Bioguided isolation of antimicrobial polyphenols from Cuspidaria convoluta leaves and their synergistic effect with antibiotics
Carola A.Torres, Mario A.Sturla, Ana M.Romero, María A.Judis
1Department of Basic and Applied Sciences, Microbiology Laboratory, Universidad Nacional del Chaco Austral, Comandante Fernández 755,Presidencia Roque Sáenz Peña, Chaco, Argentina 2Consejo Nacional de Investigaciones Científicas y Técnicas, Buenos Aires, Argentina 3Department of Basic and Applied Sciences, Laboratory of Organic Chemistry, Universidad Nacional del Chaco Austral, Argentina 4Department of Basic and Applied Sciences, Food Industry Laboratory, Universidad Nacional del Chaco Austral, Argentina
ABSTRACT Objective: To identify and isolate phenolic compounds from Cuspidaria convoluta, and to evaluate their antibacterial activity and synergistic effect with antibiotics.Methods: The crude extract was prepared by maceration with methanol (5%).The dry extract was suspended in water and fractionated successively.The most active extract was selected by its antibacterial activity and its total phenol content was determined by spectrophotometry and by HPLC-MS/MS.Bioactive fractions of the most active extract were separated by column chromatography and evaluated by bioautography.Isolated compounds were identified.Minimum inhibitory concentration (MIC) of these compounds was determined by microdilution broth method, and synergism with antibiotics (ampicillin, gentamicin and oxacillin) was tested by checkerboard and time-kill assays.Results: Coumaric acid, catechin/epicatechin, and luteolin were purified and identified from the extract.There was an increase in the antibacterial activity of antibiotics when they were combined with these compounds.The combination of luteolin and ampicillin had the most potent antibacterial activities.The MICs of oxacillin for each of methicillin-resistant Staphylococcus aureus strains were reduced between 4 and 8-fold when these strains were coincubated with sub-MIC (≤ ½ MIC) levels of these compounds, demonstrating that the combination had synergistic effect for all cases.Conclusions: Cuspidaria convoluta contains important pharmacologically active substances that can be used to improve antibiotic efficacy.
Keywords:Luteolin Ampicillin MIC reduction Antimicrobial agents
1.Introduction
Phenolic compounds are secondary plant metabolites that constitute one of the most common and widespread groups of substances in plants.They are responsible for pigmentation, growth,reproduction, and resistance to pathogens.The antibiotic properties of phenolic compounds are one of the primary defense mechanisms of plants.Most bioactive plant-based antimicrobials are phenol derivatives, controlling bacterial growth by altering their membrane permeability or reducing the pH.However, their activity is generally weak and is often non-specific.
The combination of natural drugs to treat complex infectious diseases is an approach to suppress bacterial resistance that is expected to usually develop when a single drug is used[1].This strategy is designed to combine antimicrobial compounds with different mechanisms of action for multiple targets.It could produce a synergistic antibacterial activity.This approach includes combinations of extracts with antimicrobial properties, combinations between individual natural products and combinations of extracts with antibiotics[2,3].Over the last decade, the search for synergistic interaction between plant extracts and commercial antibiotics that have already generated resistance in bacteria has increased significantly.Examples include the combination of β-lactams with α-mangostin isolated from mangosteen fruit[4]or with quercetin or kaempferol from various fruits, vegetables, and grains[5], which substantially increased the efficacy of the therapy in β-lactam resistant bacterial strains.Plant extract/antibiotic combinations not only contribute to and enhance the overall antimicrobial effect,but can also act as resistance modifying/modulating agents.For example, several extracts and essential oils of Salvia spp.and Matricaria recutita have been reported to have synergistic effects with oxacillin on methicillin resistant Staphylococcus epidermidis, greatly improving its efficacy[6].The results suggest that crude extracts from tested plants damage the cytoplasmic membrane and cause loss of intracellular components.Therefore, the authors propose that observed synergistic effects of plant extracts (essential oils) and oxacillin could be theoretically the results of the perturbation of the cell membrane coupled with the action of oxacillin.
Previously, Torres et al[7]demonstrated antioxidant,antiinflammatory and antimicrobial activities in the ethanolic extract of leaves from Cuspidaria convoluta (C.convoluta).Regarding antibacterial effect, this extract was active even against methicillinresistant Staphylococcus aureus (S.aureus) (MRSA).
The aims of this work were to identify and isolate phenolic compounds from C.convoluta, and to evaluate their antibacterial activity and synergistic effects with commercial antibiotics.
2.Materials and methods
2.1.Plant material
The plant was collected in November 2017 in Misiones (Ruta Nacional 12 y Santa Ana, 29° 10´39.274´´ S, 58° 51´22.885´´W), Argentina.The plant was identified by specialists from the Herbarium of Instituto de Botánica del Nordeste (IBONECONICET), Corrientes, Argentina, where the voucher specimen was deposited (AMG 104).
2.2.Extraction
Leaves were dried at room temperature until constant weight(10.18% humidity percentage).Then, they were triturated until particle sizes ranged between 1.70 mm and 710 µm.The crude extract was prepared by maceration (25 g) with methanol (500 mL)for 24 h using a magnetic stirrer at room temperature.The solvent was removed using a rotary evaporator (Buchi, Switzerland).Then,a dark green solid was obtained, most of which was suspended in water and then fractionated successively by n-hexane and ethyl acetate.The hexanic, ethyl acetate and water extracts were obtained and evaporated to dryness under vacuum.The extraction yield of each extract was 1.4% for hexanic extract, 3.1% for ethyl acetate extract and 2.5% for water extract.The dried extracts were stored at-20 ℃ for further use.
2.3.Selection of extract for isolation of bioactive compounds
The most active extract was selected by the determination of its antibacterial activity.The agar overlay bioautography was used according to Nieva Moreno et al[8].Thin-layer chromatography(TLC) plates (Merck, silica gel 60F254 0.2 thickness) were loaded with a spot containing 60 µg of each extract (hexanic, ethyl acetate,and water extract).Two strains of S.aureus (ATCC 25923 and ATCC 29213) were used, and were provided by the Laboratory of Clinical Analysis of the Hospital Ramón Carrillo, Sáenz Peña, Chaco,Argentina.These strains were maintained in brain heart infusion(Britania Laboratories, Argentina) containing 30% (v/v) glycerol at-20 ℃.Plates were incubated for 24 h at 37 ℃ and then sprayed with thiazolyl blue tetrazolium bromide reagent.Inhibition halos were measured.The extract with higher inhibitory effect was used for the next assays and its total phenol content was determined according to Singleton et al[9].Besides, the main phenolic compounds of the extract were also analyzed by HPLC-MS/MS.The analysis was performed in negative mode, and the identification of compounds was carried out on the basis of the m/z ratio of the quasimolecular ion, fragmentation patterns, data from the literature and/or comparison with patterns.Chromatographic separation was achieved using ACE 3 C18 analytical column (50 mm × 2.1 mm i.d.; 5 µm).Mobile phase contained 0.1% of formic acid in water and 0.1% of formic acid in acetonitrile at the flow rate of 0.3 mL/min.
Separation was carried out under the conditions of gradient elution for 30 min.This determination was performed in the Research and Development Center in Chemistry of National Institute of Industrial Technology, Buenos Aires, Argentina.
2.4.Bioguided fractionation of the extract
A portion of the selected extract (10 g) was subjected to column chromatography over silica gel (100-200 mesh).Gradient elution of increasing polarity was initiated with successive elution using hexane(100%), hexane:ethyl acetate (70:30, 50:50 and 30:70), ethyl acetate(100%), ethyl acetate:ethanol (70:30, 50:50 and 30:70), ethanol(100%), ethanol:methanol (70:30, 50:50 and 30:70), and finally only methanol (100%).Fractions having similar TLC profiles were pooled to give the major fraction and were stored in a refrigerator and evaluated by bioautography.The fractions that showed the presence of halo were subjected to further TLC and bioautographic analysis.The mobile phases used were, phase 1: toluene: ethyl acetate: glacial acetic acid (36:12:5) and phase 2: ethyl acetate:formic acid: glacial acetic acid: water (100:11:11:27).Two plates were performed simultaneously.One plate was sprayed with the polyethylene glycol reagent for natural products (NP/PEG) which was then visualized under visible light and UV at 254 and 365 nm.The second plate was used for the bioautography.At the same time,different phenolic compounds, such as gallic acid, rutin, quercetin,luteolin, and apigenin, were eluted with the different mobile phases for comparison with the separated bands in the TLC.The retention factor (Rf) values of the bands that showed antimicrobial activity were calculated.
2.5.Purification and isolation
Preparative TLC was performed and developed under conditions identical to those described in the previous section.The active bands were identified through TLC profiles by comparison with Rf values and colors of spots from the TLC previously eluted.These bands were scraped off, eluted with methanol and filtered.Subsequently,the solvent (methanol) was evaporated from each of the fractions and a precipitate was obtained which was dissolved in methanol (HPLC grade) and purity analysis was made by HPLC-DAD.The pure compounds were obtained.
2.6.Identification of isolated compounds from fractions
The compounds were tentatively identified by comparison of retention times and their UV-VIS spectra from 200 to 400 nm, as well as by the addition of an external standard.HPLC-MS/MS and IR spectroscopy confirmed the identity of the compounds.
2.7.Determination of antibacterial activity of the isolated compounds
2.7.1.Microorganisms
A total of 12 clinical isolates of ampicillin-resistant S.aureus, of which 3 were MRSA and gentamicin resistant (Sa 5307, Sa 5637,and Sa 5722) were used.In addition, Gram-negative bacteria such as Escherichia coli (E.coli) ATCC 35218, Pseudomonas aeruginosa (P.aeruginosa) ATCC 27853 and multiresistant clinical isolates of E.coli and P.aeruginosa were used.These strains were obtained from patients hospitalized at the Hospital Ramón Carrillo, Sáenz Peña,Argentina.Inocula were prepared by adjusting the turbidity of the suspension to match the 0.5 McFarland standards.
2.7.2.Determination of minimum inhibitory concentrations(MICs)
MIC values of the isolated compounds and antibiotic (ampicillin,gentamicin, and oxacillin) against the tested microorganisms were determined by the broth microdilution method[10].All compounds were dissolved in dimethyl sulfoxide (DMSO) and two-fold serial dilutions were prepared.The concentration of DMSO used in the assay was 2.9%.Then, 3 µL of these dilutions and 100 µL inoculum(~5 × 105CFU/mL) were added to each well.Growth control well contained bacterial cells and 3 µL of DMSO without any test compound, and sterile control only had growth medium.The concentrations tested for the compounds were 1 600, 800, 400, 200,100, 50 and 25 µg/mL.Plates were aerobically incubated at 37 ℃ for 16-20 h.Bacterial growth was indicated by the presence of turbidity and/or a pellet on the well bottom.The assays were performed in triplicate and as independent tests; and mean values were calculated.
2.7.3.Determination of synergistic activity between natural compounds and commercial antibiotics
The synergy between compounds and selected antibiotics(ampicillin, gentamicin and oxacillin only for MRSA strains) was studied by the checkerboard assay method[11].With the exception of ATCC strains, all bacteria mentioned in section 2.7.1 were used in this assay.The combinations were transferred to each microplate well.The concentrations used in the combinations for each antibiotic ranged from 0.19 to 1 600 µg/mL and for each natural compound between 6.25 and 1 600 µg/mL.MIC values were determined for each antibiotic and for each of these combinations.The bacterial growth and sterile controls were prepared.The fractional inhibitory concentration (FIC) was calculated as follows:
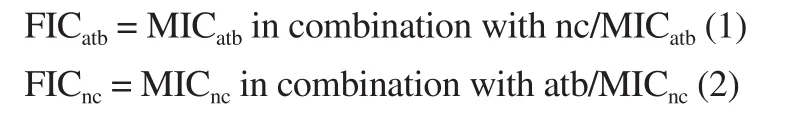
Where MICatbis MIC of antibiotics whereas MICncMIC of natural compounds.
Then, the FIC Index (FICI) was calculated according to the following equation (3):

According to Schelz et al[12]the FICI values were interpreted as either synergistic (≤0.5), additive (>0.5 and ≤1), indifferent (>1 and<4) or antagonistic (≥4).
For each combination producing synergistic interactions, 6-7 different ratios were tested.All combinations were tested in duplicates in three independent experiments, providing 6 replicates for each combination ratio.Data were presented as the mean of 6 replicates.Data for each ratio examined were plotted on an isobologram, and this was used to determine optimal combination ratios to obtain synergy.
Time-kill assays were only performed against the bacteria most resistant to antibiotics according to Petersen et al[13].Bacterial suspensions with appropriate dilution to ~ 1×105- 1×106CFU/mL of each bacterium in broth media were pre-incubated at 37 ℃.These samples were co-incubated with natural compounds or antibiotic adjusted to a series of final concentration of 1/2×MIC, 1×MIC, and 2×MIC, with the addition of broth media.Simultaneously, a growth control without natural compounds/antibiotic was also subjected to this test.In order to count viable cells (CFU), aliquots of 100 µL were taken from the culture before (0 h, positive control) and after (4, 8, 12 and 24 h) the addition of the drugs using spread plate technique.During the experiment, the mixture was maintained at 37 ℃.In addition, the natural compound and the antibiotic, with a series of equal ratio dilution from 1/4×MIC to 1/2×MIC, were combined to explore the combination kill-time according to the same procedure.Three plates were used for each sample.Synergism was demonstrated when there was a reduction of ≥2 log CFU/mL of the original inoculum[14].
3.Results
3.1.Identification and isolation of phenolic compounds from C.convoluta leaves
3.1.1.Selection of extract for isolation of bioactive compounds
The ethyl acetate extract was the most active extract in the bioautography with inhibition halos larger than 10 mm [(16 ± 1) mm].Hexanic extract did not show any activity, while the water extract showed inhibition halos of (12.0 ± 0.5) mm.The total phenol content of ethyl acetate extract was (32.27 ± 0.93) mg GAE/g of dry extract.The presence of seven polyphenolic compounds was demonstrated by HPLC-ESI-MS/MS.The phenol compounds identified were coumaric acid derivative, caffeic acid derivative, coumaric acid,catechin/epicatechin, luteolin, hydroxybenzoic acid sugar derivative and cirsiliol.
3.1.2.Bioguided fractionation of the extract
A total of 70 fractions were obtained by column chromatography, of which 6 had antibacterial activity in the bioautographic assay.These fractions demonstrated the presence of more than one compound responsible for the activity.However, only three compounds could be isolated and purified of these fractions by preparative TLC (Data were not shown).
3.1.3.Purification, isolation and identification of bioactive compounds
Compound 1 (C1) was obtained as compact powder and showed UV absorption at about 345 nm.Its mass spectrum showed a molecular ion and base peak at 165 m/z with other significant peaks at 119.The compound was identified as coumaric acid with the injection of a solution of the pure standards, which matched the feature in both retention time and fragmentation pattern, including an additional fragment at m/z 93.034.The chemical formula of C1 was C9H8O3.As p-coumaric acid is the most abundant form, the identification was attributed to the para isomer.In addition, the IR spectrum exhibited peaks at 3 385 cm-1(carboxylic acid O-H stretching), 1 674 and 1 690 cm-1(carboxylic acid C=O stretching),1 248 cm-1(carboxylic acid C-O stretching) and 1 510; 1 629 cm-1(aromatic C=C).
Compound 2 (C2) was obtained as amorphous yellow solid.C2 showed a molecular ion peak at m/z 289 and an ion fragment at m/z 245.The presence of this fragment may be caused by a neutral loss of CO2.C2 showed a UV spectrum with two maximum of absorption at 239 nm and 280 nm.These features were characteristic of catechins.The enantioseparation of catechin and epicatechin could not be performed so C2 could be catechin or its epimer epicatechin,both with molecular formula C15H14O6.The IR spectrum showed the characteristic absorption regions for O-H group (3 400 - 3 100 cm-1),C = C group around 1 600 cm-1, as well as C - O group (1 150 -1 010 cm-1).

Table 1.MIC values of isolated compounds from Cuspidaria convoluta extract and antibiotics on pathogenic bacteria.
Compound 3 (C3) was obtained as a yellow needle which showed a molecular ion peak at m/z 285, and fragmentation patterns at 241, 199, 175.In addition, similar to compound 1, a solution of the pure standard luteolin was injected and the retention times and fragmentation patterns were coincident with C3.The molecular
formula of C3 was C15H10O6, corresponding to luteolin.The IR spectra showed absorption bands for hydroxyl groups (3 405 cm-1),aromatic ring (1 441 cm-1), carbonyl group (1 655 cm-1) and hydroxyl aromatic (1 370 cm-1).

Table 2.Interactions of phenolic compounds with ampicillin (A) and gentamicin (G) against pathogenic bacteria.
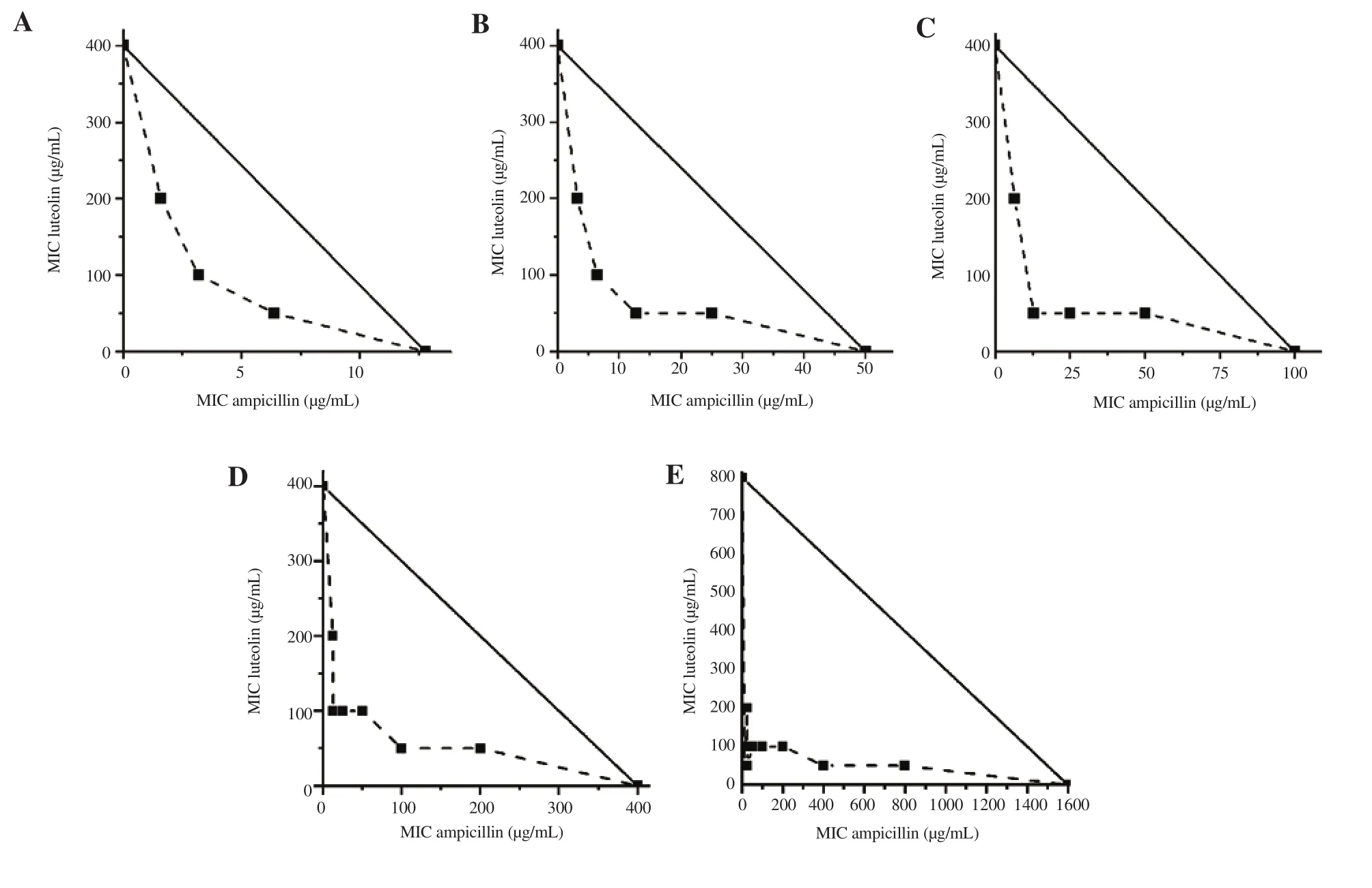
Figure 1.Isobolograms of the combinations of luteolin and ampicillin against resistant bacteria: A) S.aureus 5307, B) S.aureus 5637, C) S.aureus 5722, D)ampicillin resistant E.coli and E) ampicillin resistant P.aeruginosa.
3.2.Antibacterial activity of the isolated compounds and their synergistic action with antibiotics
3.2.1.MICs of the isolated compounds
The MIC values of all compounds are shown in Table 1.The results demonstrated that all compounds were active in the bioautographic tests, and exhibited antibacterial activity against all Gram-positive strains in concentrations of 50-800 µg/mL, even against MRSA(Sa 5307, 5637, and 5722).Moreover, these phytochemicals had moderate to weak antibacterial activities against different Gramnegative bacteria such as E.coli (400-1 600 µg/mL), and P.aeruginosa (800-1 600 µg/mL) strains.
3.2.2.Synergistic activity between natural compounds and commercial antibiotics
With respect to the synergistic effect of these compounds combined with ampicillin and gentamicin, the results are shown in Table 2.In most of these combinations, the FICI exhibited either a synergistic or an additive effect.A strong synergistic interaction was recorded against most of the bacteria with the combination of ampicillin and luteolin (FICI values between 0.08 and 0.50).Isobolograms also confirm the interaction between this antibiotic and luteolin (Figure 1).All graphs showed concave curves, which is characteristic of synergism between compounds (FICI ≤ 0.5).The combination of antibiotics with all the tested compounds led to an enhanced antimicrobial effect against S.aureus strains even up to 16 times the MIC.
The MIC values of ampicillin and gentamicin were 4-8 times lower when both were used in combination with these phenolic compounds against E.coli.Notably, the combination of ampicillin with the three compounds even showed activity against P.aeruginosa with a reduction in the MIC of the antibiotic which was between 8-64 times lower.
Based on the time-kill results, all the combinations showed synergism.However, the combination of luteolin with ampicillin was the most relevant.In this case, the synergistic effect against ampicillin-resistant E.coli and P.aeruginosa strains appeared after 8 h while in the others (coumaric acid and catechin/epicatechin combined with ampicillin and combinations with gentamicin)it appeared only after 12 hours of the trial (data are not shown).Moreover, a bactericidal effect of luteolin/ampicillin was shown at the end of the test (Figure 2).There was sustained synergistic inhibitory activity lasting for more than 24 h; bacterial colony counts were reduced by 2 log CFU/mL when compared with ampicillin alone.
Table 3 and Figure 3 show the synergistic effects of combining these polyphenols with oxacillin for each MRSA strain.This combination decreased the MIC of the antibiotic for each isolate.The MIC of oxacillin decreased by 4-8-fold for MRSA strains.These results demonstrated that the compounds were synergistic with oxacillin for each of the three MRSA strains tested in the plotted isobolograms (Figure 4).Figure 3 shows that the potency of the combined agents was more significant than individual ones;the reduction in colony count was ≥ 2 log CFU/mL over the first 12 hours of trial when the combination of luteolin and oxacillin was used.
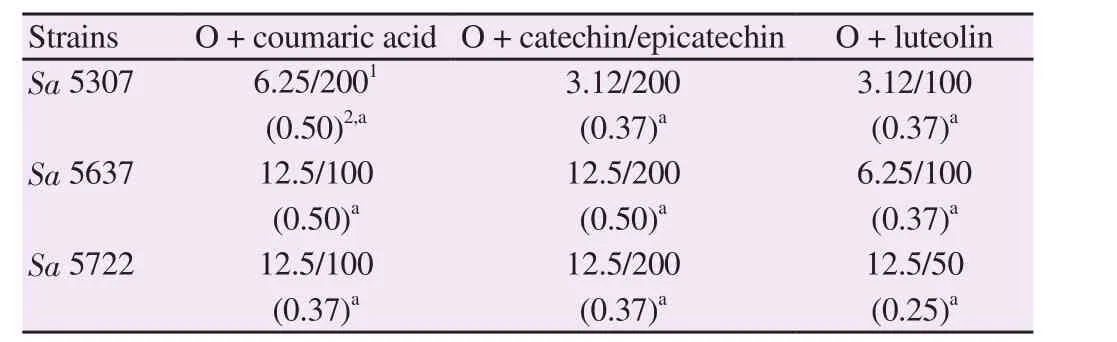
Table 3.Interactions of phenolic compounds with oxacillin (O) against MRSA.

Figure 2. Time kill curves and synergistic effect of luteolin-ampicillin combination against A) ampicillin resistant E. coli and B) ampicillin resistant P.aeruginosa. Control strain (), 2 MIC luteolin (), 2 MIC ampicillin (), MIC luteolin (), MIC ampicillin (), ½ MIC luteolin (), ½ MIC ampicillin (),combination ½ MIC luteolin and ½ MIC ampicillin ( ), combination ¼ MIC luteolin and ¼ MIC ampicillin ().

Figure 3. Time kill curves and synergistic effect of luteolin-oxacillin combination against MRSA strains, A) S. aureus 5307, B) S. aureus 5637 and C) S.aureus 5722. Control strain (), 2 MIC luteolin (), 2 MIC oxacillin (), MIC luteolin ), MIC oxacillin (), ½ MIC luteolin (), ½ MIC oxacillin ),combination ½ MIC luteolin and ½ MIC oxacillin (), combination ¼ MIC luteolin and ¼ MIC oxacillin ().

Figure 4.Isobolograms of the combinations of phenolic compounds with oxacillin against MRSA strains.A) coumaric acid-oxacillin against S.aureus 5307,B) catechin/epicatechin-oxacillin against S.aureus 5307, C) luteolin-oxacillin against S.aureus 5307, D) coumaric acid-oxacillin against S.aureus 5637,E) catechin/epicatechin-oxacillin against S.aureus 5637, F) luteolin-oxacillin against S.aureus 5637, G) coumaric acid-oxacillin against S.aureus 5722, H)catechin/epicatechin-oxacillin against S.aureus 5722 and I) luteolin-oxacillin against S.aureus 5722.
4.Discussion
Regarding the phytochemical composition of C.convoluta, Torres et al[7]reported the presence of a coumaric acid derivative, luteolin,hydroxybenzoic acid sugar derivative, and cirsiliol in its ethanolic extract.In the present work, catechin/epicatechin, luteolin, and p-coumaric acid could be isolated and identified.Regarding coumaric acid, its presence has already been reported in other representatives of Bignoniaceae family even in the Cuspidaria genus[15,16].It is also found in C.convoluta in this study.Concerning catechins, their presence in the genus Cuspidaria has not yet been reported.
In relation to the antimicrobial activity, the results of present study suggest that MIC values close to 100 µg/mL could be considered noteworthy[17].Therefore, p-coumaric acid would be the most active isolated compound with MIC values of 50 and 100 µg/mL against seven S.aureus strains.These results are in agreement with those of Lou et al[18]and Orhan et al[19], who reported antimicrobial activity of p-coumaric acid against S.aureus.However, the MIC value needed to inhibit the growth of MRSA (MIC= 1 000 µg/mL) was higher than that found in this work (MIC= 400-800 µg/mL), and the authors informed that no inhibitory effect was found against methicillin sensible S.aureus[20].On the other hand, MIC values found in this work for p-coumaric acid were higher than those reported in other paper for E.coli (MIC=80 µg/mL)[18].This difference is probably due to the different sensitivity of the clinical strains used.
The results of the synergism tests showed that the best combination was ampicillin with luteolin.These were to be expected since,in previous work, the combination of C.convoluta extract and ampicillin even showed activity against P.aeruginosa (strains Pa and F305) with a reduction in 16 times the MIC of the antibiotic[21].Several works showed that luteolin increases the efficacy of different antibiotics, since it inhibits β-lactamase in multidrug-resistant E.coli strains[22], affects the cytoplasmic membrane stability; and inhibits enzymes involved in the synthesis of folic acid[2].This could explain the results obtained with the combination of luteolin and ampicillin in this work.
In addition, our results support the findings of Hemaiswarya and Doble[23], who previously demonstrated the ability of p-coumaric acid to enhance the effect of commercial antibiotics against Gramnegative and Gram-positive bacteria.
As mentioned above, the phenolic compounds can act synergistically with different classes of antibiotics by several mechanisms[22,24-26].Concerning the synergistic effect in the luteolin/oxacillin combination against MRSA strains, Joung et al[27]have demonstrated the potential of luteolin as an active therapeutic agent against MRSA, reinforcing the possibility of substantially reducing the use of existing antibiotics.
In conclusion, p-coumaric acid, catechin/epicatechin, and luteolin were isolated from C.convoluta leaves and identified.Although these compounds are present in most plants, they have not been isolated from C.convoluta and studied yet.This contributes to the knowledge of this species, which until now, has been scarcely studied from the phytochemical perspective.
There is a synergistic interaction between the phenolic compounds isolated from C.convoluta and selected antibiotics.The synergy observed allows reducing the dose of antibiotics which translates into a decrease in the adverse effects associated with the use of these drugs.In addition, the mentioned compounds could be a solution for the multidrug resistance problem, but their mechanism of action in different microorganisms should be better understood and further explored, which will allow more effective and safer treatments to be developed than the current ones.
Conflict of interest statement
All authors declare that there is no conflict of interest.
Acknowledgments
We acknowledge the financial support from Secretaría de Ciencia y Técnica UNCAUS and Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), Argentina.We thank N.E.Cech and C.Fontán from Hospital 4 de Junio from Presidencia Roque Sáenz Peña, Chaco, Argentina for isolation and identification of bacterial strains.We also thank A.M.Gonzalez and M.M.Arbo from IBONE for plant collection and identification.
Funding
This work is financially supported by Secretaría de Ciencia y Técnica UNCAUS and by Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), Argentina.
 Asian Pacific Journal of Tropical Biomedicine2019年10期
Asian Pacific Journal of Tropical Biomedicine2019年10期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- Ethanolic extract of cashew apple inhibits lipid metabolism and ameliorates obesity in atherogenic diet-induced obese rats
- Effect of alpha-lipoic acid supplementation on blood pressure, renal oxidantantioxidant status and renal damage in spontaneously hypertensive rats
- Hydroalcoholic extract of licorice (Glycyrrhiza glabra L.) root attenuates ethanol and cerulein induced pancreatitis in rats
- Prediction of T cell and B cell epitopes of the 22-, 47-, 56-, and 58-kDa proteins of Orientia tsutsugamushi
