Hepatic flow is an intraoperative predictor of early allograft dysfunction in whole-graft deceased donor liver transplantation:An observational cohort study
Pablo Lozano Lominchar,Maitane Igone Orue-Echebarria,Lorena Martín,Cristina Julia Lisbona,María Magdalena Salcedo,Luis Olmedilla,Hemant Sharma,Jose Manuel Asencio,José Ángel López-Baena
Pablo Lozano Lominchar,Maitane Igone Orue-Echebarria,Lorena Martín,Jose Manuel Asencio,José Ángel López-Baena,General Surgery Department,Liver Transplant Unit,Hospital General Universitario Gregorio Maranon,Madrid 28007,Spain
Cristina Julia Lisbona,Luis Olmedilla,Anesthesiology Department,Liver Transplant Unit,Hospital General Universitario Gregorio Maranon,Madrid 28007,Spain
María Magdalena Salcedo,Hepatology Department,Liver Transplant Unit,Hospital General Universitario Gregorio Maranon,Madrid 28007,Spain
Hemant Sharma,Department of Transplant Surgery,Oschner Medical Center,New Orleans,LA 70816,United States
Abstract
Key words: Hepatic flow;Early allograft dysfunction;Liver transplant
INTRODUCTION
Early allograft liver dysfunction (EAD) is a condition that prematurely identifies grafts that are at risk of having marginal function after liver transplantation (LT).The development of graft dysfunction is multifactorial.The degree of impairment can range from a very mild and temporary form to a more severe and potentially deadly form unless the patient receives an early retransplantation.The regenerative capacity of the hepatic parenchyma conditions most dysfunctions to be transient[1,2].
Currently,there are a large number of predictive models for graft failure,all of which are heterogeneous because they use different criteria to select the independent variables.In general,the models have the same goal,which is to predict the development of liver dysfunction and provide an evidence-based tool that is useful during the liver graft selection process[3-5].To ensure proper function of a liver graft,the hepatocellular critical mass is needed to maintain synthetic function and adequate blood supply through the vascular tree.Hepatic flow is a determining factor in early graft function.The hepatic circulation system is highly complex due to its dual irrigation.The hepatic artery contributes 25% of the hepatic blood flow (30 mL/min per 100 g of liver mass) and provides 30-50% of the oxygen requirement of the liver.Moreover,the portal vein provides 75% of the hepatic blood flow (90 mL/min per 100 g of liver mass) and provides 50%-70% of the oxygen requirements of the liver with partially deoxygenated blood arriving from splanchnic circulation.These two systems are closely related and conform to what is known as the "hepatic arterial response buffer"[6].This mechanism explains the changes in arterial blood flow as compensatory to the changes in the portal flow so that the arterial system is able to compensate for changes of up to 25%-60% in portal flow.However,the portal system is unable to compensate for the changes in arterial blood flow[7].This buffer system remains active after LT,as shown by authors such as Cantréet al[8].The intraoperative reading of arterial and venous blood flow after LT may be useful in predicting the development of early graft dysfunction because blood flow values offer an indirect measurement of the oxygen and nutrient levels being supplied to the liver parenchyma at a given point in time.
MATERIALS AND METHODS
This is an observational study based on a single cohort of 195 consecutive patients who underwent LT with a prospective collection of data and a retrospective analysis.This study was carried out in the Liver Transplant Unit of Hospital General Universitario Gregorio Marañón,Madrid,Spain,a tertiary referral centre,during the study period between January 2008 and December 2014.The study was performed according to the International Guidelines for Ethical Review of Epidemiological Studies [Council for the International Organizations of Medical Sciences (CIOMS),Geneva,2008] and to the Declaration of Helsinki (Seoul,October,2008).The study was reviewed and approved by the Clinical Research Ethics Committee.All patients gave their written informed consent prior to study enrolment.All recruited candidates were adult patients who received an orthotopic full cadaveric donor liver transplant,including an urgent transplant,early retransplantation due to primary graft dysfunction and late retransplantation.The cases in which the intraoperative vascular blood flow measurements could not be obtained due to technical problems or those in which liver graft dysfunction was secondary to acute vascular complications were excluded.
Surgical technique
All patients underwent deceased donor LT using standard techniques without the use of venovenous bypass in favour of the piggyback technique.Anastomosis techniques related to the portal vein and hepatic artery were not modified.
Study variables were collected prospectively and recorded on an electronic case report.
Donor data
Donor data included age,cause of death,serum sodium level,ALT,AST,GGT,sex,blood group,weight,height,body mass index (BMI),body surface area (BSA) and donor risk index (DRI).Donor status was also evaluated by the need for epinephrine,evidence of shock,or the need for cardiopulmonary resuscitation or intensive care unit (ICU) stay.
Graft preservation data
Graft preservation data included cold ischaemia time and the graft preservation solution:The University of Wisconsin (UW) solution,the histidine-tryptophan ketoglutarate (HTK) solution or Celsior.
Recipient data
Recipient data included age,sex,weight,height,BSA,BMI,indications for LT,the model for end-stage liver disease (MELD) and Na,creatinine,ALT,AST and preoperative bilirubin levels.The intraoperative variables registered were surgical time,the need for red blood,platelet or plasma transfusion,and the need for cryoprecipitate.
Intraoperative measurements were performed with a flowmeter (Medistin,Norway) based on the measurement of transit time (MFTT) and Doppler technology.The Doppler effect uses the transmission of a continuous wave,and MFTT employs the transmission of pulses.By applying the Doppler concept to the components of the blood,we can measure the vessel blood flow velocity.If the sound is directed in the direction of flow,the received signal will be different depending on whether the blood components are near or far from the transducer.The sensor used by the MFTT contains two transducers and a reflector.The two transducers are located on one side of the vessel and the reflector on the opposite side;this arrangement causes a double ultrasound passage through the vessel.After performing vascular and biliary anastomoses,at the end of the procedure,the hepatic artery and portal vein flow just distal to the suture on the graft's side were sequentially measured.The absence of intraoperative blood flow or obtaining a very poor flow measurement were considered to be an indication for reviewing the arterial anastomosis after verifying the absence of the compensatory effect of the portal flow.
The duration of ICU stay,the need for mechanical ventilation and the length of hospital stay were also registered.
The modified version of the criteria for EAD into the MELD era by Olthoffet al[9]was used.EAD was defined as the presence of one or more of the following previously defined postoperative laboratory findings reflective of liver injury and dysfunction:Bilirubin level greater than or equal to 10 mg/dL on day 7,international normalized ratio greater than or equal to 1.6 on day 7,and AST or ALT level of 2000 IU/L within the first 7 d.
Follow-up
The follow-up started on the day of LT and was routinely performed at outpatient clinics.Patient follow-up was continued until August 1,2015.
Study endpoints
The primary endpoint was EAD.The secondary endpoint was postoperative 30-d mortality.
Statistical analysis
Unless otherwise stated,the data are expressed as the mean ± SD orn(%).When data were normally distributed (based on the Kolmogorov-Smirnov test),comparisons were performed using Student'st-test.The qualitative variables and risk measurements were analysed using theχ2test.Univariate and multivariate analyses of graft dysfunction were performed using a logistic regression test.Predictive analysis was conducted using receiver operating characteristic (ROC) curves.To assess the impact of the risk score on survival,Kaplan-Meier survival curve analysis was performed,and the results were compared with the log-rank test.The collected data were entered into a database created in SPSS version 20 for Mac (SPSS,Inc.,Chicago,Illinois,United States).
RESULTS
During the study period from January 2008 to December 2014,195 cadaveric liver transplant surgeries were performed in 188 patients (Figure 1).According to the Olthoff criteria for EAD,54 patients with EAD (27.70%) were identified,5 of whom underwent an urgent retransplant surgery (9.30%).Of the 54 patients who developed EAD,68.50% (37) of patients were alive at the end of the study period,while 31.50%(17) of patients died during the follow-up period.The overall mortality rates for patients with EAD were 5.60%,11.10% and 25.90% at 7 d,30 d and 6 mo,respectively.The median observation period was 39 mo.
Baseline characteristics
Demographic data and liver disease aetiologies are shown in Table 1.The characteristics of patients who reached and did not reach the endpoint (EAD) are shown in Table 2.
Cold ischaemia time was significantly higher in the group with EAD (525.74 ±153.03 min) when compared to the no-EAD group (464.94 ± 142.52 min),P= 0.01.However,no significant differences were found in the estimated graft volume and the estimated volume of the liver receptor.There were also no significant differences in the ratio of the estimated graft volume to the estimated recipient volume or in relation to other anthropometric parameters registered,such as weight,height,BMI,or BSA.Other characteristics of the donor and recipient showed no statistically significant differences between the groups.
The variables used to study the characteristics of the hospital stay were length of ICU stay,length of hospital stay and the need for mechanical ventilation.Significant differences in the time of ICU stay were found between patients who developed EAD(6.42 ± 6.50 d) and those who did not develop EAD (4.36 ± 5.04 d),P< 0.01.Likewise,the need for mechanical ventilation in patients with EAD was 7940 ± 14185 h compared to 4151 ± 11323 h in patients who showed no EAD (P= 0,02).Furthermore,the length of hospital stay in patients who developed EAD was 3567 ± 2808 d,compared to no-EAD 2618 ± 1824 d (P< 0.01).
Effect of liver blood flow on early allograft dysfunction
Table2 shows the relationship between haemodynamic hepatic blood flow and EAD.Significant differences between arterial,portal and total liver blood flow were observed.
Hepatic artery flow:HAF was significantly lower in the group with EAD (227.74 ±134.13 mL/min) than in the no-EAD group (279.67 ± 152.87 mL/min,P= 0.01).However,no significant differences were found between the groups regarding the percentage of total blood liver supply carried by HAF.The HAF variable was later categorized into two groups to label individuals in a clinically relevant manner.The cutoff value was 180 mL/min such that individuals with an HAF blood flow > 180 mL/min were considered to be normal and those with HAF blood flow < 180 mL/min were considered to have insufficient blood flow.The association of HAF with the endpoint was then analysed (OR = 2.25,95%CI:1.16-4.35,P= 0.02,Figure 2).
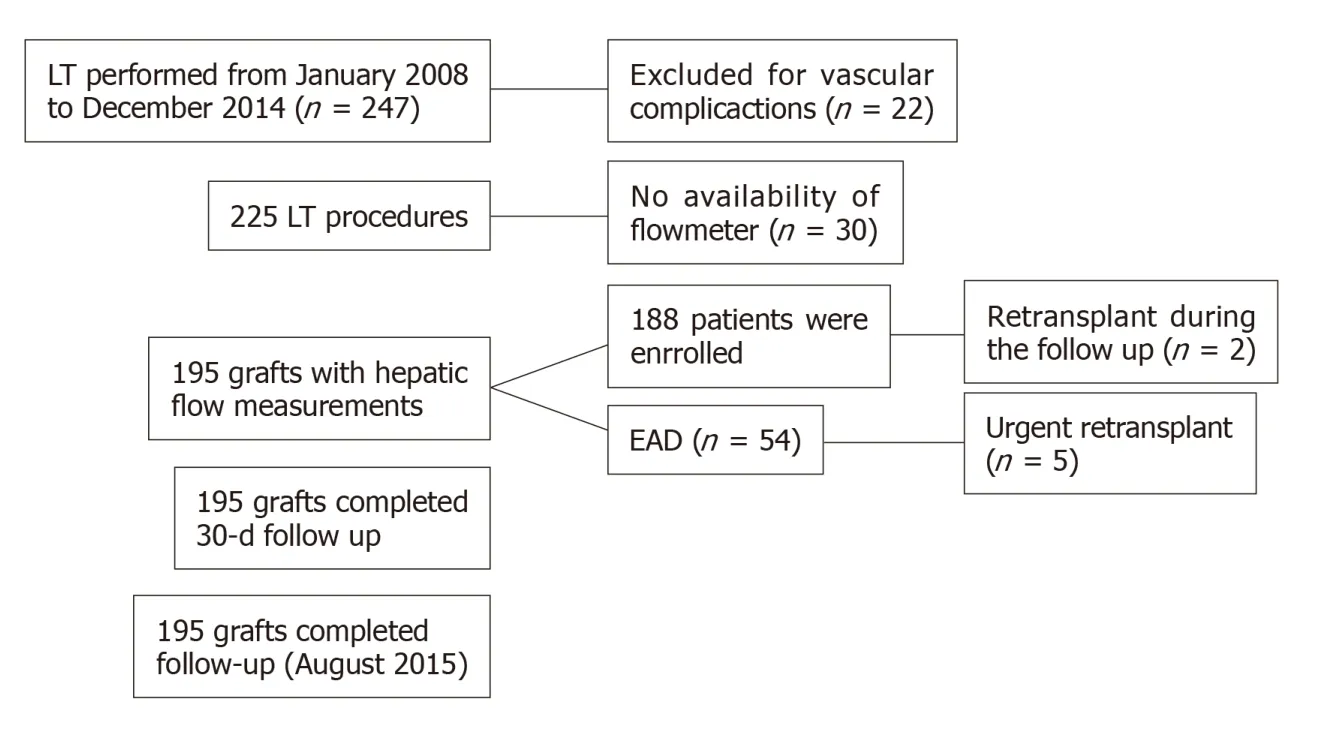
Figure1 Study flowchart.
Portal venous flow:PVF was significantly lower in the group with EAD (1363.84 ±602.06 mL/min) than in the no-EAD group (1606.73 ± 491.51 mL/min,P= 0.01).There was no difference between the EAD and no-EAD groups regarding the percentage of total blood liver supply by the portal venous flow.The PVF variable was categorized into two groups using the cutoff value of PVF < 1200 to interpret its clinical relevance with respect to the end point,and its significant relationship with the endpoint was analysed (OR = 3.36,95%CI:1.83-6.16,P< 0.01,Figure 3).
Total hepatic blood flow:THF was significantly lower in the group with EAD (1591.81 ± 631.07 mL/min) than in the group with no-EAD (1883.28 ± 513.15 mL/min).THF was categorized into two clinically relevant groups using the cutoff value of 1500 ml/min so that individuals with THF < 1500 mL/min were considered to have an inadequate THF.Its association with the endpoint was then analysed (OR = 3.05;95%CI:1.59-5.88;P< 0.01).The multivariate analysis showed that cold ischaemia time and HAF< 180 mL/min and PVF < 1200 mL/min were predictors of EAD (Table 2).
Effects of liver blood flow on 30-d patient mortality:We also evaluated the effects of our categorized blood flow variables on patient mortality at 30 d.In the univariate Cox regression analysis,only 5 variables were significantly associated with 30-d survival (HAF,PVF,THF,AST at day 1 and INR at day 1;Table 3).In the multivariate analysis,HAF < 180 mL/min and AST > 2000 UI/dL were independent prognostic factors for 30-d patient mortality.In addition to these results,the AUROC of the risk score developed showed a better diagnostic performance [area under the ROC curve(AUROC):0.814;95%CI:0.674-0.954;P< 0.01].
DISCUSSION
EAD following cadaveric donor LT affects both graft and patient survival[9].In an attempt to prevent EAD,many predictive models using donor and receptor variables,graft characteristics,intraoperative events,and functional tests have been developed[10-12].Most of these variables,which influence the risk of developing EAD,are not treatable.Therefore,the aims of new studies are to identify different treatable or preventable intraoperative variables that may aid in the decision-making process during both the surgical act and the immediate postoperative period.In this sense,the measurement of intraoperative (arterial and venous) hepatic blood flow after reperfusion as an indirect measurement of the hepatic parenchyma oxygen and nutrient input could be used as intraoperative parameters to predict EAD[13,14].These differences differ from the non-treatable variables in that hepatic inflow has the potential to be intraoperatively modified in cases in which the measured blood flow predisposes a patient to EAD and therefore worse overall outcomes of LT.
The exact definition for EAD has not yet been established because there is still plenty of variability found in the published literature.In this study,we used theOlthoff criteria to define EAD[9].Olthoff defined EAD as the presence of at least one postoperative variable previously associated with liver injury and function,such as serum levels of transaminases,bilirubin and INR.According to Olthoffet al[9],EAD increases the risk of death by ten times at 6 mo after an LT.In this study,the incidence of EAD was 27.7%,out of which 18.8% died,while only 1.8% of patients in the no-EAD group died.Graft loss occurred in 26.1% of patients with EAD.Our EAD incidence was similar to that found in previous studies,with an incidence ranging between 2%-32%.In our view,efforts should be oriented to employ intraoperative measurements of graft function,either through the measurement of blood supply or by directly measuring liver function (e.g.,blood supply,LIMAX,or clearance of indocyanine green)[15-17],to allow an early diagnosis of EAD which,in turn,would aid the decision-making process.
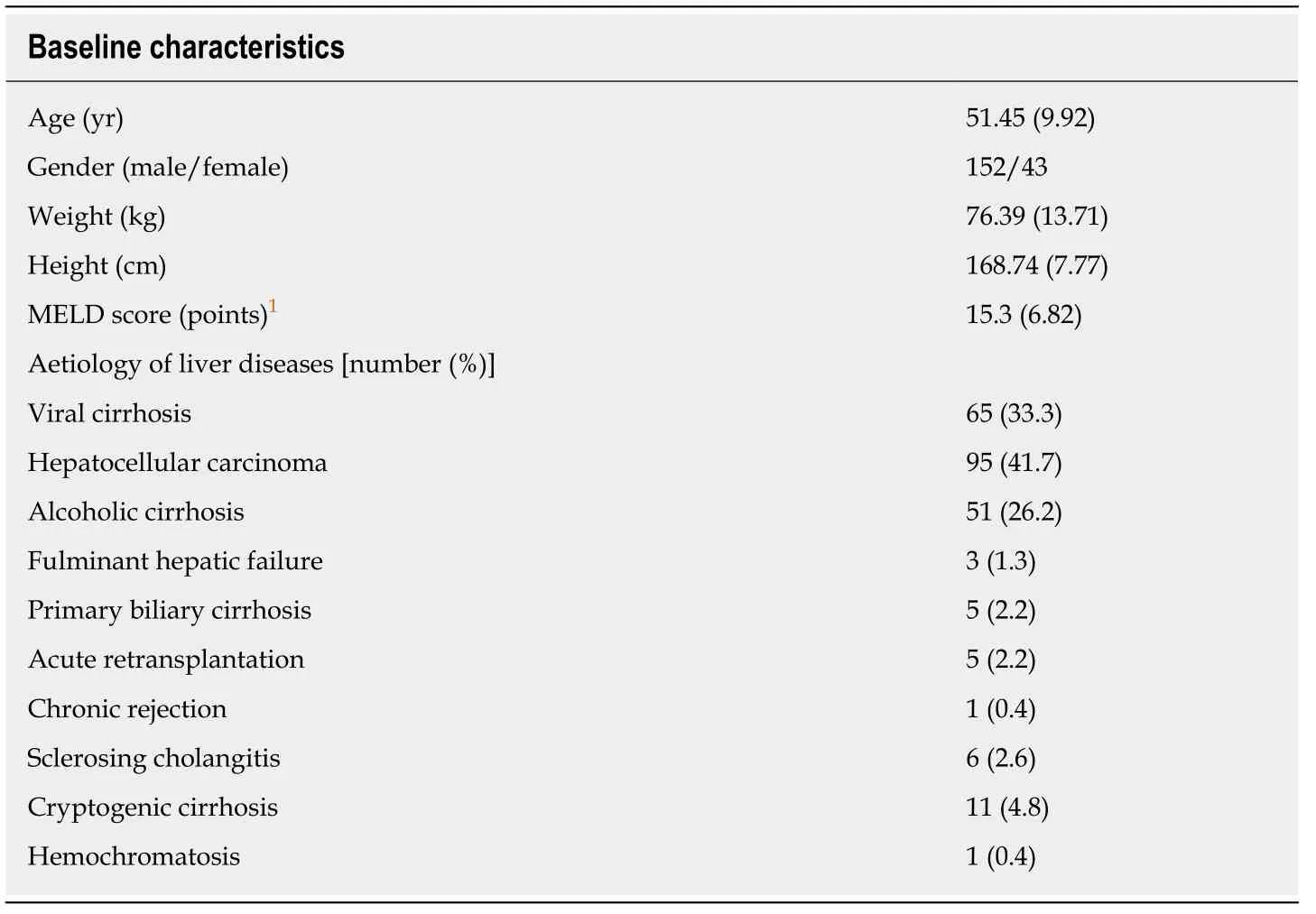
Table1 Demographic baseline characteristics
When we analysed the differences between patients with and without EAD,CIT was significantly higher in the group with EAD and was also a significant predictive factor of EAD in the multivariate analysis.The association between CIT and ischaemia-reperfusion injury has already been described.In particular,a CIT greater than 12 h is an independent variable of poor prognosis and is,therefore,associated with worse graft function[18,19].However,CIT could be considered a confusion factor due to the established relationship between prolonged CIT and ischaemia-reperfusion injury that leads to an early endothelial injury and consequently increases vascular resistance to hepatic artery flow.
The measurement of intraoperative blood flow was performed with a flow metre VeriQ,based on measuring the transit time (MFTT) and Doppler technology.This system has been validated in previous studies and generates reproducible measurements[20-22].
In our group,the mean HAF was 265.15 ± 149.45 mL/min.Vascular patency was assessed postoperatively with Doppler ultrasound.When we looked at the differences between the groups according to the presence of EAD or nor,statistically significant differences were observed.We set the cutoff in HAF < 180 mL/min because it was the best discriminatory measure between the two EAD groups (Figure 2).We note that the EAD development risk was doubled,and it became a prognostic factor for 30-d patient survival in the multivariate analysis.In the intraoperative setting,decreased HAF may guide the surgical team to undertake intraoperative tests of graft inflow modulation.First,we evaluated the patency of the anastomosis,as indicated by the absence of blood flow.Once thrombosis was ruled out,the portal drainage was occluded with the aim to observe the arterial buffer system response.If there were modifications in HAF during this response,manoeuvres such as splenectomy or ligation of the splenic artery if PVF was greater than 1300 mL/min could be performed in response to these tests[23].At other times when flow modulation is not a problem,hypotheses arise as to whether the decreased HAF is a consequence of anincrease in intrahepatic resistance.Marginal graft use is known to have poor tolerance to I/R injury.Therefore,in recent years,the use of new devices for machine liver perfusion can be useful in evaluating the increase in intrahepatic resistance that leads to worse intraoperative liver flow.As previously stated,there currently exists no agreement on a minimum value of HAF that serves as a predictor of worse outcomes[24].
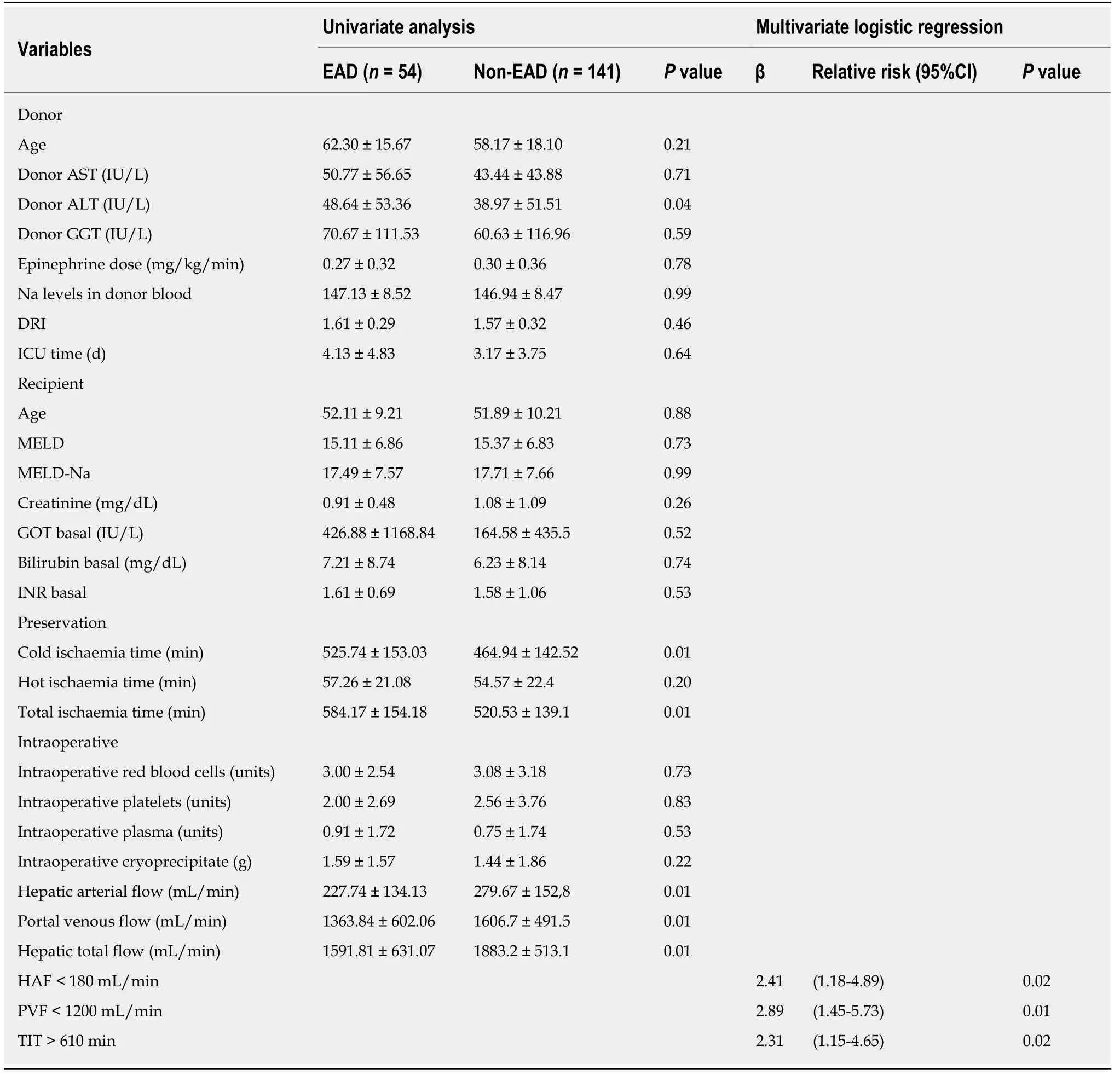
Table2 Univariate and multivariate analysis for the development of early allograft dysfunction
With respect to PVF,Lisiket al[25]suggested a link between PVF and EAD;however,their study only included 15 patients,hence its limited clinical relevance.The minimum acceptable PVF value for adequate graft function is approximately 1000 mL/min.Gastacaet al[26]showed that PVF is related to anthropometric parameters and to the patient's clinical conditions and suggests that a PVF of less than 1000 mL/min is more common in women and in patients with less advanced liver disease.Cirrhosis is associated with portal hypertension,with hyperdynamic syndrome being one of its late consequences.In our study,we found no differences in these characteristics between the groups with portal flows < 1200 mL/min and > 1200 mL/min,but there was a significant difference in terms of cardiac output.The authors note that primary non-function (PNF) is greater in the group with portal flow less than 1 L/min,but it is not associated with the development of EAD.In their study,after adjusting portal flow by graft weight (measured,not estimated),a portal flow of< 80 mL/min × 100 g was correlated with an elevated risk of PNF development and graft loss in the first year after transplantation.Our analysis showed that PVF was associated with developing EAD,with statistically significant differences when comparing both groups.A PVF less than 1200 mL/min conferred three times the risk of developing EAD and was also a prognostic factor for 30-d survival in the univariate analysis but not in the multivariate analysis.
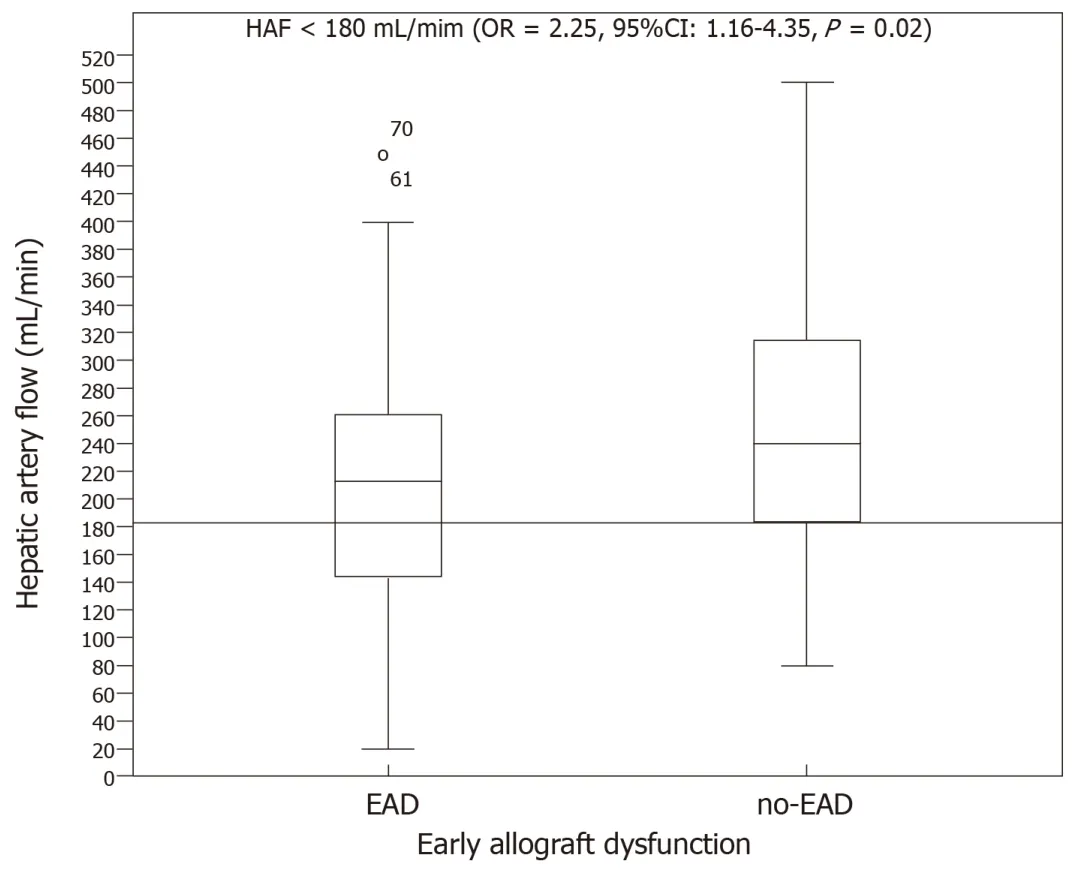
Figure2 Hepatic artery flow and development of early allograft dysfunction.
The study is,however,limited because the degree of steatosis in the grafts was not measured nor taken into count,which has been shown to behave as a risk factor for developing EAD.In this study,the information about blood flow within the graft is limited to macrovascular measurements,without taking into consideration the potential changes that occur in the microcirculation or the interaction between both factors.Microcirculatory changes occur during ischaemic phenomena of reperfusion injury.Puhlet al[14]demonstrated a significant correlation between the initial microcirculation and early graft function postoperatively.Studies have correlated measurement and microcirculation through a laser Doppler flowmeter on the liver surface with the macrovascular total hepatic flow[28].
In conclusion,we have shown that intraoperative measurements of hepatic blood flow can predict the development of EAD and that hepatic artery flow has an impact on survival at 30 d.Consequently,future efforts may focus on study strategies directed at identifying those grafts that are more susceptible to developing alterations in blood flow and how we can mend these alterations in hepatic blood flow in the intraoperative setting.
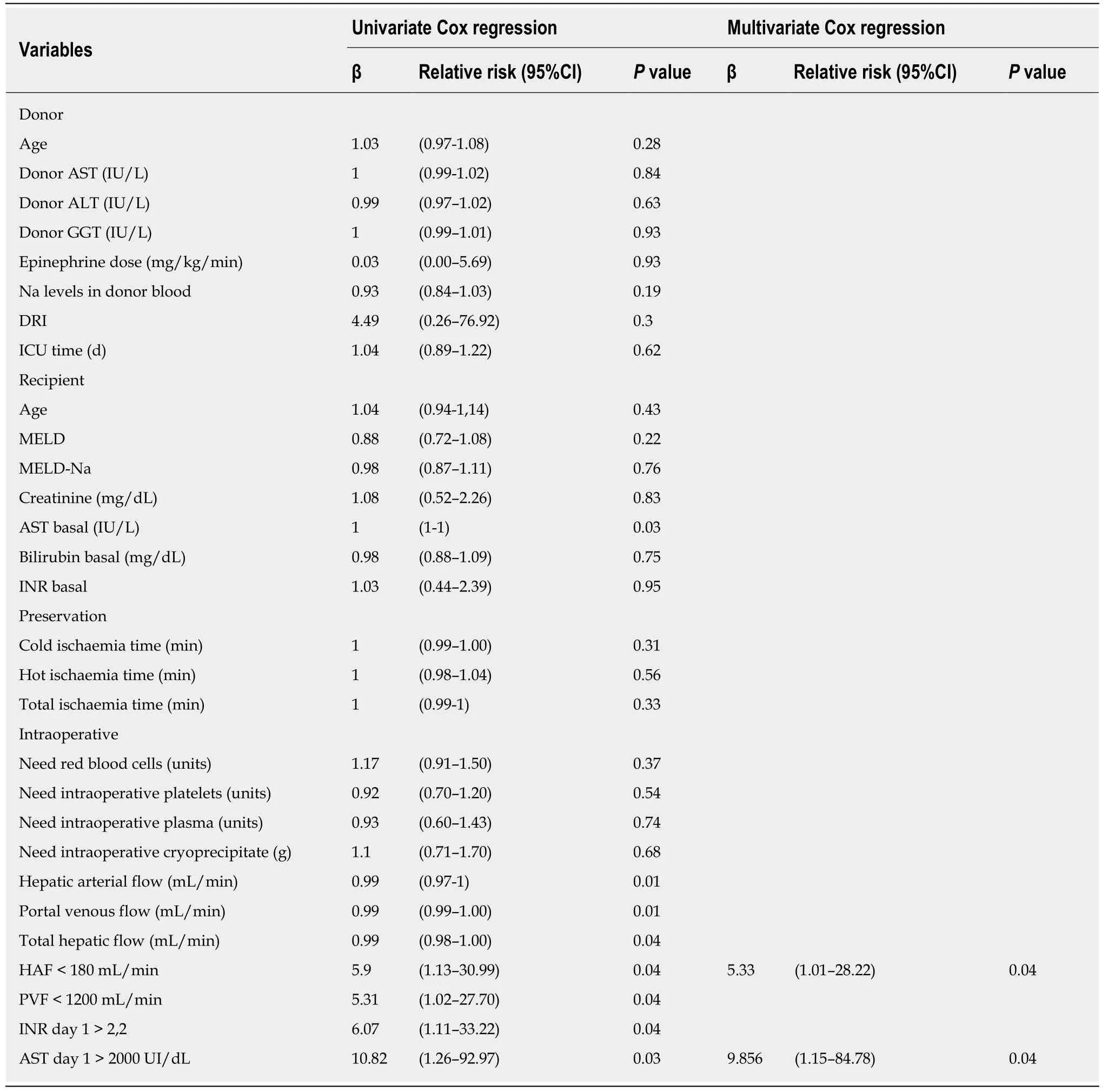
Table3 Univariate and multivariate Cox regression models of mortality at 30 d
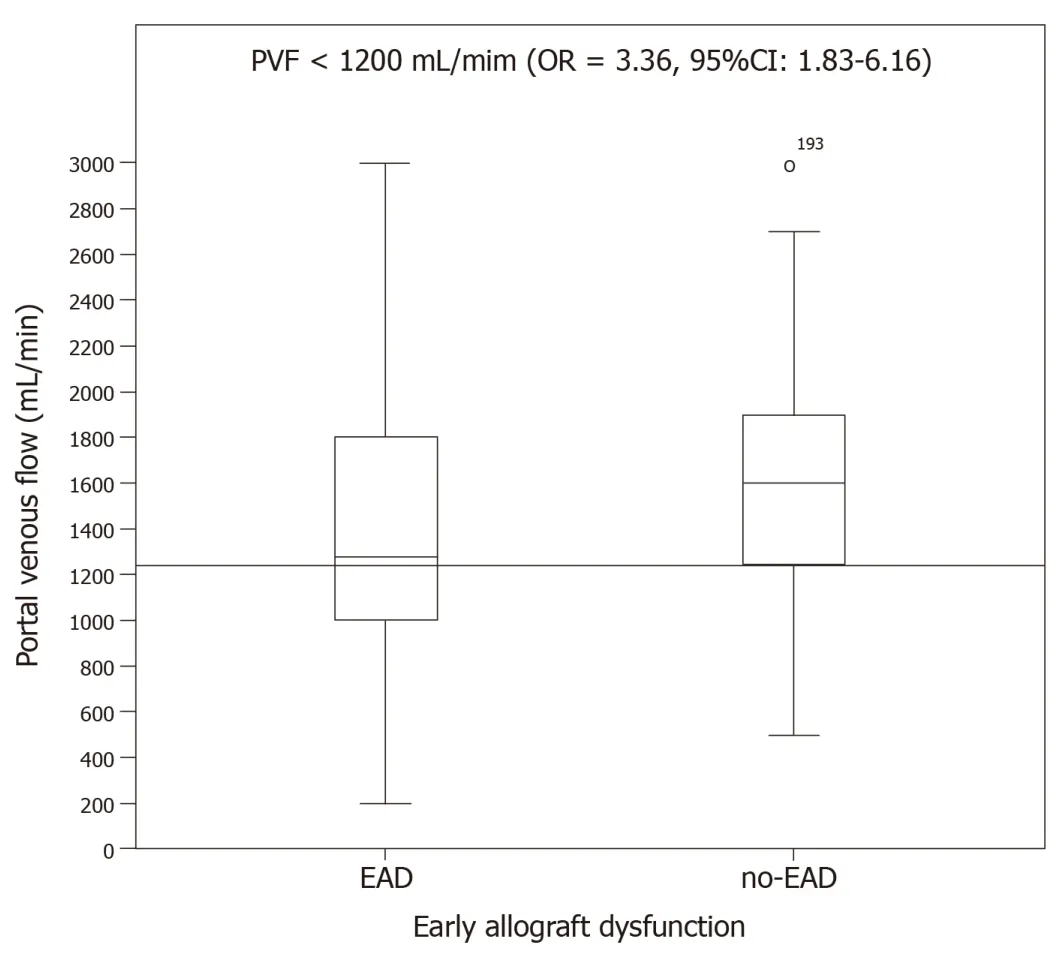
Figure3 Portal vein flow and development of early allograft dysfunction.
ARTICLE HIGHLIGHTS
Research background
Early allograft dysfunction (EAD) after liver transplantation (LT) is an important cause of morbidity and mortality.To ensure adequate graft function,a critical hepatocellular mass is required in addition to an appropriate blood supply.We hypothesized that intraoperative measurement of portal venous and hepatic arterial flow may serve as a predictor in the diagnosis of EAD.
Research motivation
EAD is a condition that can occur after implantation.The development of graft dysfunction is multifactorial.The degree of impairment can range from a very mild and temporary form to a more severe and potentially deadly form unless the patient receives an early retransplantation,which is determined by initial poor function.The regenerative capacity of the hepatic parenchyma conditions most dysfunctions to be transient.Currently,there are a large number of predictive models for graft failure,all of which are heterogeneous because they use different criteria to select the independent variables.In general,the models try to predict the development of liver dysfunction and aid clinicians in the liver graft selection process.To ensure proper function of a liver graft,the hepatocellular critical mass is needed to maintain synthetic function and adequate blood supply through the vascular tree.Hepatic flow is a determining factor in early graft function.Intraoperatively,measurable arterial and venous flow after implantation may be useful in predicting the development of EAD because blood flow values provide an indirect measurement of the oxygen and nutrient levels.A study of the intraoperative factors that may influence the development of EAD should be performed to address additional,related problems in the field.
Research objectives
To study whether hepatic flow is an independent predictor of EAD following LT.
Research methods
This is an observational coho rt study performed in a single institution.Hepatic arterial and portal venous blood flows were measured intraoperatively by transit flow.The measurement of the intraoperative flows was performed with a VeriQ™ flowmeter (Medistin,Norway).VeriQ™offers both proven transit time flow measurement and Doppler velocity measurements that are specifically designed for intraoperative blood flow and graft patency verification.The Doppler effect uses the transmission of a continuous wave,and MFTT employs the transmission of pulses.By applying the Doppler concept to the blood components,we can measure the vessel blood flow velocity.If the sound is directed in the direction of flow,the received signal will be different depending on whether the blood components are near or far from the transducer.The sensor used by the MFTT contains two transducers and a reflector.The two transducers are located on one side of the vessel and the reflector on the opposite side;this arrangement causes a double ultrasound passage through the vessel.The crystal located in the direction of flow generates a pulse of ultrasound that is captured by the glass oriented in the opposite direction.The difference in transit time depends on the volume of blood flow.Measurement probes of 5-7 mm calibre are used for the hepatic artery and 8-12 mm for the portal vein.Once the vascular anastomoses have been performed,a brief period of approximately 5 min is allowed for the intrahepatic flow to stabilize,and then the arterial and portal flows are measured sequentially at one centimetre distal to the suture on the side of the graft.In cases where the arterial intraoperative flow measured is absent or very poor,revision of the arterial anastomosis is indicated,once the absence of the portal flow compensatory effect (“hepatic arterial buffer effect”) has been proven.EAD was defined using the Olthoff criteria.Univariate and multivariate analyses were used to determine intraoperative predictors of EAD.Survival analysis and prognostic factor analysis were performed using the Kaplan-Meier and Cox regression models.
Research results
A total of 195 liver transplants were performed between January 2008 and December 2014 in 188 patients.A total of 54 (27.7%) patients developed EAD.The median follow-up was 39 mo.Portal venous flow,hepatic arterial flow (HAF) and total hepatic arterial flow were associated with EAD in both univariate and multivariate analyses.HAF is an independent prognostic factor for 30-d patient mortality.This is the first study that relies on current EAD criteria and 30-d patient survival data based on hepatic flow measured intraoperatively.
Research conclusions
In conclusion,we have shown that intraoperative measurements of hepatic blood flow can predict the development of EAD and that hepatic artery flow has an impact on survival at 30 d.
Research perspectives
Future efforts may focus on study strategies directed at identifying those grafts that are more susceptible to developing alterations in blood flow and how we can mend these alterations in hepatic blood flow in the intraoperative setting.

