Effect of Co-sensitization of Dyes with Different Acceptor Moieties for Dye-sensitized Solar Cells
ZHAO Xue, PEI Juan, LI Yingpin
(College of Sciences, Hebei University of Science and Technology, Shijiazhuang 050018, Hebei, P.R. China)
Abstract: In order to broaden the response range of dye-sensitized solar cells to the solar spectrum and improve the photovoltaic performance of the cells, two triphenylamine dyes (TR1 and TC1) containing different acceptor moieties (rhodanine-3-acetic acid (RA) and cyanoacrylic acid (CA)) were co-sensitized. The TR1 dyes adsorbed on TiO2 surface with a lied down mode, while the TC1 dyes with a standing adsorption mode. When the two dyes were co-sensitized on TiO2 in different molar ratios, TC1 would occupy part of the positions of TR1, which could expand the spectrum and inhibit the charge recombination at the same time. The electron lifetime of the co-sensitized solar cell device was longer than that of TR1-sensitized solar cell. Finally, the co-sensitized device sensitized by co-sensitizer solution in which the molar ratio of TR1 to TC1 is 5∶5 yielded short-circuit photocurrent density (Jsc) of 11.7 mA/cm2, open circuit voltage (Voc) of 704 mV, fill factor (FF) of 0.73 and the highest efficiency of 6.03%. This performance is superior to those of solar cell devices sensitized by the two dyes individually.
Key words: dye-sensitized solar cells; co-sensitization; triphenylamine dyes; photovoltaic performance; impedance
1 Introduction
Dye-sensitized solar cells (DSCs) reported in 1991 are regarded as low-cost next generation solar cells, and significant progress has been made in the past few decades[1-3]. In DSCs, the sensitizers play a crucial role due to their critical function in light harvesting and electron injection[4]. Uptill now, DSCs based on metal-complex sensitizers such as Ru complexes and Zn porphyrin photosensitizers have achieved efficiencies up to 11% and 13%, respectively[5,6]. However, the limitations corresponding to these complexes such as the rare resources of ruthenium and zinc metals, high manufacturing cost, and environmental issues would restrain their widespread applications[7]. So other organic metal-free sensitizers have attracted much attention for researchers in view of their advantages, such as larger molar extinction coefficients, higher structural flexibility, lower cost and environmentally friendliness[8,9]. It was worthy noting that the record highest efficiency of 12.5% has been achieved by Wang and coworkers[10].
Generally, metal-free organic sensitizers are constituted by donor-(πconjugated spacer)-acceptor (D-π-A) structure. However, the absorption spectra of most of the D-π-A dyes are narrow, which is not beneficial for higher short-circuit current (Jsc). Therefore, further studies are needed to develop to reduce the charge recombination and broaden the range of light-harvesting. Co-sensitization is an effective approach, by which a combination of two or more dyes adsorbed together on semiconductor surface, extending the light-harvesting ability to enhance the short-circuit photocurrent density (Jsc) and thus the efficiency (η) of DSCs[11]. Oguraetal. used a co-sensitizer composing terpyridine complex (black dye) with an indoline dye (D131) and resulted in a significantly enhanced photocurrent, leading to a device performance of 11%[12]. Koetal. explored a Ru complex (JK-142) as sensitizer in combination with an organic dye (JK-62), and positioned them on TiO2film[13]. Surprisingly a considerably improved efficiency of up to 10.2% was achieved, which is favorably superior to that of N719 (8.68%) in the same device configurations. In 2011, a DSC with a high photovoltaic performance of 12.3% was demonstrated with the co-sensitization of a zinc porphyrin dye YD2-o-C8 and a metal-free organic dye Y123 with cobalt (Ⅱ/Ⅲ)-based redox electrolyte[14]. Of course, there are also successful examples of co-sensitized DSCs using solely metal-free organic dyes. For instance, Choi et al. achieved a high performance of 8.65% by co-sensitizing JK2 and SQ1 on Al2O3-coated TiO2films[15]. Sunetal. applied cyanine dye CM203 to co-sensitize with other two organic dyes (CMR103 and HY113), theJscwas markedly increased due to the spectra response to the whole visible domain, and consequently the conversion efficiency of 8.2% was achieved[16]. In addition to much emphasis on the effect of spectral complementation ameliorating theJscmentioned in the above research, dyes in co-sensitized system also have some influence on open circuit voltage (Voc) and thenJscorη. Almost all theJscandVocof co-sensitized cells exhibit different degree of increase compared with those of DSCs based on individual dyes.
As a part of our ongoing efforts to design and synthesize triphenylamine-based organic dyes, we also interested in co-sensitizing multiple dyes for improving the performance of DSCs[17-20]. We noticed an interesting trend in which sensitizers incorporating cyanoacrylic acid (CA) moiety as the acceptor/anchoring group commonly exhibited higher Voc values than the corresponding ones bearing identical donors andπ-bridges but a different acceptor of rhodanine-3-acetic acid (RA) moiety. In the present work, the co-sensitization of two metal-free triphenylamine dyes with different acceptor moieties (RA and CA) is applied to DSCs. Furthermore, the relationship between the photovoltaic properties and triphenylamine dyes are also discussed in detail.
2 Experiment
2.1 Preparation of Dye-sensitized Nanocrystalline TiO2 Electrodes and Characterization
The two triphenylamine dyes TR1 and TC1 with different acceptor moieties were synthesized following the previous reports[20,21]. The chemical structures of these dyes are shown in Figure 1. Nanocrystalline TiO2films were prepared by coating TiO2paste onto the F-doped transparent conductive glass (F-doped SnO2, short for FTO) as described by Grätzel and coworkers[22]. Briefly, FTO substrates (10 Ω/sq, >85% transparency in the visible region, Yaohua, China) were treated with TiCl4(aq., 50 mmol/L) for 30 min, followed by screen-printing a paste consisted of TiO2(P25, a mixture of 30% rutile and 70% anatase, Degussa AG, Germany; 16%), ethyl cellulose (20-30 cp, 5%) and terpinol (79%). The film was successively fired at 450 ℃ under air for 30 min, treated with TiCl4solution and fired again to give a ~15 μm thick mesoscopic TiO2film.
Dyes TR1 and TC1 were dissolved individually in absolute methanol to form dye-sensitizer solutions with equal concentration (5×10-5mol/L) and volume (10 mL). Mixing TR1 and TC1 solutions with different volume ratios of 9∶1, 7∶3, 5∶5, 3∶7 and 1∶9 would get the co-sensitizer solutions, in which the total concentration and volume are still the same as those of individual dye solutions. Dye sensitization was carried out by immersing the TiO2electrodes in TR1 or TC1 solutions while the TiO2films were still hot. This process gave TR1/TiO2and TC1/TiO2electrodes. Co-sensitized electrodes were obtained by immersing TiO2films in the prepared mixed solutions with TR1 to TC1 molar ratios of 9∶1, 7∶3, 5∶5, 3∶7 and 1∶9, which were coded as Co1/TiO2, Co2/TiO2, Co3/TiO2, Co4/TiO2and Co5/TiO2, respectively. The absorption spectra of the dyes in methanol solutions and adsorbed on TiO2films were measured with a Jasco V-550 UV/Vis spectrophotometer.

Figure 1 Molecular structures of triphenylamine dyes TR1 and TC1
2.2 Fabrication and Characterization of Dye-sensitized Solar Cells
The dye-sensitized TiO2photoanode and the platinum coated FTO photocathode were separated by adhesive tapes (45 μm thick) to form a space, in which a redox electrolyte (0.1 mol/L LiI, 0.05 mol/L I2, and 0.6 mol/L DMPImI in acetonitrile) be introduced. The cells were finalized by firmly clamping the two electrodes together.
For photovoltaic measurements of the DSCs, a 500 W Xe lamp (400-800 nm) served as the light source in combination with a optical filter to remove ultraviolet and infrared radiation. The light intensity was held at 100 mW/cm2at the surface of the test cells with the aid of a radiant power meter (Oriel Model 70260 with 70268 Probe). I-V characteristics were recorded with a digital source meter (Keithley 2400) controlled by a computer. Monochromatic incident photon to current conversion efficiency (IPCE), plotted as a function of excitation wavelength, was recorded on a home-built system equipped with a bromine tungsten lamp and a grating spectrometer (SBP300, Zolix). The IPCE system was calibrated using a silicon reference cell (the 18th Research Institute of Electronics Industry Ministry, China). Electrochemical impedance spectra (EIS) measurements of the DSCs were carried out using a potentiostat/galvanostat/FRA (PARSTAT 2273) in the dark with the frequency range of 100 mHz to 100 kHz. The applied bias voltage and alternate current amplitude were set at -0.65 V and 0.01 V, respectively. The resulting curves were fitted to the appropriate equivalent circuit using Z-view software.
3 Results and Discussions
The absorption spectra of dyes TR1 and TC1 in absolute methanol solution are shown in Figure 2(a). The absorption peaks of TR1 and TC1 in methanol are at 470 and 410 nm. The corresponding maximum molar extinction coefficients (εmax) are 4.47×104mol-1·L·cm-1and 2.35×104mol-1·L·cm-1, which are higher than those of Ru complexes (~1.4 × 103mol-1·L·cm-1), indicating a good ability of light harvesting[23]. Noticeably, the photoresponse could complement to each other to some extent, especially in the range of 380-550 nm.
Figure 2(b) shows the absorption spectra of TiO2films sensitized by dyes TR1 and TC1, with that of pure TiO2film as a reference. In comparison to the spectra of dyes in methanol solution, the absorption spectra on TiO2films are broadened and red-shifted from 470 nm to 486 nm for TR1, and 410 nm to 431 nm for TC1, implying that the formation of dye aggregates on TiO2films[24]. Spectra of TiO2films sensitized by co-sensitizer solutions are also shown in Figure 2(b). In general, the absorption intensity ratio of dye TR1 part to TC1 part became smaller with the concentration increase of dye TC1, but with the absorption onset at almost the same position.
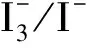
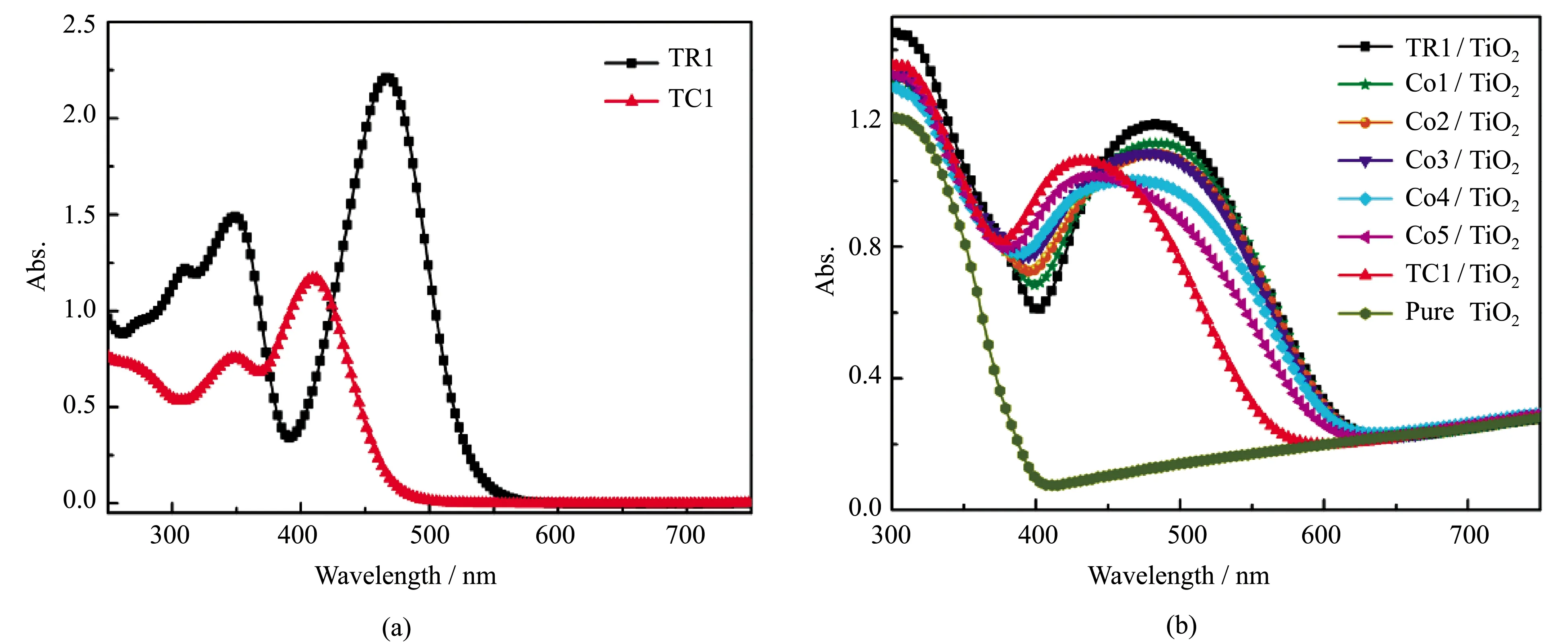
Figure 2 Absorption spectra of dyes TR1 and TC1 in methanol solution (a), and all TiO2 films sensitized in different sensitizer solutions with different volume ratios, with that of pure TiO2 film as a reference (b)
Figure 3 shows the current-voltage characteristics of DSCs sensitized by individual dyes TR1 and TC1 (DevicesA,B). The corresponding photovoltaic parameters (Jsc,Voc,FFandη) of these DSCs are summarized in Table 1. DeviceBshows significant higherVocvalue (720 mV) than that of DeviceA(633 mV), but lowerJscvalue (8.10 mA/cm2) than TR1 (9.10 mA/cm2). Many research groups, including ours, have attributed the higherVocvalues of TC dyes to their adsorption mode on TiO2[20]. TC dyes with standing adsorption mode have larger adsorption angles, and show larger dipole moments in the direction normal to the TiO2surface than TR dyes (lie down along the TiO2surface) (Figure 4). It is likely to shift the conduction band of TiO2to more negative position and generate higherVocvalues[20]. Additionally, the charge recombination is relatively reduced for DeviceB, which is in consistent with the decreased order of dark current for comparing TC1-based cell device to TR1-based one (Figure 3), and thus benefit for voltage improvement. Finally, efficiencies of 4.15% and 4.01% are obtained for Devices A and B, respectively. And dye TR1 is subjected to co-sensitize with dye TC1 because the latter could compensate for the deficit of the former in the photoresponse and charge recombination.

Figure 3 Current density-voltage characteristics of DSCs (Devices A-G) sensitized by TR1, TC1 and co-sensitizers; and dark-current of Devices A, B and E

Figure 4 Adsorption mode of the two triphenylamine dyes on TiO2 surface
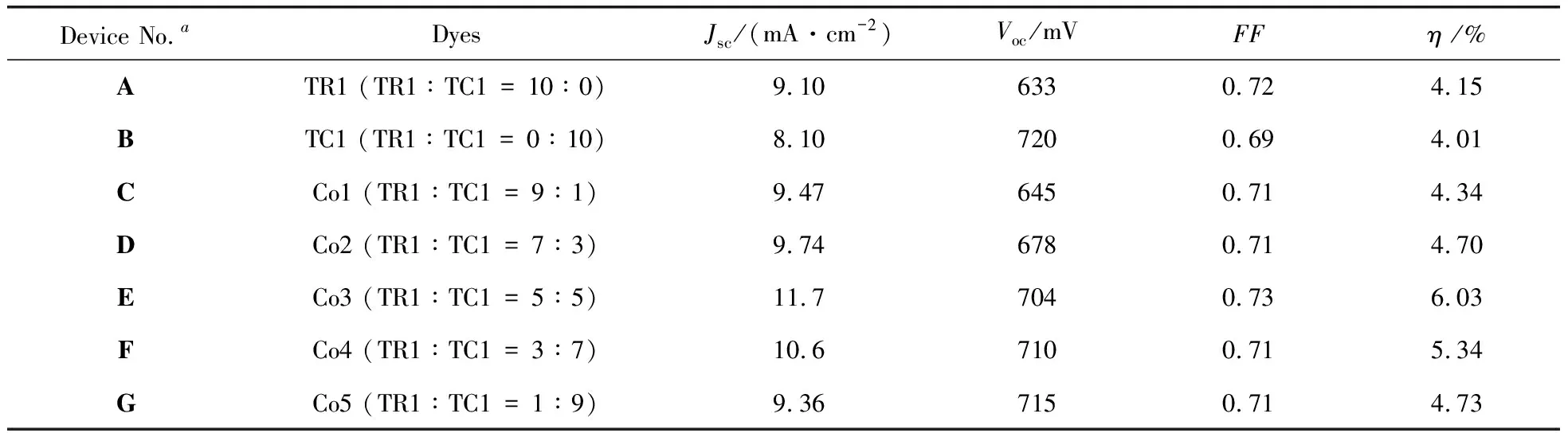
Device No.aDyesJsc /(mA·cm-2)Voc /mVFFη /%ATR1 (TR1∶TC1 = 10∶0)9.106330.724.15BTC1 (TR1∶TC1 = 0∶10)8.107200.694.01CCo1 (TR1∶TC1 = 9∶1)9.476450.714.34DCo2 (TR1∶TC1 = 7∶3)9.746780.714.70ECo3 (TR1∶TC1 = 5∶5)11.77040.736.03FCo4 (TR1∶TC1 = 3∶7)10.67100.715.34GCo5 (TR1∶TC1 = 1∶9)9.367150.714.73
a. Devices A and B are the cells sensitized by TR1 and TC1 dye solutions, while Devices C-G are the cells sensitized by the co-sensitized dyes.
DSCs sensitized by dye mixture solutions (DevicesC-G) with different molar ratios are also characterized. It can be seen from Figure 3 and Table 1 that,Vocare increasing all along with the increasing adsorbed amount of dye TC1 obviously. Because dye TC1 molecules occupy some positions of dye TR1, the charge recombination would be reduced for reasons mentioned above. For example, the dark current of DeviceEis decreased than DeviceA, but serious than DeviceB(Figure 3). And the more the TC dye, the smaller the dark current is. On the other hand,Jscvalues are not always increasing by decreasing the co-sensitizer molar ratio of the mixture solutions (from DeviceCto DeviceG). It is intriguing that the largestJscvalue reaches 11.7 mA/cm2for DeviceE. Then theJscandηbecame to decrease, but still higher than solar cells sensitized by individual dyes. The highest efficiency of 6.03% is achieved for DeviceEsensitized by the mixture solution containing equivalent concentration. These results indicate that co-sensitization of the two dyes is an effective strategy to improve the efficiency of DSCs.
Figure 5 presents the IPCE spectra of solar cells using the individual dyes (DevicesAandB). Although the photoresponse range of TC1 dye is narrower than the corresponding TR1 dye, a rather higher IPCE plateau value for TC1-sensitized solar cell (DeviceB) is performed. It is probably due to better electron injection into TiO2for TC1 dye and/or the reduced charge recombination that make the charge collection efficiency increase at the photoanode. The corresponding IPCE spectrum of cell DeviceEis displayed also in Figure 5. It is found to be broadened by covering the visible domain from 400 to 700 nm in comparison with those of solar cells sensitized by individual dyes TR1 and TC1. The peak values of 80% and 88% are at 530 and 460 nm, respectively, corresponding to the absorption spectra of dye mixtures on TiO2film.

Figure 5 IPCE spectra of Devices A, B and E

4 Conclusion
In conclusion, we demonstrated that co-sensitization by two metal-free triphenylamine dyes with different acceptor groups (TR1 and TC1) is a successful approach to improve the performance of DSCs. The Voc of the cells were increasing all along with the decreased volume ratio (also molar ratio) of TR1∶TC1 in the co-sensitized solution, resulting from the standing adsorbed TC1 dye molecules take up some positions of TR1 with lied down adsorption mode. While theJscof the cells reached the max. at 5∶5 molar ratio, which profited from the complimentary spectra response of the two dyes on the one hand, and the enhanced the electron collection efficiency for the decreased dark current on the other hand. The best efficiency of 6.03% was obtained for the cell sensitized by the co-sensitizers with 5∶5 molar ratio in mixed solution. These results shown here not only provide new vision on how to produce highly efficient solar cells using dyes with various molecular structures, but also motivate other groups to explore more efficient co-sensitizers through controlling the molecule structure to further enhance the performance of DSCs.

Figure 6 Impedance spectra of DSCs (Devices A, B and E) measured at -0.65 V bias in the dark

