Magnetically controlled growing instrumentation for early onset scoliosis: Caution needed when interpreting the literature
Kenneth Aaron Shaw,Justin M Hire,Scott Kim,Dennis P Devito,Michael L Schmitz,Joshua S Murphy
Kenneth Aaron Shaw,Department of Orthopaedic Surgery,Dwight D.Eisenhower Army Medical Center,Fort Gordon,GA 30905,United States
Justin M Hire,Department of Orthopaedic Surgery,General Leonard Wood Army Community Hospital,Fort Leonard Wood,MO 65473,United States
Scott Kim,University of Tennessee Health Science Center,Memphis,TN 38163,United States
Dennis P Devito,Michael L Schmitz,Joshua S Murphy,Department of Pediatric Orthopaedic Surgery,Children's Healthcare of Atlanta,Scottish Rite Campus,Atlanta,GA 30342,United States
Abstract
Key words:Complications;Early onset scoliosis;Magnetically controlled growing instrumentations;Keeper plate;Reoperation;Systematic review
INTRODUCTION
Early onset scoliosis (EOS) is a complex entity that has seen an evolution in its approach to surgical intervention from early definitive fusion,to non-fusion technique that allow and facilitate continued spinal growth[1].Magnetically controlled growing rods (MCGR) are one such non-fusion approach that has gained interest and support since its introduction in 2007[2].MCGR has been found to be a safe and effective nonfusion treatment for EOS[3-5],with equivalent curve correction and thoracic height growth as compared with traditional growing rods (TGR)[6].Clinical reports,however,on the outcomes and complications of MCGR have been limited to case series and cohort studies with limited patient numbers[2-23].
Thakaret al[24]preformed a retrospective review of reported studies using MCGR for the treatment of EOS.From an identified 15 studies including 336 children undergoing MCGR insertion,they identified a mean complication rate of 44.5%,with 33% of children undergoing an unplanned reoperation.However,the timeline of these studies included spanned a seven year period since the introduction of the implant[24].Over this period,the manufacturers made several alterations to the implant design,consisting first of the addition of a keeper plate in 2010 to the actuator to decrease the incidence of lost distraction,followed by alterations to the welding process in 2012,as well as expanded size options in the rod and actuator[2,25].
Early reports identified a high rate of loss of distraction due to the magnetic lengthening mechanism being unable to maintain the rod in the lengthened position.Due to the rotatory mechanism of lengthening,this inability to lock the rod in the lengthened position,the actuator was prone to unwind and shorten resulted in a loss of distraction[2,25].To combat this,a magnetic lock,the keeper plate,was applied around the lengthening mechanism to maintain the rod in place at its desired length and prevent the rod collapse identified in the early implant iterations,Figure1.However,the efficacy of the keeper plate to decrease the rate of loss of distraction has not been previously reported.
The aim of this study is to examine the reported literature on the reporting of implant iterations as well as its effect on the post-operative complication rates following MCGR implantation for the treatment of EOS,specifically the effect of the addition of the keeper plate.We hypothesized that the reporting of implant iteration would be limited and the rate of postoperative complications,specifically the rate of distraction loss,would be significantly lower in children treated with implants containing a keeper plate.
MATERIALS AND METHODS
Literature search
After obtaining institutional review board approval,a comprehensive systematic review was conducted using an internet-based search beginning with queries into the MEDLINE database for all articles between January 1,1967 and February 1,2018.The search terms included: (1) “early onset scoliosis”;(2) “magnetically controlled growing rods”;(3) “scoliosis”;and (4) “magnetically controlled growing rods complications”.The preferred reporting items for systematic reviews and metaanalyses protocol was followed for data analysis and synthesis[26].
Study selection
The abstracts of all identified articles were subsequently analyzed to determine relevance to complications associated with MCGR for early-onset scoliosis.Articles were excluded for one or more of the following criteria: Literature review or expert opinion,publication in non-English language,published prior to the year 1967,did not include pediatric patients,included fewer than 3 patients,implanted instrumentation other than MCGR.Studies reported from the same institution were further scrutinized to determine if overlapping patient cohorts were reported,excluding studies with shorter average follow-up.
A total of 49 articles were identified for further review.The full manuscripts of the remaining studies were then reviewed for the following inclusion criteria: Peerreviewed clinical studies of level I to IV evidence,involving pediatric patients undergoing surgery for implantation of MCGR,and reporting the number of perioperative complications and unplanned procedures.The references of all articles were cross-referenced as well for any additional articles that were not found on the initial search.The patient cohorts of studies with the same authors and/or institutions were scrutinized to ensure that no redundant data was collected.
Articles were further reviewed to determine the iteration of implant utilized.Since its introduction,there have been 7 main alterations to the implant design with the earliest change being the addition of a keeper plate,introduced in 2010,to correct early issues with loss of distraction[2,25].Articles were reviewed to delineate between series with and without the keeper plate based upon either direct report or time period reviewed in each study.For studies that did not specify the iteration of implant used,surgical dates were reviewed with years before 2010 defined as pre-Keeper plate series.Studies with mixed implants utilized were included in the analysis if they included > 80% of procedures with a specific implant.Studies with overlapping surgical dates were excluded.
Patient demographics (age,gender,curve etiology),construct design (number of rods implanted,technique,anchors placed),and the frequency and number of lengthening’s were extracted from each article.Complication rates were recorded for each study.Complications were classified as either major or minor,with major complications defined as complications necessitating cessation of treatment (failure of distraction) or revision surgery (implant failure to include rod breakage,screw pullout,proximal junctional kyphosis,deep surgical site infection,or sequela that did not resolve without significant interventions).Minor complications were defined as prominent hardware,superficial surgical site infection,or issues that required minimal intervention without repeat surgical intervention.Reoperation or need for revision surgery was recorded as a separate variable.
Data analysis was performed using SPSS statistical package version 24 (SPSS Inc,Chicago,IL,United States).Significance was set atP< 0.05.Descriptive statistics were generated.Univariate analyses were used to compare overall complication rates by implant iteration,specific complication rates,and to identify risk factors for post-operative complications.
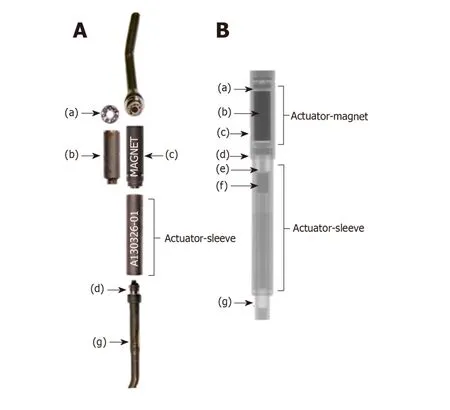
Figure1 Clinical and radiographic image of a magnetically controlled growing rod after sectioning.A: Clinical image of a magnetically controlled growing rod after sectioning;B: Radiographic image of a magnetically controlled growing rod after sectioning.The keeper plate (label c) is seen in its position around the magnet (label b).The Figureis adapted from Panagiotopoulou et al[31].
RESULTS
A total of 49 studies were identified for manuscript review.After review of the manuscripts,26 were excluded (7 mechanical failure studies,6 cost comparison studies,3 imaging studies,2 case reports,2 editorial,2 non-human studies,2 animal studies,1 case series,and 1 review article).Of the remaining 23 clinical articles,3 additional studies were excluded (1 each with insufficient patient number,overlapping patient samples,combined MCGR/Shilla technique) leaving 20 clinical studies for review.Of these 20 studies,an additional 5 studies were excluded due to mixed implant iterations leaving 15 studies that met inclusionary criteria,consisting of 11 case series and 4 cohort studies,Figure2.
From the 15 clinical articles,a total of 271 children were identified (7.87 years ± 1.54 years,46.8% male) with an average of 26.4-mo follow-up.Curve etiology is summarized in Table1,with idiopathic (32.8%) reported as the most common,and an average curve magnitude of 61.3 degrees.Pre-keeper plate implants were utilized in 3 studies with remaining 12 post-Keeper plate implants.The majority of cases were primary MCGR implantations (74.7%)vsconversion procedures (25.2%).Dual rod instrumentation (76.4%) was the most common construct,with children undergoing an average of 7.85 lengthening’s.
From the identified 271 children,115 (42.4%) experienced a post-operative complication,Table2.Of the 115 complications,95 (82.6%) were defined as major,with an average major complication rate of 80% per study.Complications were not subdivided according to curve etiology.Failure of distraction was the most common complication,occurring in 14% of children,followed by implant failure (including rod breakage and implant failure not otherwise characterized) in 8.86%,and screw/hook pullout (8.12%),Table2.Of the 115 children with a postoperative complication,69 patients (27.9% of overall cohort) required an unplanned reoperation.The most common reason for reoperation was the inability to distract (n= 20),followed by proximal instrumentation pullout with or without proximal junctional kyphosis (n=19),rod breakage (n= 19),wound dehiscence/infections (n= 6),prominent hardware(n= 2),and 3 unlisted procedures.
Univariate analysis of complications between implant iterations identified that complication rates significantly decreased with the addition of the keeper plate (35.7%vs80.6%,P= 0.036,Table2).Additionally,there was a statistically significant decrease in the rate of distraction failure in the keeper plate cohort (8.1%vs40.8%,P= 0.02).There was not difference in reoperation rates between implant iteration cohorts(25.5% without keeper platevs27.1% with keeper plate,P= 0.92).Identified studies did not provide information for revision surgeries according to type of instrumentation (single rodvsdual road),or by proximal anchor type (ribvsspine) or number of proximal anchor points.Given the paucity of available data,a subgroups analysis was foregone.Summary of articles included for analysis is shown in the Table3.
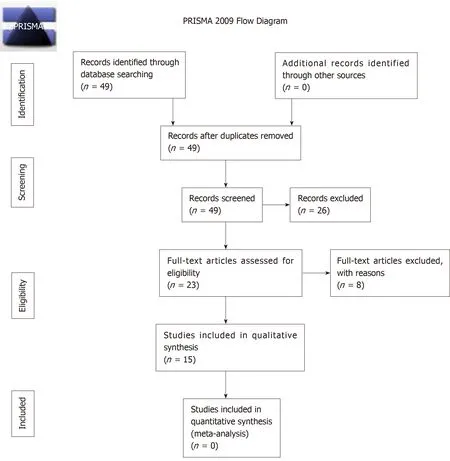
Figure2 The preferred reporting items for systematic reviews and meta-analyses flowchart depicting protocol for reviewing studies considered for inclusion.PRISMA: Preferred reporting items for systematic reviews and meta-analyses.
DISCUSSION
Through this systematic review,we identified that children treated with all types of MCGR implants for EOS have a 42.4% rate of postoperative complications at an average of 26.4-mo follow-up after implantation,with failure of distraction being the most common complication seen in 14%.The implant iteration was found to significantly affect complication rates with the keeper plate-enabled implants significantly decreasing the rate of postoperative complications (35.7%vs80.6%).However,of the 20 studies published at the time of this review,25% included mixed implants iterations in their retrospective reviews.
Complications in the treatment of EOS are not infrequent,given the patient age and the necessity to accommodate continued growth of the thorax and spine.TGR instrumentation preceded MCGR in the treatment of EOS,with well-reported complication profiles.Besset al[27]reported that 58% of patients developed at least one complication during their treatment duration,with higher rates of complications with the use of single rod fixation,decreasing patient age,and with each additional lengthening procedure.Yanget al[28]identified underlying scoliosis etiology,prior rod failure,single rod constructs,stainless steel rods,small diameter rods,and tandem connector variables as risk factors for rod failure with TGR.Additionally,the requirement for repeat surgical interventions for lengthening increase the rate of wound and other complications 24% for each additional lengthening procedure[28].
MCGR was developed in an attempt to meet the need for continued spinal growth and curve correction while attempting to decrease the risk of post-operativecomplications.MCGR functionally lengthens the spinal construct through the application of an external magnet which induces a rotatory motion to the actuator,which is threaded,resulting in elongation[2].Akbarniaet al[6]performed a casematched comparison of children with EOS treated with MCGR and TGR,finding equivalent curve correction and thoracic height gain.Although the MCGR cohort had less overall surgical procedures,the incidence of unplanned reoperation secondary to post-operative complications was not affected,with 75% of MCGR reoperations occurring secondary to unspecified implant failures.

Table1 Summary of patient and surgery characteristics for identified patients undergoing magnetically controlled growing rods instrumentation,n (%)
Unique to MCGR is the risk of rod distraction failure[29],which accounts for between 25%-35% of unplanned surgical procedures[4,29].The current findings reinforce previous studies[24],that these instances are not isolated,with loss of distraction accounting for 33% of all complications,and 28.9% of reoperations.Numerous mechanisms for distraction failure have been identified in the literature,to include: Fracture of the actuator pin,wear of the extending bar,debris in the actuator,damage to the radial bearings,and O-ring seal failure[30,31].Loss of distraction ranged in the reported articles,accounting for between 0% to 100% of complications,and affecting between 0% and 100% of patients/series (average 14.86%patients/series)[2-22,29,32].
The only identified risk factor for complication was the use of a pre-keeper plate implant,with an 80.6% complication rate compared with 35.7% in keeper plate enabled implants.The necessity for the keeper plate was identified early following the induction of MCGR due to tendency for the actuator to unwind and shorten resulted in a loss of distraction[2,25].To combat this,a magnetic lock,the keeper plate,was applied around the lengthening mechanism to maintain the actuator in the desired lengthen position and prevent rod collapse[25].With regard to distraction failure,this decreased to a rate of 8.1% from 40.8% with the introduction of the keeper plate.This data indicates that the keeper plate was successful as designed to lock the magnetic actuator in its lengthening position,resisting the tendency to unwind and shorten following distraction.
An important implication of this data is in the future reporting of clinical outcomes of MCGR and the synthesis of the current published literature in systematic reviews.Since the introduction of MCGR technology,the product has gone through a continual process of quality improvement,evident by the seven iteration changes to date[1,9].This study is the first to report on the effect these iteration changes have on post-operative complications,specifically the introduction of the keeper plate to reduce rod distraction failure.Despite this fact,25% of the published clinical articles included mixed implant iterations in their analysis.Given these significant differences,future studies and systematic reviews need to include implant iterations in their data reporting and analysis for postoperative complications to avoid contaminating the results of more recent MCGR implant iterations.
This study is not without its limits.As a systematic review,the strength of the findings are solely dependent on the quality and rigor of the studies included in the analysis,which in this instance is comprised largely of level IV case series and four level II cohort studies.As a newer surgical technique,there is also the risk for performance bias between the 2 study cohorts,which could also impact the rate ofpostoperative complications.This is further confounded by the temporal relationships between included studies.The concern for overlapping patients in the identified studies was mitigated by close inspection of the study methods.However,several studies reported data from multi-center databases[1,2,15,18]and as such,the risk for overlapping information is present.
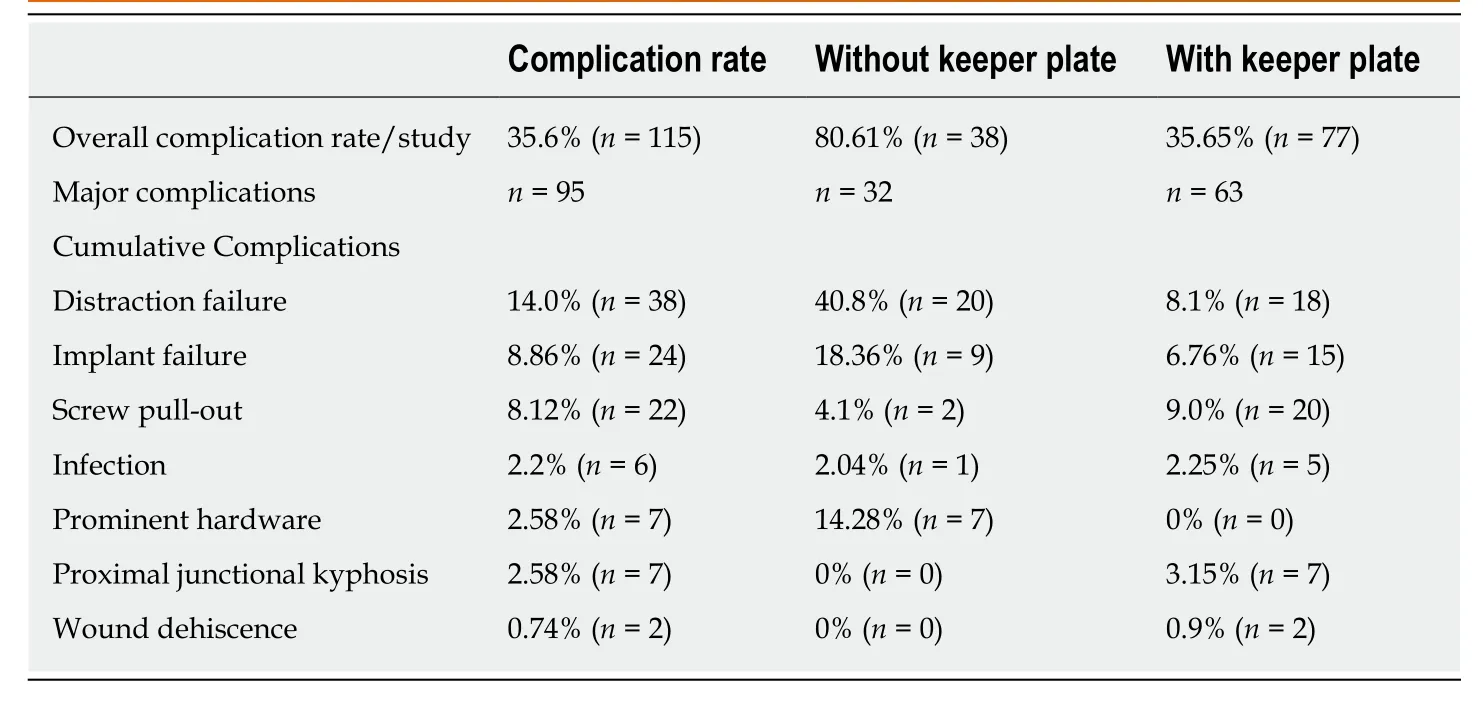
Table2 Summary of hardware related complications following magnetically controlled growing rods instrumentation for early onset scoliosis
A number of the identified risk factors for post-operative complications,include patient age,curve etiology,number,and type of proximal and distal fixation points,as well as type of implantation (primaryvsconversion),were not able to be investigated due to a lack of reporting in the original studies.The average follow-up in this review consisted of 26 mo.Given that the average patient age at time of MCGR implantation was 7.87 years,these results do not account for the full extent of the child’s treatment course and may underestimate the long-term complication profile.Additionally,there is no standard method for reporting complications for children treated with MCGR,leading to variable methods of reporting in the identified studies.
Given these identified deficiencies in standardized complication reporting,we recommend future studies also consider MCGR complication reporting according to patient and treatment variables (underlying diagnosis,number of rods,type of implantation,type and number of proximal anchorage points,occurrence of complication by number of lengthenings) and classify complications into the following categories: Permanent mechanical distraction failure,temporary distraction failure,rod breakage unrelated to the distraction mechanism,proximal anchorage failure,infectious/wound complication,and hardware prominence.These six categories represent the most common post-operative complications,while also identifying complications requiring an alteration in the planned treatment course.
In conclusion,this systematic review identified that 271 children undergoing MCGR implantation for the treatment of EOS,resulting in a cumulative 42.4% rate of post-operative complications,87% of which required a cessation in the planned treatment course or a reoperation.The introduction of the keeper plate significantly decreased the rate of post-operative complications to 35.7% and the rate of distraction failure.However,of the 20 clinical articles reporting on the outcomes of MCGR in EOS,25% included mixed implant iterations highlighting the need for strict.Further research is needed to investigate the effects of subsequent implant iterations as well as the long-term outcomes of treatment.
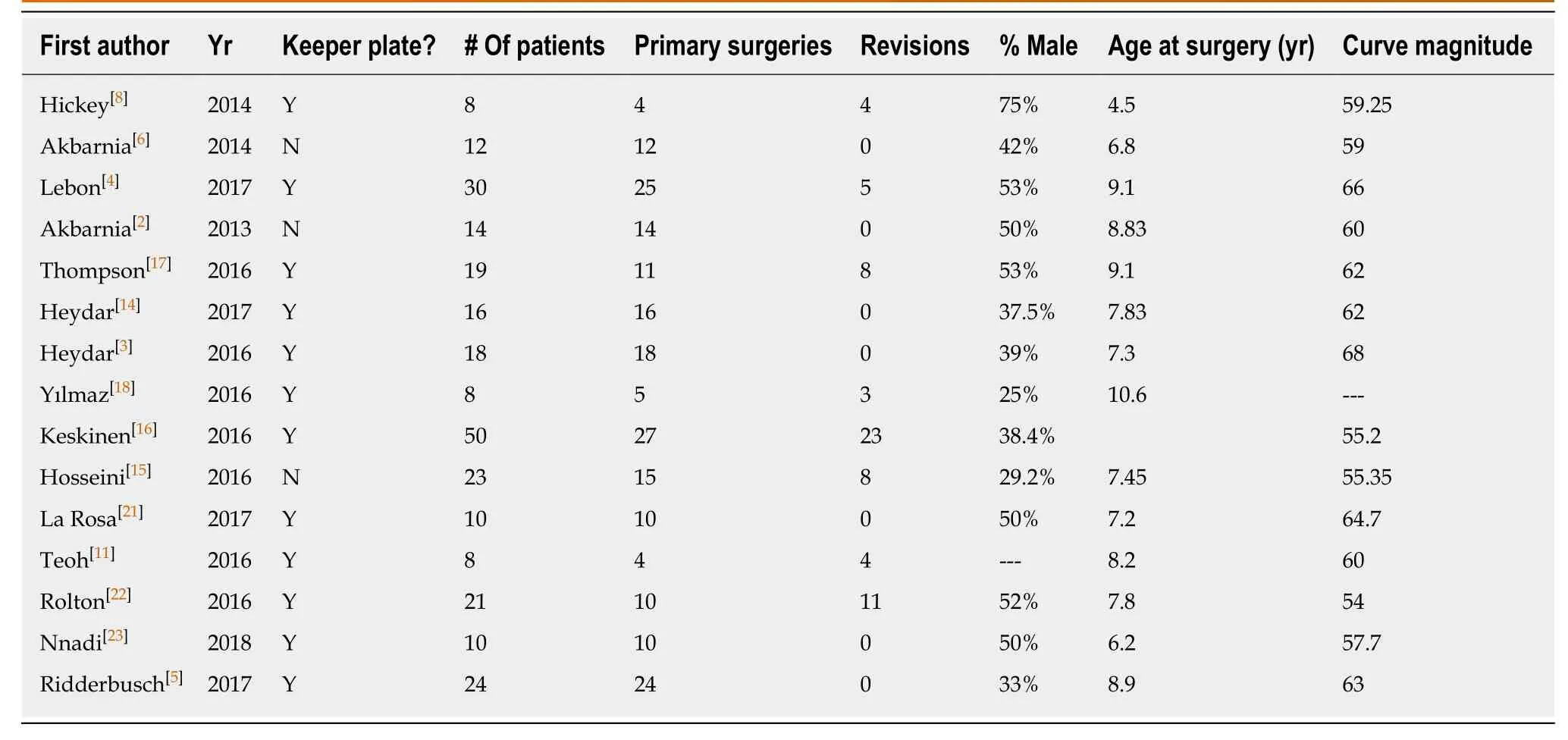
Table3 Summary of articles included for analysis
ARTICLE HIGHLIGHTS
Research background
Although the outcomes of using magnetically controlled growing rods (MCGR) to treat early onset scoliosis (EOS) has been reviewed,these studies do not take into account important implants modifications,termed iterations,that were made due to early on postoperative complications is not well reported or understood.
Research motivation
To gain a deeper understanding of how modification to MCGR after affected patients outcomes for the treatment of EOS and the implications of these effects on the reporting of future MCGR.
Research objectives
To assess the effect of MCGR implant iterations on post-operative complications in EOS.
Research methods
A systematic review was performed to identify studies investigating MCGR specifically for the treatment of EOS,refined to those reporting the implant iteration,specifically the incorporation of the keeper plate to the implant design.Articles with mixed implant iteration usage were excluded.Complications following surgery were recorded as well as potential risk factors and compared between implant cohorts.
Research results
Although 20 articles were identified for inclusion,5 (25%) included mixed implant iteration leaving a total of 271 patients identified through 15 clinical studies that met inclusion criteria.Pre-keeper plate implants were utilized in 3 studies with a total of 49 patients.Overall,115(42.4%) post-operative complications were identified,with 87% defined as major.The addition of the keeper plate significantly decreased the rate of post-operative complications per study (35.7%vs80.6%,P= 0.036),and the rate of distraction failure (8.1%vs40.8%,P= 0.02).Unplanned reoperation occurred in 69 (26.7%) patients but was not different between implant iteration cohorts (25.5% without keeper platevs27.1% with keeper plate,P= 0.92).
Research conclusions
MCGR implant with Keeper plates have less post-operative distraction failures.Of the currently published studies,25% include mixed implant designs.Future studies reporting on MCGR outcomes should include implant iteration in their analysis.MCGR implant with Keeper plates have less post-operative distraction failures.Of the currently published studies,25% include mixed implant designs.Studies included mixed implant iterations could be artificially inflating postoperative complication rates.Have more recent implant modification exhibited similar effects on MCGR outcomes.Twenty-five percent of currently published studies on MCGR outcomes included mixed implant iterations which could be artificially inflating complication rates.The addition of the keeper plate has decreased the incidence of distraction failure in the treatment of EOS.Understanding implant design gives important insight to understanding how they affect patient outcomes.
Research perspectives
Future studies should include implant iterations in the reporting of MCGR outcomes for the treatment of EOS.Long-term follow-up of children treated with MCGR for EOS.Subdividing MCGR outcomes by implant iteration will help ensure complications rates are not artificially inflated.

