specified system.In addition to driving force,there are also some other factors which can affect induction time.
3.3.1.Guest molecule size
Barrer and Edge[75]first carried out a study on the formation of clathrate hydrate with three different gases (argon,krypton,and xenon).They demonstrated that an induction period was observed for the krypton hydrate formation process,which did not appear in the formation of argon or xenon hydrate.The induction period lasted for almost an hour,and the conversion the krypton to form hydrate could be negligible.However,they did not give a reasonable explanation in their work.The same conclusion that induction time can be markedly influenced by guest molecules was also found by Falabella[76]in his experiment.He conducted investigations on both the kinetic properties and the equilibrium conditions of hydrates of krypton,carbon dioxide,ethane,ethylene,methane and ethane,formed from ice.An apparatus similar to that of Barrer and Edge[75]was used to duplicate the unusual induction period for krypton.The results indicate that there is a clearly defined induction period for both krypton and methane hydrate formation process,while it is not observed in other systems.Subsequently,Sloan and Fleyfel[33]conducted a further investigation in this aspect and found that the induction time was significantly related to the size ratio of the guest molecules to crystal cage,which was confirmed by Skovborg et al.[59]in 1993.They determined the induction time of methane and krypton hydrate formed from ice in terms of the structure perspective,and pointed out that the crystal cage size ratio between 0.81 and 0.89 was susceptible to the induction period.The above observations indicate that ethane and carbon dioxide would not show the induction period,which was proved incorrect by Natarajan et al.[61].
3.3.2.Chemical additives
Many researchers have investigated the influence of chemical additives on the nucleation and growth of gas hydrates.Their findings suggest that proper additives can considerably reduce the induction time and promote the formation of gas hydrates.
For example,Linga et al.[77]investigated the impact of the addition of tetrahydrofuran(THF)on the hydrate formation of CO2/N2mixed gas.The results demonstrate that THF can shorten the induction time and substantially reduce the operating pressure for the equilibrium formation for CO2/N2system.Subsequently,Li et al.[57]studied the capture of CO2from a binary mixture via forming clathrate hydrate with the help of tetra-n-butyl ammonium bromide(TBAB).They suggested that the addition of TBAB to CO2/N2binary mixture system would reduce the induction time and accelerate the hydrate formation process.Apart from thermodynamics promoters,there are another kind of chemical additives that do not change hydrate equilibrium conditions,but could dramatically increase both the hydrate formation rate and the amount of water converted into hydrate.The chemical additives holding such characteristics are called kinetic promoters,such as,sodium dodecyl sulfate(SDS).As shown by Feklistov[78],SDS prevents the hydrate particles from agglomerating,and thereby inhibits the formation of a rigid hydrate film at the liquid-gas interface,which can enhance the mass-transformation of gas molecules.
In addition,some additives may have dual effects,i.e.,inhibition or promotion,on hydrate nucleation and/or growth,which depends on their concentrations in the solution.Based on the modeling results,Ke et al.[79]found that polyvinylpyrrolidone(PVP)showed dual effects of the inhibition and promotion on hydrate nucleation rate under the experimental pressures.The switch from a promoting to an inhibiting effect on nucleation rate was seemingly not affected by pressure or cooling rate,but rather was dependent on the degree of subcooling,ΔT,as shown in Fig.7.Abay and Svartaas [80]previously observed similar dual effects of methanol at ultralow concentrations(0.00015 wt%-0.002 wt%)on structure I methane hydrate.They also reported the promoting effect of PVCap at 0.005 wt%-0.05 wt%on structure II hydrate nucleation.
Some additives,e.g.,surfactants or polymers,in the aqueous solution also play important roles in inhibiting the nucleation of gas hydrates.Two major mechanisms have been proposed for explaining the inhibition effect of thermodynamic inhibitors(KHIs)on hydrate nucleation,namely,the adsorption-inhibition mechanism and perturbationinhibition mechanism[81-88].The former assumes that the presence of polymeric KHI molecules would adsorb onto and cover the surface of clustering embryos thus preventing hydrate nucleation.The latter assumes that KHI molecules would perturb the water phase,preventing water molecules from effectively gathering and forming complete cavities[89].In addition,the inhibition performance of PVP was first reported by Perrin et al.[90].Subsequently,a series of KHIs have been developed and reported,such as Gaffix VC-713 and polyvinylcaprolactan(PVCap).
3.3.3.Ambient disturbance
Exerting an ambient disturbance,such as stirring and spraying,is an effective way to accelerate nucleation and shorten the induction period.These methods promote hydrate nucleation by increasing the gasliquid interfacial area and eliminating heat.As an illustration,Skovborg et al.[59]presented experimental data on hydrate formation kinetics for methane,ethane,and their mixture in a stirred batch reactor.They pointed out that the induction time is obviously dependent on the stirring rate.The induction time appears to be inversely proportional to the stirring rate in a particular range,which was also confirmed by Herri et al.[91].In particular,the influence of the fluid shear rate on the induction time for CO2-THF hydrate formation was investigated [92].Experimental results indicated that the average induction time decreases dramatically with the increase of shear rate.It is worth noting that the agitation could only have a significant influence on the induction time over a certain degree.Jensen et al.[58]demonstrated that the change in stirring rate can only exert little influence on the induction time of propane hydrate,especially at a high stirring rate.
3.3.4.Presence of extra surfaces
The presence of extra surfaces can shorten the induction time.Experiments show that the presence of a solid wall affects the nucleation potency of gas hydrates [93].In the work of Liu et al.[94],iron rods were used in the autoclave,and it was suggested that the metal surfaces may play a key role in promoting the growth of hydrates.In a recent novel study,silver nanoparticles were separately added to methane[53]and ethane[54]hydrate system and observable decreases in the induction time were observed.These results indicate that the presence of silver nanoparticles in the system leads to heterogeneous nucleation and increases the nucleation probability.Meanwhile,these nanoparticles significantly enhance the capability of both the mass transfer and heat transfer in the aqueous solution.
As porous media can provide a high internal surface area,they are widely used to improve the hydrate nucleation and formation rate.It was reported by Seo et al.[95],Adeyemo et al.[96]and Sun and Mohanty[97]that porous media could significantly improve the gasliquid contact area and interaction,thus forming hydrate easily within the pores.Linga et al.[98]evaluated hydrate formation rate in a bed of silica sand and in a stirred vessel,respectively.The results show that hydrate forms faster in the fixed bed of silica sand than in the stirred vessel.Subsequently,Bagherzadeh et al.[99]studied the formation of methane hydrate in the bed of silica sand with different particle sizes,and demonstrated that the smaller the silica particle size is,the faster hydrate forms.The research conducted by Liu et al.[100]shows the average induction time of THF hydrate is inversely proportional to the particle size of porous media.
As mentioned above,the presence of extra surfaces,by adding metal bar,porous media or nanoparticles,exhibits a great advantage in strengthening mass transfer and heat transfer in the solution.This not only could significantly increase hydrate formation rate,but also would become a promising research topic in the future.
3.4.Memory effect
There are a few outstanding scientific puzzles emerging in studies on the nucleation of hydrates,one of which is the so-called memory effect.The memory effect is attributed to the observations that gas hydrate forms faster and more easily when the water is obtained from decomposed hydrate than when it is obtained from fresh water.Hence,the phenomenon that the thermal history of water molecules could shorten the induction time of hydrates is called the memory effect by researchers.There are many reports regarding the existence of this effect.Several hypotheses have also been proposed to explain this effect.

Fig.7.The nucleation rate is a function of subcooling in DIW and 0.005%PVP,respectively[78].
3.4.1.Experimental studies on the memory effect
A seminal work on the memory effect could trace back to the early discussion of clathrate hydrate science by Roozeboom[101]in 1884,who postulated a structural memory effect.After that,Gilpin[102]reported that the thermal history of water can exert an influence on the induction time for ice crystals.Later,Makogon[103]first proposed the idea that clathrate hydrate could be formed rather easily from decomposed hydrate water.He considered the state of water as a primary variable for hydrate formation for the first time.Since then,researchers were interested in the study of the memory effect of clathrate hydrate.As mentioned in the first section,the thermal history of water,one of the variables of the induction period,can affect the hydrate induction times.There are examples of this trend from the experimental studies by Vysniauskas and Bishnoi[62]and Moudrakovski et al.[104].Furthermore,in the work of Giavarini et al.[105],the formation kinetics of propane hydrate by using melting ice and fresh water were investigated.Under identical operating conditions,the formation of propane hydrate from melting ice was instantaneous,while that from fresh water took a long time.Parent and Bishnoi[71]conducted a series of experiments to identify the variables of the methane hydrate nucleation process.In their experiment,three water samples with different thermal histories were used to investigate hydrate nucleation events.The results indicated that hydrate induction time was influenced by the thermal history of water.In addition,when studying the formation of tetrahydrofuran(THF)hydrate in porous media,Liu et al.[100]drew a similar conclusion that the thermal histories of water could reduce the induction time.In a recent work,Zhao et al.[106]developed experimental procedures to study the memory effect of different structured hydrates.The results indicate that the memory effect occurs between different structures of hydrates,which reduces the induction time as well.
Another important area involving memory effect is how this effect depends on the thermal and kinetic histories in the nucleation of clathrate hydrate.It was reported by Link et al.[107]that when methane hydrate is decomposed by warming it to a slightly higher temperature(298.15 K),the memory effect does not appear in the next reformation experiments.Similar results were also reported by Zhang et al.[108]in their research on coal mine methane separation.Another interesting experiment was conducted to investigate the influence of the fractional addition of pre-structured water on CO2hydrate nucleation in the untreated distilled water[109].The authors revealed that even a small fractional addition(e.g.,5 vol%-35 vol%)of thawed water can reduce the overpressure for nucleation by an average of 50%.Sefidroodi et al.[110]made a similar conclusion by investigating the memory effect of cyclopentane hydrate in different thermal treatments.Various degrees of superheating and time intervals were investigated to figure out the conditions where the memory effect would disappear.It was found that the memory effect still existed in the condition of superheating above the equilibrium temperature (7.7 K)within no more than 2-3 K.Meanwhile,at 1.5 K superheating for 20 h or 8.4 K superheating for 20 min,the memory effect was not always observed.Subsequently,detailed studies were conducted by Martinez de Baños et al.[111]and Sowa and Maeda [112],respectively.In the former,a millifluidic method was first used to gain physical insight into the memory effect of cyclopentane hydrate.The results show that the memory effect of hydrate reformation fades with the increase of melting temperature and/or the duration.The latter work applied a statistical method to study the memory effect in methane-propane mixed gas hydrate system.The superheating temperature and the effect of heating time were studied in several sample cells.It was concluded that the existence of the memory effect can cause a significant bias in the stochastic nucleation event and the memory effect fades as the superheating temperature increases.However,there is no clear effect of the heating time.Further quantitative studies on these variables are expected to be performed in the future.
It is notable that there are several abnormal experimental studies on the memory effect,such as the influence of an unusual kinetic inhibitor on this effect or,in some experiments,no memory effect even being observed.The first report that molecules can inhibit the memory effect was conducted by Zeng et al.[113]in 2006.The authors tested the inhibition activities of two different antifreeze proteins on THF clathrate hydrate formation.A striking result was obtained that the antifreeze proteins can eliminate the memory effect.Explanations for this unusual phenomenon will be discussed in detail in the next section.Soon after,Lee and Englezos[114]carried out a study on the influence of unusual kinetic inhibitors on the formation of methane-ethane mixture.The inhibitors were used integrated with polyethylene oxide (PEO),exhibiting a synergistic effect[115].Similar results were obtained that the presence of kinetic inhibitors can reduce the memory effect to some degree,and the extra addition of PEO will enhance this inhibiting effect.On the other hand,the impact of the memory effect on the performance of kinetic hydrate inhibitors was investigated [116].The expected subcoolings of the kinetic inhibitor systems were reduced to some extent.For the special case of the memory effect,there was an experiment on THF hydrate formation and reformation,showing no evidence for the existence of the memory effect[69].Interestingly,some experimental data do not support the hypothesis of memory effect.The authors attributed these phenomena to inherent stochastic nature of heterogeneous nucleation and to the protocols with too few runs[69].They suggested that the melting samples were simply not supercooled adequately,rather than a memory effect existing in the water.
In summary,the memory effect occurs sometimes but does not always appear for all hydrate systems.All these observations are attributed to the complexity of this phenomenon.As a result,experimental and theoretical studies on the mechanism of the memory effect are still a challenging topic for the future.
3.4.2.Mechanism on the memory effect
Based on the aforementioned experimental studies,researchers summarized three types of mechanisms for the memory effect,which are the residual structure mechanism,guest supersaturation mechanism and impurity imprinting mechanism.These mechanisms are discussed below[32].
3.4.2.1.Residual structure mechanism.The first and most popular mechanism for the existence of the memory effect is the residual structure mechanism[70,117,118].In this mechanism,residual structures remain in the liquid phase after gas hydrate dissolution,which promote the reformation of hydrate.The residual structure mechanism,first addressed by Makogon[103],was proposed for the reduction of induction time,and additional work by several researchers followed[62,71].Such residual structures probably refer to polyhedral clusters or partial hydrate cages,whose sizes are smaller than the critical size nuclei.Molecular dynamics simulations of methane hydrate by Rodger[119]demonstrated that there are both water-like and ice-like residual structures existing in the decomposition water,but showed no evidence of clusters of ordered water molecules.Similarly,macroscopic experimental analysis on the nucleation of carbon dioxide hydrate suggested that a metastable residual structure remains in water,which may be the polyhedral water cluster encaging the gas molecule[117].Evidence from an NMR/MRI study of THF clathrate hydrate [120]and from research on TBAB semiclathrate hydrate formation[121]confirmed the existence of residual structure.In the latter,the conformations of TBAB and the O--H stretching bands were not detected yet by the Raman spectra,so the authors concluded that the residual structures may be very small and do not respond to Raman measurements.Unfortunately,the residual structure was not able to be observed even in neutron diffraction experiments on methane hydrate decomposition[122].
3.4.2.2.Guest supersaturation mechanism.In the guest supersaturation mechanism,the memory effect is attributed to supersaturation of guest molecules after the decomposition of clathrate hydrate.It is notable that the supersaturation is ubiquitous during the formation and dissociation of gas hydrate.Computer simulations of methane hydrate nucleation [123]demonstrated that the areas rich in methane molecules nucleate more easily,and no memory of residual crystalline structures is required for hydrate reformation.Therefore,there was no residual structure in the solution after hydrate dissociation.Subsequently,molecular dynamics simulations of methane hydrate decomposition[96,124,125]were conducted to study the conditions for the release of methane into the liquid phase and the formation of bubbles.The results demonstrated that methane molecules are released from the cages,aggregate in the water phase,and then approach the limit of supersaturation,and finally the bubbles form.The spontaneously formed methane nanobubbles can create regions in high concentrations of methane molecules and further affect the thermodynamic state of the solution.In addition,the nanobubbles can act as nucleation centers for hydrate reformation,accelerating the nucleation rate [126,127].Recently,Uchida et al.[128,129]observed the existence of micro-and nanobubbles(MNBs)after hydrate dissociation in the experiment and confirmed the significant role of MNBs on the memory effect.In the study,transmission electron microscope(TEM)was used to observe the decomposition of ethane hydrate in the solution and identify the existence of MNBs,as shown in Fig.8.Then,the induction time of ethane hydrate reformation was measured with and without the addition of such MNBs solution.It is worth mentioning here that MNBs help reduce the induction time and produce a memory effect.
3.4.2.3.Impurity imprinting mechanism.The impurity imprinting mechanism was proposed primarily for the memory effect in heterogeneous nucleation systems by Zeng et al.[113,130].In the experiments,the homogeneous nucleation temperature of micron sized droplets in a THF-water solution was measured to be 243 K,which is close to that observed for water.For the THF-water system,additives such as antifreeze protein did not affect the homogeneous nucleation temperature.Hence,the authors believed that the additives had no effect on the homogeneous nucleation temperature,except for that in the system involving impurities.Therefore,the memory effect was investigated in the experiment under heterogeneous nucleation conditions,indicating that the impurities act as nucleation sites.Based on the above discussion,a mechanism of memory effect for heterogeneous nucleation was postulated,called the impurity imprinting hypothesis.In this hypothesis,the memory effect occurs owing to the alteration of the surface states of impurity particles.Once hydrate crystals nucleated on the surface,the surface was imprinted.These surface imprints can remain in the solution after hydrate decomposition for some time,thus offering the memory effect a certain lifetime,depending on the operating temperature.The inhibitors can be adsorbed onto the impurity particle surfaces,blocking the nucleating sites and eliminating the memory effect.
The mechanism of the memory effect is still under debate,and none of the above hypotheses can account for all the aspects.Further experimental investigations and theories are still required.
4.Future Directions and Perspectives
Insights into the mechanism of hydrate nucleation and growth are of significance for the exploration of in situ natural gas hydrates,the development of hydrate-based technologies as well as hydrate relevant flow assurance.All these issues involve complicated systems,e.g.,gas-water in porous sediments,gas-water-liquid hydrocarbon,and systems containing multiple guests,promoters or inhibitors.However,most of the existing studies on the mechanisms of hydrate nucleation focused on the simple hydrate former-water systems,as shown above.With the considerable progress in molecular dynamics (MD)simulations,we have obtained critical information about hydrate nucleation from these simple systems.In addition,helpful insights into the effect of kinetic inhibitors on hydrate nucleation could also be obtained.Although the studies summarized above give us useful knowledge on the mechanism of hydrate nucleation,several challenges and limitations in the experiments and simulations still exist.For example,the spatial and time scales of these simulations are still insufficient to compare with their experimental counterparts.Therefore,the following aspects need to be considered in future studies.

Fig.8.TEM images of micro-and nanobubbles(MNBs)in the replica after CH4hydrate dissociation.(a),(b)One hour after hydrate dissociation;(c),(d)3 h after hydrate dissociation.Each scale bar represents 500 nm[129].
(1)The basic physical properties,such as wave velocity of the hydrate-bearing sediment,depend on not only the saturation of the hydrate but also the local distribution of hydrate within the sediments.It has been well proven that the hydrates covering the sand particles can consolidate sediment and make significant contribution to the increase of wave velocity compared to those located freely in the pores of sediments.As the wave velocity is a critical parameter in the exploration of natural gas hydrate,it is important to understand the mechanism of hydrate nucleation of the hydrates in sediments,especially the first location of hydrate nucleation,and the interaction modes of hydrate with sand surfaces.
(2)The memory effect,whether it is a favorable or negative factor or a nuisance,plays an important role in the applications of clathrate hydrate.However,the fundamental explanation of the memory effect is still under debate and none of the existing hypotheses can account for all the aspects.With the development of computational and experimental research methods,the fundamental molecular-level understanding of this effect is valuable.Further investigations and theories are still required.
(3)For systems containing chemical additives,compared with kinetic inhibition,investigations on the kinetic promoting mechanisms of some surfactants are quite insufficient.Nevertheless,these mechanisms are important for the development of hydrate-based technologies,thus deserving further attention.
(4)As mentioned in the above review,some of the additives may have dual effects on the nucleation of gas hydrates,including promotion and inhibition,which depends on their concentrations in the solutions and degrees of subcooling.However,in industrial production,the dual effects,which will increase the risk of inappropriate determination of the dosage of additives,should be avoided.For example,some new developed inhibitors exhibit good performance in labs,but adversely affect the formation of gas hydrate in large-scale flow loops.Hence,it is quite important to figure out the mechanism of the dual effects of an additive,as well as the relationship between additive dosage and performance.In this aspect,molecular simulation will play an increasingly important role in the future.
 Chinese Journal of Chemical Engineering2019年9期
Chinese Journal of Chemical Engineering2019年9期
- Chinese Journal of Chemical Engineering的其它文章
- Decomposition behaviors of methane hydrate in porous media below the ice melting point by depressurization☆
- Research progress in hydrate-based technologies and processes in China:A review☆
- Methane hydrates:A future clean energy resource
- Progress and trends in hydrate based desalination(HBD)technology:A review☆
- Extraction of methane hydrate energy by carbon dioxide injection-key challenges and a paradigm shift
- A short review on natural gas hydrate,kinetic hydrate inhibitors and inhibitor synergists☆
