Counting stripes:revision of the Lipinia vittigera complex(Reptilia,Squamata,Scincidae)with description of two new species from Indochina
Nikolay A. Poyarkov Jr., Peter Geissler, Vladislav A. Gorin, Evgeniy A. Dunayev, Timo Hartmann, Chatmongkon Suwannapoom
1 Department of Vertebrate Zoology,Biological Faculty,Lomonosov Moscow State University,Moscow 119991,Russia
2 Joint Russian-Vietnamese Tropical Research and Technological Center,Nghia Do,Cau Giay,Hanoi,Vietnam
3 Museum Natur und Mensch,Freiburg 79098,Germany
4 Zoological Museum,Moscow State University,Moscow 125009,Russia
5 Zoological Research Museum Alexander Koenig,Bonn 53113,Germany
6 School of Agriculture and Natural Resources,University of Phayao,Phayao 56000,Thailand
ABSTRACT We provide an integrative taxonomic analysis of the Lipinia vittigera species complex from mainland Southeast Asia. Based on examination of external morphology, color pattern, and 681 base pairs of the cytochrome oxidase subunit I (COI) mitochondrial gene, we demonstrate the presence of four morphologically distinct lineages of Lipinia in Vietnam, Cambodia, Thailand, and Malaysia,showing a sequence divergence ranging 15.5%-20.4%. All discovered lineages are discretely diagnosable from one another by a combination of scalation traits and color patterns. A review of the published distribution data and a re-examination of available type material revealed the following results: (1) distribution of L. vittigera (Boulenger,1894) sensu stricto is restricted to Sundaland and the Thai-Malay Peninsula south of the Isthmus of Kra; (2) L. microcercus (Boettger, 1901) stat. nov. is elevated to full species rank; the species has a wide distribution from central and southern Vietnam across Cambodia to eastern Thailand; we regard Lygosoma vittigerum kronfanum Smith, 1922 and Leiolopisma pranensis Cochran, 1930 as its junior synonyms; (3) Lipinia trivittata sp. nov. occurs in hilly areas of southern Vietnam, Cambodia, and eastern Thailand; and (4) Lipinia vassilievi sp. nov.is currently known only from a narrow area along the Vietnamese-Cambodian border in the foothills of the central Annamite Mountain Range. We further provide an identification key for Lipinia occurring in mainland Southeast Asia.
Keywords: Lipinia microcercus stat. nov.; Lipinia trivittata sp. nov.; Lipinia vassilievi sp. nov.;Vietnam; Thailand; Cambodia; Biogeography;mtDNA;COI-barcoding
INTRODUCTION
The scincid genus Lipinia Gray, 1845 is an assemblage of arboreal and terrestrial lizards currently comprising at least 29 valid species (Grismer et al., 2016; Uetz & Hošek, 2019).Many species of Lipinia were originally described as members of the genus Lygosoma Hardwicke and Gray, 1827. The genus was revived from its synonymy by Mittleman (1952).Lipinia skinks inhabit a large area from the Andaman and Nicobar Islands of India in the northwest, Mentawai and Sumatra Islands of Indonesia, eastward through Indochina and the Malay Peninsula to Borneo, the Philippines,numerous islands of the Indo-Australian Archipelago to New Guinea, and further eastwards throughout much of the South Pacific, including the archipelagos of Palau, Fiji, Samoa, and French Polynesia (Adler et al., 1995; Grismer et al., 2016;Günther, 2000; Linkem, 2013). Lipinia records east of Vanuatu have been suggested to be the result of anthropogenic introduction (Austin, 1999). Despite their significant diversity in Australasia, a biogeographic origin of the genus in Southeast Asia or the Philippines has been suggested (Austin, 1998).
Members of the genus Lipinia are characterized by the following combination of natural history traits and morphological characters: diurnal, arboreal, semi-arboreal, or secretive terrestrial lifestyle; small body size (snout vent length (SVL) to 58 mm); lower eyelid generally with transparent window (absent in some taxa); auricular lobules absent; body scales smooth; basal subdigital lamellae usually expanded; postorbital absent; vomers fused; pterygoid teeth absent; dorsal color pattern typically pale (rarely dark)middorsal stripe anteriorly (lacking in some taxa) (Das &Austin, 2007; Das & Greer, 2002; Grismer, 2011a, 2011b).Despite the overall morphological similarity, recent study has shown Lipinia to be a widely polyphyletic assemblage within the scincid tribe Sphenomorphini (Grismer et al., 2016;Linkem, 2013), and a stable phylogenetic hypothesis for Lipinia is still lacking.
In mainland Southeast Asia, Lipinia skinks occur widely in central to southern Indochina from easternmost Myanmar and northern Thailand southwards to southern Laos, central and southern Vietnam, Cambodia, and further southwards to the Thai-Malay Peninsula and Singapore (Figure 1). Boulenger(1894) described Lygosoma vittigerum from Mentawai (or Mentawei) Archipelago near the western coast of Sumatra,Indonesia. Shortly after, Boettger (1901) described Lygosoma(Leiolopisma) microcercum from Phuoc Son in Annam (now in Quang Nam Province, central Vietnam), noting that the new species appeared to be close to L. vulcania and L. pulchella from the Philippines. Annandale (1905) recorded a brightly colored tree-dwelling skink from Tavoy, Tenasserim (now Dawei, Tanintharyi Region, Myanmar) and identified it as Lygosoma pulchellum. Later, Smith (1922) described a new subspecies of Boulenger's Lygosoma vittigerum from Daban near Langbian in southern Vietnam as Lygosoma vittigerum kronfanum. Furthermore, Smith (1922) reported that this form also occurred in peninsular Siam (now Thailand) and differed from the nominate subspecies from the Mentawai Islands both in scalation and coloration pattern; among other characters,the main difference to the mainland form was the presence of five distinct light stripes on the dorsum and flank, instead of one prominent vertebral light stripe in the nominate form.However, Smith (1922, p. 209) noted certain geographic variation in this character and reported a juvenile specimen from northern Siam (now Thailand) with three dorsal light stripes and predicted that " …further collections from this region may establish a race with 3 light stripes only". Soon after, Cochran (1930) analyzed the herpetological collections from Siam and described a new species of the genus Leiolopisma Duméril & Bibron from Pran (now Pran-Buri,Prachuap Khiri Khan, Thailand) and Doi Angka (Chiang Mai,Thailand)-L. pranensis, noting its affinity to L. vittigerum, to which it differed in midbody scale row count, number of subdigital lamellae,and distinct coloration.
Subsequently, in his review of lizards of British India and adjacent territories, Smith (1935) also assigned L. vittigerum to the genus Leiolopisma, and listed two subspecies: L. v.vittigerum in Tenasserim, northern, western, and southern Siam (Thailand), and the Malay Peninsula (including L.pranensis as a synonym without any justification for this decision), and L. v. microcercum in southern and central Annam (thus formally recognizing synonymy of his kronfanum with microcercum of Boettger, 1901). The two-subspecies taxonomy of Smith (1922, 1930, 1935) was well established for almost a century without any significant changes, with Indonesian, Malayan, and south-Thai populations traditionally assigned to the subspecies L. v. vittigerum (Boulenger, 1894),and Indochinese populations assigned to L. v. microcercum(Boettger, 1901) (note: after resurrection of the genus Lipinia by Mittleman, 1952, the gender of species names should be modified as L. v. vittigera and L. v. microcercus; for details see Etymology sections of respective species). However, the border between the ranges of the two forms and their evolutionary relationships remained unclear. Stuart (1999)mentioned the presence of distinct morphs of Lipinia in southern Laos. Recently, Grimser (2011b) reported on morphologically distinct types of L. vittigera in the Malay Peninsula and its offshore islands. More recently, Grismer et al. (2016) provided a preliminary phylogenetic tree for Lipinia,which included three specimens of L. vittigera from the Malay Peninsula and Indochina, each represented by a significantly divergent mtDNA lineage. However, the taxonomic value of these differences was not clear.
During our herpetological surveys in eastern Indochina over the last decade, we encountered a number of skink specimens tentatively identified as L. v. cf. microcercus.However, these specimens showed significant variation in scalation characters and body coloration, with three main color morphs recorded: i. e., (1) five-striped with narrow vertebral stripe; (2) three-striped with wide vertebral stripe;and (3) spotted with wide vertebral stripe. These forms were all found in close geographic proximity but not in sympatry(Figure 1), indicating that our knowledge of the taxonomy of Lipinia skinks in Indochina is far from complete. In the present paper, we provide descriptions of the available type material of the L. vittigera species complex and apply integrative approaches, including morphological and chromatical analyses together with COI DNA-barcoding, to assess the taxonomy of this enigmatic group of skinks.

Figure 1 Map of Indochina,showing known distribution of the genus Lipinia and survey localities
MATERIALS AND METHODS
Sample collection
Fieldwork was carried out in southern and central Vietnam (by N.A.P. and P.G.) and Cambodia (by P.G., T.H., and E.A.D.)from 2009 to 2017. Specimens of Lipinia spp. were collected on tree trunks, on buildings, or on the ground (while foraging)by hand or using a rod with a loose loop. Specimens were euthanized by 20% benzocaine and muscle tissue samples were taken and stored in 96% ethanol for subsequent genetic analysis. Specimens were subsequently preserved in 70%ethanol and deposited in the herpetological collections of the Zoological Museum of Moscow State University (ZMMU),Moscow, Russia, and the Zoologisches Forschungsmuseum Alexander Koenig (ZFMK), Bonn, Germany. Additionally, we isolated DNA from the ethanol-preserved specimens in ZMMU and ZFMK, resulting in 14 morphologically examined populations of L. cf. vittigera and 15 barcoded populations (25 specimens in total). The morphologically and genetically examined populations are presented in Figure 1.
All applicable international, national, and/or institutional guidelines for the care and use of animals were strictly followed; all animal collection protocols complied with the current laws of Vietnam, Cambodia, and Thailand. Specimen collection protocols and animal use were approved by the Institutional Ethical Committee of Animal Experimentation of the University of Phayao, Phayao, Thailand (certificate number UP-AE59-01-04-0022 issued to Chatmongkon Suwannapoom) and strictly complied with the ethical conditions of the Thailand Animal Welfare Act.
Morphological data and analyses
The following measurements were taken with digital vernier calipers (to the nearest 0.1 mm), following Bucklitsch et al.(2012), Das &Austin (2007), and Grismer et al. (2014): snoutvent length (SVL); tail (original or regenerated) length (TaL);trunk length (TrunkL), from posterior end of forelimb insertion to anterior part of hindlimb insertion, measured when stretched out; head length (HL), distance between posterior margin of parietal and snout-tip; head width (HW), measured across retroarticular process of mandibles; head height (HH),measured as greatest transverse depth of head, taken posterior of orbital region; snout length (SL), from anterior corner of eye to tip of snout; snout-tympanum length (STL),distance from snout tip to anterior border of tympanum; snoutforelimb length (SFlL), from tip of snout to anterior forelimb insertion, with limb held at right angles to body; eye-nostril distance (END), distance from anterior corner of eye to posterior border of nostril; horizontal eye length (EL), distance between anterior and posterior corners of eyelid; maximum diameter of tympanum (TYD); forelimb length (FLL), from anterior junction of forelimb and body wall to tip of fourth finger, with limb held at right angles to body; hind-limb length(HLL), from anterior junction of hind limb and body wall to tip of fourth toe,with limb held at right angles to body.
Nomenclature of head scales follow Taylor (1935); among other meristic features of pholidosis, we examined the following characters, which showed variation in representatives of the L. vittigera species complex:frontonasal width greater than its length (FNW); prefrontals in contact or not (PFC); frontal in contact with which supraoculars (FSO); number of supraoculars (SOC);frontoparietal in contact with which supraoculars (FPS);number of nuchals (Ncl); number of supraciliaries (Scil);number of postsupraoculars (pretemporals) (PSPO); number of postsuboculars (PSBO); number of supralabials (SLab);number of supralabials immediately below eye (SLO); number of infralabials (ILab); number of midbody scale rows (MSR);number of middorsal (vertebral) scales (MdS); number of ventrals in transverse rows (Vent); number of enlarged precloacals (PrCl); number of subdigital lamellae on fourth finger (SDL4F); number of subdigital lamellae on fourth toe(SDL4T); and number of light stripes on dorsum and flanks(LStr). Nomenclature of body coloration included description of dark and light markings on body and head, as shown in Figure 2: middorsal light stripe (MDLS); paravertebral dark stripes (PVDS); dorsolateral light stripe (DLLS); lateral dark stripe (LDS); lateral light stripe (LLS); ventrolateral dark stripe(VLDS); and dark temporal markings (DTM). Comparative material examined is listed in Appendix I. Sources of additional comparative data on character states and distribution of other species of Lipinia are from Bourret (2009);Brongersma (1942); Bucklitsch et al. (2012); Cochran (1930);Das (1997, 2010); Das & Austin (2007); de Rooij (1915);Grismer (2011b); Grismer et al. (2014, 2016); Günther (2000);Koch (2012); Loveridge (1948); Smith (1935); Taylor (1917,1922);Werner(1910);and Zweifel(1979).
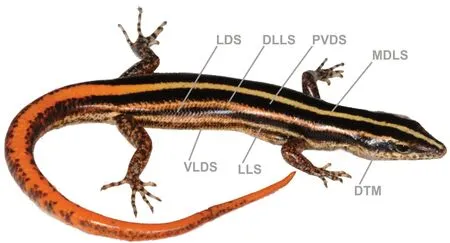
Figure 2 Terminology describing coloration of Lipinia used in present paper
We examined all type specimens in the L. vittigera complex that were traceable in museum collections today. Museum abbreviations, where available, follow Leviton et al. (1985,1988), including ZMMU, ZFMK, Field Museum of Natural History (FMNH), Chicago, USA; Senckenberg Museum Frankfurt (SMF), Germany; Museo Civico di Storia Naturale"Giacomo Doria" (MSNG), Genova, Italy; Institute of Ecology and Biological Resources (IEBR), Hanoi, Vietnam; University of Kansas, Museum of Natural History, Division of Herpetology(KU), USA; California Academy of Sciences, Department of Herpetology (CAS), USA; Museum of Vertebrate Zoology,University of California (MVZ), USA; and National Museum of Natural History, Division of Amphibians and Reptiles (USNM),USA. Other abbreviations include: Prov.: Province; Div.:Division; Dist.: District; Mt.: Mountain; NP: National park; WS:Wildlife sanctuary; NBCA: National Biodiversity and Conservation Area.
One-way analysis of variance (ANOVA) was used for morphometric comparisons between species; a significance level of 95% was used for all statistical tests. Principal component analysis (PCA) was used to determine if the examined populations were separated from each other in morphospace and if this coincided with the mtDNA lineages recovered with molecular phylogenetic analysis. Sexes were analyzed together due to the small sample size for two out of the four analyzed lineages; juvenile specimens were excluded from analysis; a total of 30 specimens were included in the PCA. All meristic characters used in the PCA were logtransformed and scaled to their standard deviation prior to analysis to normalize their distribution and ensure characters with very large or very low values did not over-leverage the results due to intervariable nonlinearity and to ensure the data were analyzed based on correlation not covariance.Tail length(TaL) was excluded from the PCA as tails were broken or regenerated in many specimens. All other metric (n=13) and meristic (n=16) characters were subjected to PCA using Statistica v8.0(StatSoft,Inc.,2007).
DNA isolation, polymerase chain reaction (PCR), and sequencing
Total genomic DNA was extracted from 95% ethanolpreserved muscle tissues using standard phenol-chloroform extraction protocols (Hillis et al., 1996). Total DNA concentration was estimated in 1 μL using NanoDrop 2000(Thermo Scientific, USA) and consequently adjusted to 100 ng DNA/μL.
We amplified 655 bp of cytochrome oxidase subunit I (COI),a mitochondrial barcoding marker widely used in vertebrates,including both reptiles and amphibians (Murphy et al., 2013;Nagy et al., 2012; Smith et al., 2008) and proven to be useful for species identification in various groups of lizards(Hartmann et al., 2013b; Nazarov et al., 2012, 2014; Neang et al., 2018; Solovyeva et al., 2011, 2018). Primers used for PCR and sequencing included ReptCOI-F (5'-TNTTMTCAACNAAC CACAAAGA-3') and ReptCOI-R (5'-ACTTCTGGRTGKCCAAA RAATCA-3') (Nagy et al., 2012). The PCR assays were performed in 25 μL reactions using 50 ng of genomic DNA,10 pmol of each primer, 15 nmol of each dNTP, 50 nmol additional MgCl2,Taq PCR buffer (10 mmol/L Tris-HCl, pH 8.3,50 mmol/L KCl, 1.1 mmol/L MgCl2, and 0.01% gelatin), and 1 U of Taq DNA polymerase. The PCR conditions included an initial denaturation step of 5 min at 95 ° C, 5 cycles of denaturation for 1 min at 94 °C, primer annealing for 40 s at 45 ° C, and extension for 1 min at 72 ° C, 30 cycles of denaturation for 1 min at 94 °C, primer annealing for 40 s at 53 °C, and extension for 1 min at 72 °C, and a final extension step for 5 min at 72 °C. The PCR products were visualized by agarose electrophoresis in the presence of ethidium bromide and purified using 2 μL from a 1: 4 dilution of ExoSapIt(Amersham, UK) per 5 μL of PCR product prior to cycle sequencing. The obtained fragments were sequenced in both directions for each sample, and a consensus sequence was generated. Sequencing was performed in both directions using the same primers as used in PCR on an ABI3730xl Automated Sequencer (Applied Biosystems, USA) in Evrogen Inc., Moscow. The newly obtained sequences were aligned and deposited in GenBank under the accession Nos.MK463827-MK463852, MK843792-MK843793, and GU657766-GU657767 (Supplementary Table S1). Sequences of three other Lipinia species used for comparisons were obtained from GenBank(Supplementary Table S1).
Phylogenetic analyses
The COI gene fragment was applied in the present study mainly as a barcoding marker for species identification rather than a tool for reconstructing phylogenetic relationships among species. Information on voucher specimens and GenBank accession Nos. used in phylogenetic analyses are summarized in Supplementary Table S1. In total, 58 COI fragment sequences of Scincidae, including 53 sequences of Lipinia spp. (including 25 sequences representing target L.vittigera species complex) and sequences of outgroup taxa,including representatives of scincid genera Lygosoma,Eutropis, Tropidophorus, Sphenomorphus, and Eumeces were included in the final alignment with a total length of 681 bp.Nucleotide sequences were initially aligned in the program MAFFT v6 (Katoh et al., 2002) with default parameters, and then checked by eye in BioEdit 7.0.5.2 (Hall, 1999) and slightly adjusted. Mean uncorrected genetic distances (Pdistances) between sequences were calculated with MEGA 7.0(Kumar et al.,2016).
Matrilineal genealogy was inferred using Bayesian inference (BI) and maximum likelihood (ML) algorithms. We conducted BI analysis in MrBayes 3.1.2 (Huelsenbeck &Ronquist, 2001; Ronquist & Huelsenbeck, 2003). The dataset was divided into three codon-partitions of the COI gene;MODELTEST v.3.06 (Posada & Crandall, 1998) was used to estimate the optimal evolutionary models for each partition.The best-fitting model selected for the COI dataset was GTR+Gamma for the first and third codon positions and HKY+I for the second codon position. Metropolis-coupled Markov chain Monte Carlo (MCMCMC) analyses were run with one cold chain and three heated chains for twenty million generations,with sampling every 2 000 generations. Five independent MCMCMC runs were performed and 1 000 trees were discarded as burn-in. Stationarity from each run was checked using TRACER v1.6 (Rambaut & Drummond, 2007) to ensure an effective sample size(ESS)above 200 for all parameters.
The ML analysis was conducted using RAxML (http://embnet.vital-it.ch/raxml-bb/; Stamatakis et al., 2008), with the ML trees searched using default priors and the GTR+Gamma model of evolution for all codon positions. Confidence in node topology was tested by posterior probability (PP) for BI trees(Huelsenbeck & Ronquist, 2001) and by non-parametric bootstrapping with 1 000 replicates (ML BS, see Felsenstein,1985) for ML trees. We a-priori regarded tree nodes with bootstrap (ML BS) values of ≤70% and Bayesian posterior probabilities (BI PP) values >0.95 as sufficiently resolved; ML BS values between 70% and 50% (BI PP between 0.95 and 0.90) were treated as tendencies and nodes with ML BS values below 50% (BI PP below 0.90) were regarded as unresolved(Felsenstein,2004;Huelsenbeck&Hillis,1993).
RESULTS
Genetic differentiation of Indochinese Lipinia
Sequence data:The final alignment of the COI gene contained 681 aligned characters, including 422 conserved sites and 257 variable sites, of which 252 were parsimonyinformative. The transition-transversion bias (R) was estimated as 1.577 (all data given for ingroup only).Nucleotide frequencies were 22.72% (A), 28.92% (T), 27.86%(C),and 20.50%(G).
Genetic diversity and geographic distribution of mtDNA
haplotypes:The phylogenetic analysis results of the examined Lipinia species are presented in Figure 3. We also calculated a tree with more outgroup taxa, representing different groups of lygosomine skinks (Supplementary Figure S1).

Figure 3 Bayesian inference tree of Lipinia derived from analysis of 681 bp of partial COI gene sequences
Both the BI and ML phylogenetic analyses resulted in essentially similar topologies. Phylogenetic relationships within the genus Lipinia remained fundamentally unresolved in our analyses, whereas monophyly of species-level groups was highly supported (1.0/100, hereafter node support values are given for BI PP/ML BS, respectively) (Figure 3). In the additional analysis involving more outgroup taxa, monophyly of the genus Lipinia was not supported, and all higher-level taxonomic relationships between this genus and other lygosomine skink genera (i.e., Lygosoma, Eutropis,Tropidophorus, and Sphenomorphus) remained unresolved(Supplementary Figure S1). Monophyly of the L. vittigera species complex was not supported in either analysis (Figure 3;Supplementary Figure S1).
Our analyses revealed unexpectedly high genetic diversity within the Indochinese Lipinia skinks, contradicting current taxonomy of the group. In Indochina, four major mtDNA lineages were recovered (lineages A-D; see Figure 3 for phylogenetic tree and Figure 1 for geographic distribution of revealed lineages):
(1) Lineage from southern peninsular Thailand (Surat Thani Province), corresponding to L. vittigera sensu stricto (see below)(lineage C,see Figure 3).
(2) Lineage from Kon Tum Province of Vietnam and adjacent part of Ratanakiri Province of Cambodia,corresponding to the spotted form of L. cf. vittigera (lineage B,see Figure 3). This lineage forms a well-supported clade with L. vittigera sensu stricto lineage C from southern Thailand(1.0/100).
(3) Lineage recorded from lowland and hilly areas of central and southern Vietnam, as well as from across Cambodia, fivestriped form specimens, corresponding to the traditionally recognized subspecies L. v. microcercus (lineage A, see Figure 3).
(4) Lineage recorded from hilly areas in southern Vietnam and Cambodia, corresponding to the three-striped form of L.cf.vittigera(lineage D,see Figure 3).
The three mtDNA lineages of L. cf. vittigera recorded from Indochina, north of the Isthmus of Kra, correspond to distinct morphotypes and color forms and were never recorded in the same biotope. However, their ranges appear largely overlapping (see Figure 1) and the geographic distance between two localities where different mtDNA lineages/morphotypes occur is as little as 15 km (e. g., between localities 11 and 22 in Figure 1). While the five-striped form(lineage A, L. v. microcercus) widely occurs in southern Indochina from the central Annamites to southern Vietnam and Cambodia (possibly also penetrating into Thailand), the three-striped form (lineage D) is confined to hilly areas of southern Annam (Langbian Plateau), Cambodia,southernmost Vietnam (and possibly southeast Thailand),whereas the spotted form (lineage B) is restricted to a narrow area in the central Annamites on the border of Vietnam and Cambodia(Figure 1).
Genetic distances:The uncorrected P-distances among and within the studied mtDNA fragments for the examined Lipinia species are shown in Supplementary Table S2. Intraspecific genetic distances in all examined species were below the level P=3.5% (in the three-striped form, lineage D). The interspecific uncorrected genetic P-distances between Lipinia species varied from 7.1% (between L. noctua (Lesson) and Lipinia sp. from Palau) to 23.4% (between Lipinia sp. from Palau and spotted form, lineage B). Genetic differentiation among the four lineages of L. vittigera sensu lato from Indochina was surprisingly high and varied from 15.5%(between lineages B and C) to 20.4% (between lineages B and D). This degree of pairwise divergence is high, notably greater than the genetic divergence observed between many recognized species of Scincidae (e.g., Murphy et al., 2013;Nagy et al., 2012; Neang et al., 2018; Okamoto & Hikida,2012).
Systematics
Recent study demonstrated that the genus Lipinia likely does not represent a monophyletic group, but rather is an assemblage of distantly related sphenomorphine skinks(Grismer et al., 2016; Linkem, 2013). Reconstructing phylogenetic relationships among members of the Lipinia assemblage would require much broader taxon sampling and a multilocus approach combining data from several mtDNA and nuDNA markers. In the present paper, we applied COI DNA-barcoding, which is a useful tool for uncovering cryptic diversity in squamate reptiles, including members of the family Scincidae. We did not aim to discuss phylogenetic relationships of Indochinese Lipinia or use COI-barcoding solely for assessment of their genetic diversity and distribution of mtDNA lineages.
The four mtDNA lineages revealed within the L. vittigera species complex were highly divergent, with uncorrected genetic distances exceeding P=15.5%. The PCA results corroborated these findings and indicated that each mtDNA lineage occupies a unique position in morphospace that did not overlap with any other species in the ordination of the first two principle components (PC) (Figure 4). PC1 accounted for 28.24% of the variation in the dataset, with loading for snouttympanum length, snout-forelimb length, head width, head height, and snout-vent length, whereas PC2 accounted for an additional 16.05% of the variation, with loading for frontonasal width, snout length, number of light stripes on dorsum and flanks, and head length (Figure 4; Supplementary Table S3).These results suggest deep differentiation of the four Indochinese lineages of Lipinia skinks, not only in genetic, but also in morphological characters. Additional differences in morphological and color characters not amenable to statistical analyses are discussed in the comparison sections of each species and summarized in Table 1.

Figure 4 Two-dimensional plots of first two factors of principal component analysis (PCA) with convex hull polygons showing morphospatial relationships of species in the Lipinia vittigera species complex (Photos by Nikolay A. Poyarkov, Evgeniy A.Dunayev,Eduard A.Galoyan,and Vitaly L.Trounov)

Table 1 Morphological comparison between Lipinia vittigera species complex members found in Indochina and adjacent territories
The data on genetic divergence, together with congruent differentiation of mtDNA lineages in morphospace revealed by PCA and other morphological differences compiled below,support our hypothesis that at least four distinct species, as described in the following taxonomic accounts, should be recognized within the Indochinese members of the L. vittigera species complex,two of which appear to be new to science.
Lipinia vittigera sensu stricto (=Lygosoma vittigerum)(Boulenger,1894)
Figures 5A-B,7A-B,8-9;Tables 1,2.
Chresonymy
Lygosoma vittigerum Boulenger, 1894, p. 615; Sworder, 1933,p.102;

Figure 5 Photos of Indochinese Lipinia species in life(part I)

Figure 6 Photos of Indochinese Lipinia species in life(part II)
Leiolopisma vittigerum—Barbour,1912,p.187;
Leiolopisma vittigerum vittigerum — Smith, 1935, p. 306;Taylor,1963,p.1029;Bourret,2009,p.274;
Lygosoma(Leiolopisma)vittigerum—Smith,1937,p.224;
Lygosoma (Scincella) vittigerum vittigerum — Grandison,1972,p.82;
Lipinia vittigera — Greer, 1974, p. 11; Manthey &Grossmann, 1997, p. 266; Cox et al., 1998, p. 116; Grismer et al., 2002, p. 27; Pauwels et al., 2003, p. 28; Nguyen et al.,2005, p. 59; Das & Austin, 2007, p. 66; Grismer, 2011a, p.149;Grismer,2011b,p.606;Chan-ard et al.,2015,p.111;
Lipinia cf.vittigera—Teo&Rajathurai,1997,p.415;
Lipinia vittigera(?)—Onn et al.,2010,p.140;
Lipinia vittigera vittigera — Das, 2010, p. 237; Grossmann,2010,p.2;Bucklitsch et al.,2012,p.325.
Lygosoma vittergerum—Grismer,2011b,p.606(ex errore).

Figure 7 Head pholidosis of holotype specimens of Indochinese Lipinia in lateral(left)and dorsal(right)aspects
Holotype:MSNG 55855, adult male (Figure 8), collected by Elio Modigliani from Sereinu (=Sipora), Mentawei, Sumatra(Indonesia) (Figure 1, locality 1). A re-description of the holotype was published by Bucklitsch et al.(2012).
Paratypes:None.
Diagnosis:Based on the holotype, additional specimens examined from Penang (Malaysia) and peninsular Thailand(Appendix I) as well as literature data from peninsular Malaysia and Singapore (Grismer, 2011b): small (SVL to 44 mm) species of Lipinia, differentiated from congeners by the following combination of external traits: external ear opening present; lower eyelid bearing large transparent spectacle; 28-30 midbody scale rows; 48-56 middorsal scales between parietals and point above vent; 15-16 subdigital lamellae under finger IV; 20-26 lamellae under toe IV; prefrontals in punctiform or broad contact; seven supralabials, seven infralabials; broad middorsal light stripe from snout tip to tail base; two paravertebral dark stripes from supraoculars toward tail, continuing on anterior part of tail;flanks dotted brown,without any distinct dark or light stripes.
They didn t awake till it was pitch dark, and Hansel comforted his sister, saying: Only wait, Gretel, till the moon rises, then we shall see the bread-crumbs I scattered20 along the path; they will show us the way back to the house

Figure 8 Holotype of Lygosoma vittigerum Boulenger,1894(MSNG 55855,male)in preservative
Etymology:Although not stated by Boulenger (1894), the species name "vittigerum" is derived from Latin "vitta" for a head band used by Roman priests during rituals and "gero" (to wear), as a reference to the banded dorsal pattern of this species. Due to the feminine gender of the genus name Lipinia,the species epithet has to be adapted to"vittigera".
Description of holotype:Measurements and counts of holotype are presented in detail in Table 2. Head scalation of holotype is detailed in Figure 7A-B.

Figure 9 Specimen of Lipinia cf.vittigera(Boulenger,1894)(ZMMU R14477,male)in preservative
SVL 36.9 mm (Figure 8), TaL 4.5 mm, largest parts missing. Snout acute, SL 4.1 mm; nostrils oriented laterally,oval, situated closer to snout tip than to orbit, END 3.0 mm;head elongated, HL 10.1 mm, HW 4.8 mm, HL/HW ratio 2.1,flattened, HH 3.4 mm, HL/HH ratio 3.0; rostral broad, visible in dorsal view (Figure 7A-B); frontonasal almost as wide as long; frontal elongated, arrow-shaped, wider anteriorly;prefrontals large, not in contact medially, laterally and posteriorly in contact with loreals, first presupraocular, first supraocular, frontal and frontonasal; two frontoparietals in broad median contact; interparietal arrow-shaped, wider anteriorly; parietals in contact behind interparietal, anteriorly in contact with postsupraoculars, fourth supraocular, nuchals,and frontoparietals; seven nuchals; four supraoculars; three presupraoculars, visible from above; seven supraciliaries;nostrils located within nasals; postnasal absent; two loreals,slightly elongated, second longer than first; two enlarged presuboculars, separating supralabials III and IV from eye;seven supralabials, supralabial V largest, in contact with orbit;two postsuboculars, separating supralabial VI from orbit;seven postsupraoculars (pretemporals); one primary temporal; two secondary temporals, dorsal largest; two postsupralabials; lower eyelid bearing large transparent window; scales on upper row of lower eyelid small, 14 in number; mental wider than long; one postmental, in contact with first infralabial and anterior portion of second infralabial;seven infralabials; three pairs of chinshields, first pair in contact medially, second pair separated by one scale, third pair separated by three scales; external ear opening visible and subcircular (Figure 7A-B).
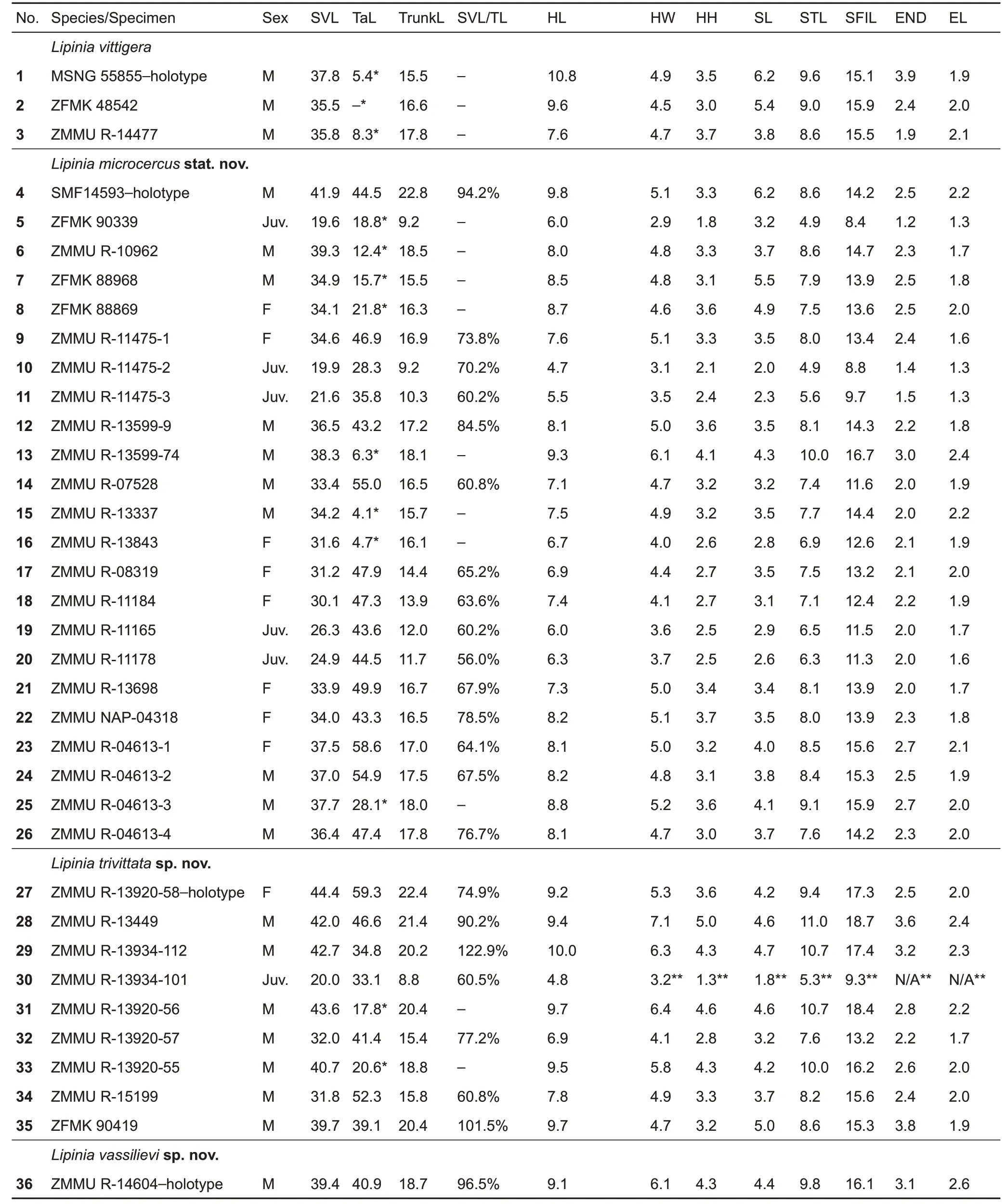
Table 2 Morphometric and meristic data of examined specimens of Lipinia vittigera species complex of Indochina and adjacent territories(All measurements are in mm)

Continued

Continued
Body slender (Figure 8), TrunkL 17.1 mm; head slightly distinct from neck and body; 56 middorsal scales from parietal to point above vent, scales in four median longitudinal rows enlarged; ventrals in 58 rows, counted from first postgular to preanal scales; body scales smooth, subcycloid; 30 scales around midbody; four slightly enlarged preanals; Limbs slender, pentadactyl, and clawed; forelimb and hindlimb meeting when adpressed; subdigital lamellae under finger IV:15/16; subdigital lamellae under toe IV: 25/25; all subdigital lamellae enlarged.
Coloration in preservative:MDLS cream (Figure 8), from snout to tail base, widening at point above vent and merging into light dorsal coloration of tail. DTM indistinct dark mottling in temporal region (Figure 8D). MDLS on midbody about two scales wide. Two black PVDS from supraoculars to tail base,one scale wide at midbody, merging into brown lateral tail coloration. Outer margin of PVDS straight; flanks and gular region light fawn, dotted with small brown spots. Labials and surrounding of outer ear opening fawn with brown markings.Dorsal surface of remaining part of tail cream, becoming darker on lateral sides. Dorsal surface of limbs grayish fawn scattered with irregular brown spots. Toes and fingers light fawn with dark brown blotches on joints. Ventral surface of throat,trunk,limbs,and tail cream.
Coloration in life:Based on specimen (ZMMU R-14477, see Figure 5A-B) from Phanom District, Surat Thani Province,peninsular Thailand: MDLS fawn yellow on head, neck, and anterior half of trunk, gradually turning into fawn orange toward tail; PVDS brownish black; flanks and lateral surfaces of head light golden brown, scattered with lighter fawn and dark brown spots; dorsal surfaces of limbs golden brown with light orange complexion and small brown dots; digits fawn with dark brown spots on joints; labials fawn white; throat and belly white; tail fawn orange; lateral surfaces of tail marbled in brown. DTM, indistinct dark mottling in temporal region (Figure 5B,see Figure 9D for the same specimen in preservative).
Type locality:Sereinu Island (=Sipura), Mentawai Archipelago, west of Sumatra; see Boulenger (1894) and Bucklitsch et al.(2012)for discussion.
Distribution:See Figure 1. In mainland Southeast Asia, L.vittigera sensu stricto seems to be restricted to an area south of the Isthmus of Kra, though additional research on the Thai-Malay Peninsula populations is needed to confirm this assumption. Distribution of the species in peninsular Malaysia and its offshore islands was reviewed by Grismer (2011b).The species has been reported from: Peninsular Thailand: "Tasan,Isthmus of Kra" (Smith, 1935); Nakhon Si Thammarat Prov.:Khao Ram Rome Mt. (P. Pawangkhanant, personal communication); Surat Thani Prov.: Phanom (KUH 328480);Phanom Dist. (ZMMU R-14477; see material examined,Appendix I); Peninsular Malaysia: Pulau Pinang (Penang)(ZFMK 48542); Lata Tembaka, Terengganu; Jor, Perak, Kuala Teku, Pahang, Kepong and Ulu Langat, Selangor; and Endau Rompin and Gunung Panti, Johor (Denzer & Manthey, 1991;Grandison, 1972; Grismer, 2011b; Norsham & Ong, 2001;Onn et al., 2010; Smith, 1922; Tanner, 1953; Wood et al.,2004); Seribuat Archipelago: Pulau Aur and Pulau Babi Besar,Johor, and Pulau Tioman, Pahang (Grismer et al., 2006);Singapore (Baker & Lim, 2008); Mentawai Archipelago(Boulenger, 1894) (MSNG 55855); as well as in Sumatra and northern Borneo (Das, 2010). Distribution records from north of the Isthmus of Kra often lack a clear assignment to one of the former subspecies, i.e., L. v. vittigera or L. v. microcercus.Thus, those records need further research to verify species assignment (see account on L. microcercus stat. nov.). Smith(1935) and Taylor (1963) published several records of L. v.vittigera from northern and eastern Thailand: "Chantaboon (=Chantaburi); Rehang district; Meh Lem, Meh Wang in N.Siam". However, without having seen the reference specimens, it is not possible to decide to which species of the L.vittigera complex those records belong.
Natural history:Grismer (2011a, 2011b) summarized several field observations from peninsular Malaysia and its offshore islands, with the species occurring in lowland and hill dipterocarp forests(primary and secondary)from 0 to 600 m a.s.l., and with a preferred microhabitat including trunks of large trees up to a height of 10 m. Reproduction was described by Goldberg & Grismer (2014): gravid females were recorded in March; females lay two to three eggs; hatchlings were observed in July.
Remarks:Our study suggests that the range of L. vittigera sensu stricto is restricted to the Mentawei Archipelago,Sumatra, the Thai-Malay Peninsula south of Kra Isthmus, and Borneo. The northern extent of its distribution is unknown and requires further studies. However, certain morphological variation was observed even within our limited sampling on L.vittigera sensu stricto. No data on variation of Bornean populations of L. vittigera are available. Grismer (2011b)reported color variation between specimens from Pulau Tioman, Pahang, which exhibited thin vertebral stripes only one scale wide, whereas specimens from peninsular Malaysia showed wider vertebral stripes, usually two scale rows wide.These data suggest that diversity of the L. vittigera complex in Peninsular Malaysia and Sundaland may still be underestimated. Thus, further morphological and molecular studies are needed to address these questions.
Lipinia microcercus stat. nov. (=Lygosoma microcercum)(Boettger,1901)
Figures 5C-H,7C-F,11-13;Tables 1-2.
Chresonymy
Lygosoma(Leiolopisma)microcercum Boettger,1901,p.49;
Lygosoma pulchellum (partim) — Annandale, 1905, p. 145(preliminary,see taxonomic comment below);
Leiolopisma pulchellum(partim)—Taylor,1922,p.212;
Lygosoma vittigerum kronfanum Smith, 1922, p. 208 (see taxonomic comment below);
Leiolopisma vittigerum kronfanum—Schmidt,1928,p.80;
Leiolopisma pranensis Cochran, 1930, p. 18 (preliminary,see taxonomic comment below);Smith,1930,p.126;
Leiolopisma vittigerum microcercum — Smith, 1935, p. 308;Bourret, 1939, p. 52; Taylor, 1963, p. 1030; Ho & Nguyen,1981, p. 140; Semenov et al., 1983, p. 72; Bourret, 2009,p.275;
Lipinia vittigerum microcercum — Bobrov, 1992, p. 19;Bobrov,1995,p.15;
Lygosoma microcercum—Bobrov,1995,p.15;
Scincella vittigerum(partim)—Nguyen&Ho,1996,p.40;
Scincella vittigerum kronfanum — Nguyen & Ho, 1996,p.40;
Scincella vittigerum microcercum — Nguyen & Ho, 1996,p.40;
Lipinia vittigera microcerca — Bobrov & Semenov, 2008, p.64; Nguyen et al., 2009, p. 252; Bucklitsch et al., 2012, p. 325;Vassilieva et al.,2016,p.175;
Lipinia vittigera microcercum—Grossmann,2010,p.2;
Lipinia vittigera (partim) — Nguyen et al., 2005, p. 59;Stuart et al., 2006, p. 147; Stuart & Emmet, 2006, p. 15;Grismer et al., 2008, p. 22; Hartmann et al., 2013b, p. 48;Jestrzemski et al.,2013,p.96;Grismer&Quah,2019,p.234;
Lipinia vittigera kronfanum — Das, 2010, p. 237 (treated as a valid subspecies).
Holotype:SMF 14593, adult male (Figure 10), collected by Hans Fruhstorfer from "Phuc-son in Annam" (=Phuoc Son District in Quang Nam Province), Vietnam (see comment below for discussion on type locality).
Paratypes:None.
Diagnosis:Small (SVL to 41.9 mm) species of Lipinia,differentiated from congeners by the following combination of external traits: external ear opening present; lower eyelid bearing large transparent spectacle; 28-32 midbody scale rows; 48-58 middorsal scales between parietals and point above vent; 18-21 subdigital lamellae under finger IV; 24-32 lamellae under toe IV; prefrontals in broad contact; seven supralabials, seven infralabials; thin middorsal light stripe from snout tip to tail base; two paravertebral dark stripes from supraoculars toward tail, continuing on anterior part of tail; two distinct dorsolateral light stripes; broad lateral dark stripe from temporals to anterior part of tail; one distinct lateral light stripe,separated from belly by narrow ventrolateral dark stripe or longitudinal patch of dark spots.
Etymology:Referring to its short tail compared to other lygosomine skinks, Boettger (1901) provided the species epithet "microcercum". He treated this as a flexible adjective by adjusting it to the neutral gender of the genus name Lygosoma. However, such an adjective does not exist in the Latin language. The noun "cercus" is a Latinized version of the ancient Greek "κέρκος (kérkos)" in female gender,meaning "tail". When used as a noun in apposition, the species epithet cannot be reflected and therefore must be used as"microcercus".
Redescription of holotype:Measurements and counts of holotype are presented in detail in Table 2. Head scalation of holotype is detailed in Figure 7E-F. Adult male (Figure 10),SVL 41.9 mm,TaL 44.5 mm.
Snout acute, SL 6.2 mm; nostrils oriented laterally, oval,situated closer to snout tip than to orbit, END 2.5 mm; head long, almost twice as long as wide, HL 9.8 mm, HW 5.1 mm,HL/HW ratio 1.92, flattened, HH 3.3 mm, HL/HH ratio 2.97;rostral broad, visible in dorsal view, posterior border almost straight, contact zone with nasals slightly emarginated,frontonasal wider than long; frontal elongated, arrow-shaped,wider anteriorly; prefrontals large, in broad contact medially(Figure 7F), laterally and posteriorly in contact with loreals,enlarged presupraocular, first supraocular, frontal and frontonasal; two frontoparietals in broad median contact;interparietal arrow-shaped, wider anteriorly; parietals in contact behind interparietal, anteriorly in contact with postsupraoculars, nuchals, and frontoparietals; six nuchals;four supraoculars; one enlarged presupraocular, visible from above; seven supraciliaries; nostrils located within nasals;postnasal absent; two loreals, slightly elongated, second longer than first; two enlarged presuboculars, separating supralabials III and IV from eye; seven supralabials,supralabial V largest, in contact with orbit; three postsuboculars, separating supralabial VI from orbit; six postsupraoculars (pretemporals); one primary temporal; two secondary temporals, dorsal largest; two postsupralabials;lower eyelid bearing large transparent spectacle (Figure 10F);scales on upper row of lower eyelid small, 11 in number;mental wider than long; one postmental, in contact with first infralabial and anterior portion of second infralabial; seven infralabials; three pairs of chinshields, first pair in contact medially, second pair separated by one scale, third pair separated by three scales; external ear opening visible and subcircular(Figures 7E,10E).
Body slender, TrunkL 22.8 mm; head slightly distinct from neck and body; 56 middorsal scales from parietal to point above vent, scales in four median longitudinal rows enlarged;ventrals in 58 rows, counted from first postgular to preanal scales; body scales smooth, subcycloid; 28 scales around midbody;six slightly enlarged preanals;tail relatively long,TaL/SVL ratio 0.94, tip rounded; tail gradually tapering to point;median row of subcaudals enlarged; Limbs slender,pentadactyl, and clawed; forelimb and hindlimb meeting when adpressed; Subdigital lamellae under finger IV: 21 (Figure 10G); subdigital lamellae under toe IV: 26 (Figure 10H); all subdigital lamellae enlarged(Figure 10G-H).

Figure 10 Holotype of Lygosoma(Leiolopisma)microcercum Bottger,1901(SMF 14593,male)in preservative
Coloration in preservative:See Figure 10; MDLS whitish,from head to tail base, widening at point above vent and merging into light brownish coloration of tail. MDLS on midbody about one scale wide, covering interior halves of two paravertebral scale rows. Two brownish PVDS from snout to tail base, about two scales wide, continued by row of eight irregular brown blotches on both sides of anterior half of tail.Two fawn DLLS, starting on supraoculars, running down to point above vent, merging with MDLS and light brownish tail coloration. Laterally, on flanks, stripes sharply bordered by blackish brown LDS, starting at nostrils, extending to hind margin of hindlimbs. Ventral margin of LDS less sharp,sometimes frayed. LLS cream, only separated from light cream ventral trunk surface by faint narrow patch of brown spots (VLDS) between axilla and groin. DTM as wide dark band, about two times wider than DLLS above, running from posterior margin of eye to temporal region and further posteriorly on body flanks, joining with LDS (Figure 10E).Labials and surrounding of outer ear opening fawn white with some faint irregular brown pigmentation. Dorsal surface of tail light brown, becoming darker on lateral sides.Anterior third of tail with eight dark brown lateral blotches flanking light brown median tail surface. Dorsal surface of limbs brown, scattered with irregular darker spots. Toes and fingers light fawn with dark brown blotches on joints. Ventral surface of throat, trunk,limbs, and tail fawn, somewhat darker on tail and posterior part of trunk than on throat.
Coloration in life:Description of life coloration is based on an adult male specimen (ZMMU R13698, Yok Don, Dak Lak,Vietnam), see Figure 5E-F. MDLS whitish beige on head and anterior parts of trunk, gradually turning light orange on tail;PVDS and LDS brownish black; DLLS whitish beige with light orange complexion toward tail base; LLS grayish white; VLDS grayish brown; limbs and digits dark brown, scattered with lighter beige and slightly orange spots; digits whitish with dark brown spots on joints; labials bright white with dark gray markings; throat and belly white; tail bright orange, with increasing intensity toward tail tip; lateral surfaces of tail orange,interrupted by partly connected dark brown patches.
Variation:To assess morphological variation, we investigated 23 recently collected specimens from central and southern Vietnam and from Cambodia. Selected traits and measurements are shown in Table 2. We also examined photos of type specimens of Lygosoma vittigerum kronfanum Smith, 1922 (Figure 11) and Leiolopisma pranensis Cochran,1930 (Figure 12). In general, all examined specimens correspond quite well to the description of the holotype,although certain variation was observed in number of supraoculars contacting frontal and number of superciliary scales. Coloration features appeared quite stable within L.microcercus stat. nov. (Figure 13). No statistically significant differences in morphological characters between sexes were observed.
Distribution:Distribution is shown in Figure 1.This species is known with certainty from central and southern Vietnam,Cambodia, and extreme south of Laos. As we tentatively synonymize Leiolopisma pranensis Cochran, 1930 from Tenasserim with L. microcercus stat. nov., its distribution may extend further westward to northern and western Thailand north of the Isthmus of Kra; occurrence of this species in easternmost Myanmar is expected(Figure 1).
It is likely that the records of "L. vittigera" from Tanintharyi Division of Myanmar correspond to this species; it was recorded from the following localities: Tavoy (=Dawei, Dewei)(Annandale, 1905); Yepone, Yebyu Township, Dewei Dist.,Tanintharyi Div., Myanmar (CAS 243723); and Kawthaung Township, Tanintharyi Div., Myanmar (CAS 229605). However,the taxonomic status of these populations needs to be clarified.
Records of the genus Lipinia from Thailand require further specimen examination for correct identification; members of the L. vittigera complex were recorded from all over the country, including: Phetchaburi Prov.: Kaeng Krachan NP;Prachuap Khiri Khan Prov.: Pran (=Pran Buri) (USNM 75591);Kui Buri NP; Uthai Thani Prov.: Huai Kha Khaeng WS; Chiang Mai Prov.: Doi Angka Mt. (USNM 76850); Chiang Mai (FMNH 177050); Doi Suthep-Pui NP, Mae Takhrai NP (P.Pawangkhanant, personal communication); Nakhon Ratchisma Prov.: Mueang Nakhon Ratchisma Dist. (KUH 328481); Khao Yai NP; Nan Prov.: Tambol Auan,Amphoe Pua(FMNH 270715); Loei Prov.: Phu Kradueng NP; Saraburi Prov.: Muak Lek, Chet Sao Noi NP; Sa Kaeo Prov.: Pang Sida NP; Ubon Ratchathani Prov.: Ubon (FMNH 177615) (P.Pawangkhanant, personal communication). Further studies are essential to clarify species identity of the Thai populations of the L.vittigera complex.
Records of Lipinia from Laos are poorly documented but include Xepian NBCA, Champasak Prov. (Teynié et al., 2004).In addition to the localities presented in Figure 1 (see Appendix I for details), existing records from Cambodia (as L.vittigera) include: Pursat Prov.: Veal Veang, Phnom Samkos WS (NCSM 80292); Kratie Prov.: Sambour, Koh Kring Island(MVZ 258369); Kampong Speu Prov.: Phnom Sruoch Dist.(FMNH 261862); Ratanakiri Prov.: Ta Veng Dist. (FMNH 262984); Koh Kong Prov., Thmar Baing Dist. (FMNH 263362);Sihanoukville Prov. (FMNH 270597) (Stuart & Emmet, 2006;Stuart et al., 2006, 2010). Hartmann et al. (2013a) also mentioned a photo record of a Lipinia specimen from northwestern Kulen Prum Tep WS (KPWS, Oddar Meanchey Province, Trapeang, Prasat District: ZFMK-PA SE 30;N14.7467°, E104.8100°, 99 m a.s.l.); based on coloration this specimen can be reliably assigned to L.microcercus.
In addition to the localities verified by genetic and morphological analyses (see Figure 1 and Appendix I for details), L. microcercus stat. nov. was documented as L. v.microcercus from the following localities in Vietnam: Thua Thien-Hue Prov.:A Luoi (AMNH R-154622), Hue environs; Da Nang: Hai Van mountain pass, Bach Ma NP; Quang Nam Prov.: Hien, Nam Giang, Tay Gianh, Phuoc Son, Ngoc Linh NP; Kon Tum Prov.: Ngoc Linh NP, Kon Tum; Gia Lai Prov.:Buon Luoi (FMNH 252169), K Bang: SOn Lang, Chu Se; Dak Lak Prov.: Buon Ma Thuot (MVZ 222200), Yok Don NP; Lam Dong Prov.: Lang Bian, Da Ban, Bao Loc (USNM 90386), Cat Loc; Khanh Hoa Prov.: Hon Ba NP; Binh Phuoc Prov.: Nghia Trung; Dong Nai Prov.: Cat Tien NP, Ma Da; Ba Ria - Vung Tau Prov.: Binh Chau-Phuok Buu NP; Tay Ninh NP: Lo Go-Xa Mat NP; Kien Giang Prov.: Phu Quoc NP(Bobrov & Semenov,2008; Nguyen et al., 2009). The record of "L. vittigera" from Ha Tinh Prov. (Vu Kuang NP) by Semenov (2001) is doubtful;see Bobrov(1992,1995)and Bobrov&Semenov(2008).

Figure 11 Paratype of Lygosoma vittigerum kronfanum Smith,1922(FMNH 196010,field number M.A.Smith 4514,male)in preservative
Natural history:A brief description of the biology of this species in Vietnam was presented by Bobrov & Sememov(2008) and Vassilieva et al. (2016). The species was recorded from diverse types of clear forest habitats, including disturbed areas and rural and suburban landscapes in lowland, hilly, and submontane regions up to 1 200 m a.s.l. (Vassilieva et al.,2016). It is an arboreal species, which can be quite common locally. Specimens were usually observed during the day while the lizards foraged on trunks of large trees or, occasionally, on walls of wooden buildings located within the forest or at forest edges. Occasionally, specimens were observed on the ground, presumably crossing from one tree trunk to another.These lizards are active at temperatures above 20 °C, and usual hide under bark or in small tree hollows on rainy days(Bobrov & Sememov, 2008). Diet includes spiders and various small insects; in Cat Tien NP, a male specimen (ZFMK 88969)was observed catching ants on the walls of a wooden stilt hut on the forest edge at Bau Sau Lake at a height of 5 m above ground. They are occasionally observed in groups of 2-3 on the same tree trunk. Waving movements of their brightly colored reddish or bright-orange tails is a commonly observed display behavior. They are oviparous, laying 2-4 eggs per clutch; hatching is observed during the rainy season. Sexual maturity is reached at the end of the first year, shedding occurs in January(Vassilieva et al.,2016).
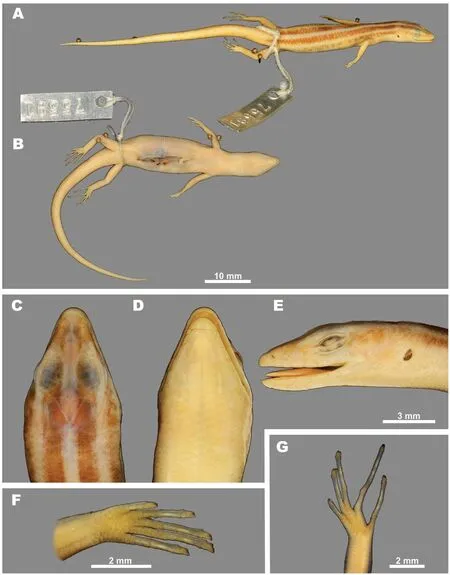
Figure 12 Holotype of Leiolopisma pranensis Cochran,1930(USNM75591,male)in preservative
Comparisons:Comparisons are based on original descriptions or descriptions provided in broader faunal and taxonomic publications (citations in the Materials and Methods section above).

Figure 13 Specimen of Lipinia microcercus stat.nov.(Boettger,1901)(ZMMU NAP-04318,male)in preservative
Morphological comparisons between the four members of the L. vittigera species complex from mainland Southeast Asia are summarized in Table 1. Lipinia microcercus stat. nov. can be differentiated from all other members of the L. vittigera species complex by the following opposing combination of characters (see Table 1 for details): L. vittigera sensu stricto(Boulenger, 1894) from Peninsular Malaysia, Mentawai Islands, Sumatra, and Borneo: 15-16 subdigital lamellae under finger IV; MDLS broad (two scales wide) from snout tip to tail; PVDS not as broad as MDLS; flanks with dark and pale spots (LDS, LLS absent or indistinct); DTM present, indistinct dark mottling or spots; Lipinia trivittata sp. nov. (herein) from southern Vietnam, Cambodia, and eastern Thailand: 20-26 subdigital lamellae under finger IV; MDLS broad (two scales wide); PVDS not as broad as MDLS, not continuing on tail;LDS only present on anterior third of trunk; DTM as narrow black stripe about as wide as DLLS; Lipinia vassilievi sp. nov.(herein) from central Vietnam: 10-11 supraciliaries; 14-15 subdigital lamellae under finger IV; 19-21 subdigital lamellae under toe IV; MDLS broad (two scales wide); PVDS not as broad as MDLS; LDS and VDSL dissolving into two rows of irregular dark blotches; DTM narrow black stripe, about as wide as DLLS.
In the following, L. microcercus stat. nov. is compared with other congeners bearing a light middorsal stripe by listing their opposing character combinations: L. albodorsalis (Vogt) from New Guinea: SVL to 54 mm; 22-25 midbody scale rows;dorsum pale yellow with dark lateral stripe; L. infralineolata(Günther) from Sulawesi and Sangihi Islands: SVL to 49 mm;22 midbody scale rows; L. leptosoma (Brown & Fehlmann)from Palau Islands: 7-10 lamellae under toe IV; L. longiceps(Boulenger) from New Guinea, Fergusson, Misima, and Trobriand Islands: 24 midbody scale rows; snout strongly elongated; dorsum light brown with two dorsolateral stripes converging on tail base; tail golden yellow; L. macrotympanum(Stoliczka) from Andaman and Nicobar Islands: 21-23 midbody scale rows; prefrontals separated; hindlimbs and forelimbs not meeting when adpressed; L. miangensis(Werner) from Pulau Miang, Kalimantan Timur, Borneo: 24 midbody scale rows; back golden with two dark brown longitudinal stripes from snout to tail; L. nitens (Peters) from Sarawak, Borneo: SVL to 33.6 mm; external ear opening replaced by scaly dimple; back metallic green with pale yellow vertebral stripe; L. noctua from South Pacific islands(allochthonous) and Indonesia: Maluku and Papua Province;Papua New Guinea, Admiralty Islands, Bismarck Islands, and Solomon Islands: dorsum brown with light vertebral stripe,starting with wide and contrasting light spot on head; dark paravertebral stripes with irregular borders, often broken into row of irregular dark blotches; L. pulchella from the Philippines: 22-26 midbody scale rows; 24-31 lamellae under toe IV; only one light whitish stripe present on dorsum, from snout to tail tip, becoming yellowish from midbody toward tail;L. pulchra (Boulenger) from New Guinea: dorsum black with five greenish light stripes; L. rabori (Brown & Alcala) from the Philippines (Negros Island): SVL to 51.0 mm in males and 54.8 mm in females; 22 midbody scale rows; L. relicta(Vinciguerra) from Indonesia: Mentawai Archipelago, SVL to 56 mm; 20 midbody scale rows; 16-18 lamellae under toe IV;tail thick; light vertebral line present and labials each bearing small white spot; lateral dark stripe present from eye to insertion of hindlimbs; L. rouxi (Hediger) from New Ireland,northeast Papua New Guinea, and Bismarck Archipelago:dorsum brown, dark paravertebral stripes, with irregular borders, often broken into row of irregular dark blotches; L.septentrionalis Günther from Indonesia (Papua Province): 24-26 midbody scale rows; one cream vertebral stripe,broadening toward tail; L. subvittata (Günther) from Sulawesi,Java, the Philippines (Mindanao Island): SVL 47 - 56 mm;dorsal longitudinal stripes extending to forearm region; 22 midbody scale rows; L. vulcania from the Philippines(Mindanao and Luzon Islands): 17 lamellae under toe IV;limbs not meeting when adpressed; dorsum brownish,scattered with dark brown spots; dark lateral stripe, scattered with whitish or yellowish spots.
The following congeners can be distinguished from L.microcercus by the absence of a light middorsal stripe: L.auriculata (Taylor) from the Philippines (Negros and Masbate Islands); L. cheesmanae (Parker) from New Guinea; L.inexpectata Das & Austin from Borneo and its northern offshore islands; L. nototaenia (Boulenger) from West Papua;L. occidentalis Günther from Papua Province, New Guinea; L.sekayuensis Grismer, Ismail, Awangm, Rizal & Ahmad from Peninsular Malaysia; L. semperi (Peters) from the Philippines(Mindanao and Camiguin Islands); L. surda (Boulenger) from Peninsular Malaysia; L. venemai (Brongersma) from Indonesia: Papua Province; and L. zamboangensis (Brown &Alcala)from the Philippines(Mindanao Island).
Remarks:Lipinia microcercus stat. nov. was ranked as a subspecies of L. vittigera (Boulenger, 1894) by Smith (1935).However, the genetic differentiation presented in this study as well as the morphological differentiation documented herein indicate that L. microcercus has to be treated as a valid species.
Remarks on type locality of Lygosoma (Leiolopisma)microcercumBoettger, 1901: Boettger (1901) noted the type locality as "Phuc-son in Annam". A recent literature review enabled an exact localization of this village. The type specimen belonged to a herpetological collection sent to Frankfurt by the German naturalist Hans Fruhstorfer in November and December 1899. Hans Fruhstorfer travelled through Indochina for about three years (1899-1901), visiting all provinces of the so-called Union Indochinoise (Lamas,2005). Though mainly focused on collecting insects and molluscs, Fruhstorfer also noted in his published travelogue(Fruhstorfer, 1905) several reptiles and amphibians. In the case of the holotype specimen of Lygosoma microcercum, a concurrent reference in Fruhstorfer (1901) can be ascribed,where he mentions a remarkable lizard: "Viel Aufsehen erregte der Fang einer Eidechse mit rothem Schwanz und drei goldenen Linien über dem Rücken, wahrscheinlich Lygosoma sanctum Dum." (p. 130) (translation: "The catch of a lizard caused a certain sensation. It had a red tail and three golden lines on its back, probably Lygosoma sanctum Dum."). This note, the only one which can be assigned to a specimen of the genus Lipinia, belongs to an entry from 24 November 1899 in "Phuc son, Gebiet der Moi" (Fruhstorfer, 1901).Fruhstorfer entered Annam at the harbor of Tourane (today Da Nang, Quang Nam Province) on 13 November 1899 and left for a collection trip (six weeks) into the hinterland of Tourane,to "Phuc son" and "Thu-bon" on 15 November 1899. Though Fruhstorfer's transcription of "Phuc son", which was adopted by Boettger (1901), slightly differs from the modern "Phuoc Son", it can be stated with certain reliability that the type locality of Lygosoma microcercum can be restricted to the modern Phuoc Son District in Quang Nam Province, Central Vietnam(Figure 1,locality 6).
Taxonomic status of Lygosoma vittigerum kronfanum
Smith, 1922: Smith (1922) described the new subspecies Lygosoma vittigerum kronfanum based on six specimens collected at "Daban, Langbian plateau, S. Annam in March 1918". However, all diagnostic characters provided by Smith(1922) are within the character range of Lygosoma microcercum Boettger, 1901:TL 93 mm; SVL 34 mm; midbody scale rows 28-32; limbs overlapping when adpressed;prefrontals in contact, forming broad median suture; five "welldefined light stripes". The description of coloration in preservative also fits the coloration pattern of L. microcercusstat. nov.,as described above: "Black above, with 5 greenishwhite dorsal stripes, namely, a vertebral one from tip of the nose to the root of the tail, a dorsolateral pair from the upper eyelid to above the thigh, and a lateral pair from the upper lip to the groin"(Smith,1922,p.209).
Smith (1935) himself then placed Lygosoma vittigerum kronfanum into synonomy of Leiolopisma vittigerum microcercum (Boettger, 1901), referring to a personal comment of Robert Mertens (unpublished), the successor of Oskar Boettger in SMF in Frankfurt: "Dr. Mertens has compared my kronfanum with the type of microcercum, and has confirmed my suspicion that the two are identical" (Smith,1935, p. 308). At least one paratype of L. vittigerum kronfanum is still present in today's FMNH collection. The handwritten labels indicate that FMNH 196010 (see Figure 11)is one of four paratypes (collection numbers: 2453-2456)present in the private collection of Smith). The fate of the holotype (Smith's private collection number 2417) and other paratypes remains unknown. Das (2010) erroneously listed L.v. kronfanum as a valid subspecies. Based on morphological accordance, we follow Smith (1935) in recognizing L. v.kronfanum as a junior synonym of L. microcercus (Boettger,1901)stat.nov.
Taxonomic status of Leiolopisma pranensisCochran,1930: Cochran (1930) described Leiolopisma pranensis from Siam (now Thailand) based on two specimens, including the holotype USNM 75591 from Pran (now Pran Buri, Prachuap Khiri Khan Prov.) and paratype USNM 76850 from Doi Angka Mt. (Chiang Mai Prov.). Soon after, Smith (1935) synonymized this taxon with Leiolopisma vittigerum vittigerum, although without any justification for this decision. This opinion was later accepted by subsequent researchers (see Taylor, 1963,p.1029).
We examined photos of the holotype (Figure 12) of Leiolopisma pranensis Cochran, 1930, and we doubt that this taxon is close to L. vittigera sensu stricto. In general, external morphology of the holotype USNM 75591 agrees well with L.microcercus stat. nov. (see Table 1 for comparison): SVL 38.0; TaL/SVL ratio 1.26; TrunkL/SVL ratio 0.53; SL/SVL ratio 0.12; STL/SVL ratio 0.24; SFIL/SVL ratio 0.37; FLL/SVL ratio 0.29; HLL/SVL ratio 0.39; frontonasal wider than long;prefrontals in contact (see Figure 7D); frontal contacting 1+2 supraoculars; frontoparietals contacting 2+3+4 supraoculars;supraciliaries 7-8; MSR 30; MDSR 54; ventrals 56. In coloration (Figure 12), the holotype of Leiolopisma pranensis is also similar to L. microcercus stat. nov. with narrow MDLS(one scale wide), PDS slightly wider than MDLS, not continuing on tail (although coloration on tail is possibly faded as the specimen appears slightly bleached); DLLS distinct with regular borders,one scale wide.
However, the holotype of Leiolopisma pranensis shows some differences when compared with the holotype of L.microcercus stat. nov.: 14 subdigital lamellae under finger IV;21 subdigital lamellae under toe IV; fifth supralabial not contacting orbit (Figure 7C); LDS dissolving into row of irregular dark blotches, not reaching groin; VLDS only present as narrow row of irregular small spots. Unfortunately, our phylogenetic analysis lacked samples from western Thailand,including the vicinity of the Leiolopisma pranensis type locality,so the taxonomic value of these differences is not clear and requires further study. Nevertheless, the available information indicates that Leiolopisma pranensis is notably different from
L. vittigera sensu stricto and more closely resembles L.microcercus stat. nov. Further molecular and morphological data on Lipinia populations from western Thailand are needed for future research. We herein propose to place Leiolopisma pranensis Cochran, 1930 into tentative synonomy of L.microcercus(Boettger,1901)stat.nov.
Lipinia trivittata sp.nov.
Figures 6A-D,7G-H,14;Tables 1,2.
Chresonymy
Lygosoma vittigerum kronfanum (partim) — Smith, 1922, p.209(tentative chresonymy;see taxonomic comment below);
Lipinia vittigera (partim) — Mahony, 2008, p. 239(?);Grismer et al.,2011,p.62(?);Vassilieva et al.,2016,p.167.
Holotype:ZMMU R13920-58, adult female (Figure 14),collected by Eduard A. Galoyan from a tree trunk in a pine forest in Chu Yang Sin NP, Dak Lak Province, Vietnam(N12.40611°,E108.35361°;950 m a.s.l.)on 30 March 2013.
Paratypes:ZMMU R13920-55-57, three adult males with same collection information as holotype; ZMMU R13449, adult male collected by Nikolay A. Poyarkov on the edge of a forest road in Dac Ca River valley, Bu Gia Map NP, Binh Phuok Province, Vietnam (N12.1931°, E107.2121°; 545 m a.s.l.) on 16 April 2009; ZMMU R13934-112, adult male, and ZMMU R13934-101, juvenile, collected by Eduard A. Galoyan and Anna B. Vassilieva from Loc Bac forest (operated by Loc Bac Forest Enterprise), Loc Bao Commune, Bao Lam District, Lam Dong Province, Vietnam (N11.73806°, E107.70694°; 850 m a.s.l.) on 7 April 2013; ZFMK 90419, adult female, collected by T. Hartmann from Kulen Prum Tep WS, Preah Vihear Province, Kulen District (N13.8851°, E104.8820°; 70 m a.s.l.)on 10 July 2009.
Diagnosis:Small (SVL up to 44.4 mm) species of Lipinia,differentiated from congeners by the following combination of external morphological traits: external ear opening present;lower eyelid bearing large transparent spectacle; 28-32 midbody scale rows; 48-58 middorsal scales between parietals and point above vent; 20-26 subdigital lamellae under finger IV; 29-33 lamellae under toe IV; prefrontals in broad contact; seven supralabials, seven infralabials;middorsal light stripe from snout tip to tail base; two paravertebral dark stripes, continuing on anterior part of tail;two dorsolateral light stripes, distinct on head and anterior third of trunk; narrow lateral dark stripe from loreals to anterior half of trunk.

Figure 14 Holotype of Lipinia trivittata sp.nov.(ZMMU R13920-58,female)in preservative
Etymology:The species epithet is an adjective in nominative singular (feminine gender) derived from the Latin "tri-" for three and "vitta" for a head band (see above), referring to its prominent three stripes (one light middorsal stripe and two dark paravertebral stripes)in dorsal view.
Description of holotype: Measurements and counts of holotype are presented in detail in Table 2. Adult female, SVL 44.4 mm(see Figure 14),TL 59.3 mm.
Snout acute, SL 4.2 mm; nostrils oriented laterally, oval,situated closer to snout tip than to orbit, END 2.5 mm; head long, almost twice as long as wide, HL 9.2 mm, HW 5.3 mm,HL/HW ratio 1.74, flattened, HH 3.7 mm, HL/HH ratio 2.5;rostral broad, visible in dorsal view, posterior border of rostral waved, contact zone with nasals slightly emarginated;frontonasal wider than long; frontal elongated, arrow-shaped,wider anteriorly; prefrontals large, in broad contact medially(Figure 7H), surrounded by loreals, enlarged presupraocular,frontal, first supraoculars (punctiform contact) and frontonasal;two frontoparietals in broad median contact; interparietal arrow-shaped, wider anteriorly; parietals in contact behind interparietal, surrounded by postsupraoculars, nuchals,interparietal, and frontoparietals; six nuchals; four supraoculars; one enlarged presupraocular, visible from above; nine/eight supraciliaries; nostrils located within nasals;postnasal absent; two loreals, slightly elongated, second longer than first; two enlarged presuboculars, separating supralabial III and IV from orbit; seven supralabials,supralabial V largest, in contact with orbit; three postsuboculars, separating supralabial VI from orbit; six postsupraoculars (pretemporals); one primary temporal; two secondary temporals, dorsal largest; two postsupralabials;lower eyelid bearing large transparent spectacle; scales on upper row of lower eyelid small, 10 in number; mental wider than long; one postmental, in contact with first infralabial and anterior portion of second infralabial; seven infralabials; three pairs of chinshields, first pair in contact medially, second pair separated by one scale, third pair separated by three scales;External ear opening visible and subcircular(Figure 7G).
Body slender (Figure 14A-B), TrunkL 22.4 mm; head slightly distinct from neck and body; 56 middorsal scales from parietal to point above vent, scales in four median longitudinal rows enlarged; ventrals in 66 rows, counted from first postgular to preanal scales; body scales smooth, subcycloid;28 scales around midbody; four slightly enlarged preanals;Tail relatively long, TaL/SVL ratio 1.33, tip rounded; tail gradually tapering to point; median row of subcaudals enlarged; Limbs slender, pentadactyl, and clawed; forelimb and hindlimb meeting when adpressed; Subdigital lamellae under finger IV:24 (Figure 14F); subdigital lamellae under toe IV: 32/33(Figure 14G);all subdigital lamellae enlarged.
Coloration in preservative:MDLS whitish, from head to tail base, widening at point above vent and continuing on dorsal surface of tail. MDLS on midbody about two scale rows wide,covering both paravertebral scale rows. Two black PVDS from snout to tail base, about two scales wide, narrowing on tail base, running into thin brown stripe within anterior fourth of tail. Two fawn DLLS, starting on supraoculars, running down to point above forelimb insertion, merging with brownish flank on anterior part of trunk. LDS starting at nostrils, running out into diffuse stripe on anterior third of trunk. LLS and VLDS absent. DTM as narrow black stripe ca. same width as DLLS above, running from posterior margin of eye to temporal region and further posteriorly on body flanks, joining with LDS(Figure 14E). Labials, gular region, and flanks cream,scattered with brown pigmentation, darker on head, becoming lighter on flanks. Dorsal surface of tail whitish, lateral surface light brown. Dorsal surface of limbs light brown, scattered with irregular darker spots on hands and feet. Toes and fingers light fawn with dark brown blotches on joints. Ventral surface of throat, trunk, limbs, and tail white. Subdigital lamellae and ventral scales on hands and feet dark brown.
Coloration in life:MDLS whitish beige on head and anterior parts of trunk, gradually turning into light orange beige on tail(Figure 6A). DLLS and flanks grayish brown, slightly golden.Limbs and digits light grayish, scattered with dark brown spots. Lateral surfaces of tail irregularly mottled in light brown and light orange beige. DTM as narrow dark brown stripe running from posterior corner of eye posteriorly(Figure 6B).
Variation:For variation of traits in paratypes see Table 2. In general, members of the type series well agree with the description of the holotype. Certain variation was observed in prefrontal positions, which are broadly contacting with each other in most specimens, but slightly touching each other in ZMMU R-13934-101 and ZMMU R-13920-57 and are not in contact in ZMMU R-13934-112. Paratype ZMMU R13449 has MDLS wider than PVDS and brighter orange coloration of tail,becoming light orange toward sacrum (Figure 6C). Paratype ZFMK 90419 shows a brighter coloration of light dorsal stripes, with dorsal tail surface being intensely orange. A specimen from Khao Soi Dao NP, Thailand (Figure 6D, not collected) shows a very similar coloration pattern, with even brighter orange-reddish coloration of tail and MDLS; based on this photo record, we assume that this specimen belongs to Lipinia trivittata sp. nov. We did not reveal any sexually dimorphic characters in this species.
Distribution:Distribution of the new species is presented in Figure 1. Lipinia trivittata sp. nov. is reliably reported from hilly and mountainous areas of southern Vietnam-Langbian Plateau and its foothills (Dak Lak, Lam Dong and Binh Phuok Provinces), as well as from the Bay Nui Hills in southern Vietnam (An Giang Province) and northern Cambodia: Kulen Prum Tep NP (Preah Vihear Province). Sequences of a tissue sample from a non-collected specimen originating from Phu Quoc Island of Vietnam confirm the presence of Lipinia trivittata sp. nov. on the island (Figure 1, locality 24). A photo record of Lipinia trivittata sp. nov. from Khao Soi Dao NP in Chanthaburi Province of eastern Thailand (Figure 6D)indicates that the range of the species is likely wider than currently known and possibly extends to the Cambodian part of the Cardamoms. Further studies are required to clarify the distribution of the new species.
Natural history:Forest arboreal species, occurring throughout primary and old secondary montane and hill dipterocarp forests to disturbed forests, bamboo groves, and pine montane forests, known from elevations of 300 to 1 100 m a.s.l. In the type locality (Chu Yang Sin NP, Dak Lak Province, Vietnam), the new species was observed in montane pine forests dominated by Pinus kesiya Royle ex Gordon; and observed in the day climbing on logs or trunks of large trees while foraging for small insects (mostly ants). In Bu Gia Map NP, Binh Phuok Province, a specimen of the new species was collected on the ground while it was crossing a road in a bamboo grove at midday. In Cambodia, the female paratype was collected from the southeastern part of KPWS while it was actively foraging on a fallen tree trunk at midday in a disturbed semi-evergreen forest at 70 m a.s.l.; this locality is located ~60 km from the L. microcercus record in KPWS.Gravid females were not recorded. Juveniles were observed in May-June.Clutch size is unknown.
Comparisons:Comparisons are based on the original descriptions or descriptions provided in broader faunal and taxonomic publications (citations in the Materials and Methods section above). For distribution notes of each of the compared congeners, see the comparison section within the L.microcercus account above as well as Figure 1.
Morphological comparisons between the four members of the L. vittigera species complex from mainland Southeast Asia are summarized in Table 1. In general, Lipinia trivittata sp.nov. resembles the other members of the Southeast Asian L.vittigera complex. However, the new species can be differentiated based on the following opposing combination of characters (see Table 1 for details): L. vittigera sensu stricto(Boulenger, 1894): 15-16 subdigital lamellae under finger IV;flanks with dark and pale spots; LDS absent or indistinct;Lipinia vassilievi sp. nov. (herein): 10-11 supraciliaries; 14-15 subdigital lamellae under finger IV; 19-21 subdigital lamellae under toe IV; LDS and VDSL dissolving into two rows of irregular dark blotches; L. microcercus (Boettger, 1901) stat.nov.: MDLS narrow (one scale wide at midbody), PVDS continuing on tail; distinct LDS present from temporal region to anterior parts of tail.
Lipinia trivittata sp. nov. can be compared with other congeners bearing a middorsal light stipe by their opposing character combinations:L.albodorsalis:SVL up to 54 mm;22-25 midbody scale rows; dorsum pale yellow with a dark lateral stripe; L. infralineolata (Günther): SVL to 49 mm; 22 midbody scale rows; L. leptosoma: 7-10 lamellae under toe IV; L.longiceps: 24 midbody scale rows; snout elongated; back light brown with two dorsolateral stripes converging on tail base;tail golden yellow; L. macrotympanum: 21-23 midbody scale rows; prefrontals separated; hindlimbs and forelimbs not meeting when adpressed; L. miangensis: 24 midbody scale rows; back golden with two dark brown longitudinal stripes from snout to tail; dark line from eye to insertion of forelimb; L.nitens: SVL to 33.6 mm; external ear opening replaced by scaly dimple; back metallic green with pale yellow vertebral stripe; L. noctua: dorsum brown with light vertebral stripe,starting with wide and contrasting light spot on head; dark paravertebral stripes with irregular borders, often broken into row of irregular dark blotches; L. pulchella: 22-26 midbody scale rows; and 24-31 lamellae under toe IV; only one light whitish stripe present on dorsum, from snout to tail tip,becoming yellowish from midbody toward tail; L. pulchra:dorsum black with five greenish light stripes; L. rabori: SVL to 51.0 mm in males and 54.8 mm in females; 22 midbody scale rows; L. relicta: SVL to 56 mm; 20 midbody scale rows; 16-18 lamellae under toe IV; tail thick; light vertebral line present and labials each bearing small white spot; lateral dark stripe present from eye to insertion of hindlimbs; L. rouxi: dorsum brown, dark paravertebral stripes, with irregular borders, often broken into row of irregular dark blotches; L. septentrionalis:24-26 midbody scale rows; light middorsal stripe as well as brown paravertebral stripes each bearing narrow black contour; L. subvittata: SVL 47-56 mm; dorsal longitudinal stripes extending to forearm region; 22 midbody scale rows; L.vulcania: 17 lamellae under toe IV; limbs not meeting when adpressed; dorsum brownish, scattered with dark brown spots; dark lateral stripe, scattered with whitish or yellowish spots.
Remaining congeners can be distinguished from Lipinia trivittata sp. nov. by absence of light middorsal stripe: L.auriculata; L. cheesmanae; L. inexpectata; L. nototaenia; L.occidentalis; L. sekayuensis; L. semperi; L. surda; L. venemai;and L.zamboangensis.
Remarks:In his description of Lygosoma vittigerum kronfanum, Smith (1922) reported on geographic variation in number of stripes on dorsum in Indochinese Lipinia: in addition to the typical form (L. vittigera sensu stricto from Sumatra and Malayan Peninsula) with one distinct vertebral stripe on dorsum and Indochinese form (now treated as L.microcercus stat. nov.) with five stripes on dorsum, he reported a juvenile specimen from northern Siam (now Thailand) with three dorsal light stripes and stated that further sampling may show this three-striped form to be taxonomically distinct. We were unable to locate and examine this specimen;however, we assume that it may possibly represent the first discovery of Lipinia trivittata sp. nov. Some records of L.vittigera from Cambodia (Grismer et al., 2011:62; Mahony,2008, p. 239) and southern Vietnam (Vassilieva et al., 2016, p.167) may also correspond to this species, though this requires further confirmation.
Lipinia vassilievi sp.nov.
Figure 6E-F;Figure 7I-J;Figure 15;Tables 1,2.
Holotype:ZMMU R14604, adult male (Figure 15), collected by Dimitry F. Fedorenko and Anna B. Vassilieva in a montane forest in Chu Mom Ray NP, Kon Tum Province, Vietnam(N14.49583°,E107.71947°;810 m a.s.l.)on 30 March 2015.
Diagnosis:Small (SVL to 39.4 mm) species of Lipinia,differentiated from congeners by the following combination of external traits: external ear opening present; lower eyelid bearing large transparent spectacle; 30 midbody scale rows;56 middorsal scales between parietals and point above vent;15/14 subdigital lamellae under finger IV; 21/19 lamellae under toe IV; prefrontals in broad contact; seven supralabials,seven infralabials; broad middorsal light stripe from snout tip to tail base; two paravertebral dark stripes from supraoculars toward tail, continuing on anterior part of tail; two distinct dorsolateral light stripes; lateral dark stripe from temporals to groin dissolving into row of irregular black spots; one lateral light stripe, separated from belly by row of large black spots between ear opening and groin.

Figure 15 Holotype of Lipinia vassilievi sp.nov.(ZMMU R14604,male)in preservative
Etymology:The name of the new species is a Latinized patronymic adjective in genitive plural, possessive form of the family name Vassiliev. This species is named in honor of Prof.Boris D. Vassiliev, a professor of herpetology from the Department of Vertebrate Zoology of Lomonosov Moscow State University for the last 50 years; he has nurtured and educated several generations of Russian herpetologists,including three co-authors of the present paper. In the last 30 years, he has participated in several expeditions to central and southern Vietnam.
Description of holotype:Adult male, SVL 39.4 mm (Figure 15A-B), TaL 44.5 mm; Snout acute, SL 4.4 mm; nostrils oriented laterally, oval, situated closer to snout tip than to orbit, END 3.1 mm; head elongated, HL 9.1 mm, HW 6.1 mm,HL/HW ratio 1.5, slightly flattened, HH 4.3 mm, HL/HH ratio 2.1; rostral broad, visible in dorsal view, posterior border almost straight, contact zone with nasals slightly emarginated,frontonasal wider than long; frontal elongated, arrow-shaped,wider anteriorly; prefrontals large, in contact medially (Figure 7J), laterally and posteriorly in contact with loreals, enlarged presupraocular, first supraocular, frontal, and frontonasal; two frontoparietals in broad median contact; interparietal arrowshaped, wider anteriorly; parietals in contact behind interparietal, anteriorly in contact with postsupraoculars, fourth supraocular, nuchals, and frontoparietals; seven nuchals; four supraoculars; one enlarged presupraocular, visible from above; 10/11 supraciliaries; nostrils located within nasals;postnasal absent; two loreals, slightly elongated, second longer than first; two enlarged presuboculars, separating supralabials III and IV from eye; seven supralabials,supralabial V largest, in contact with orbit; two postsuboculars,separating supralabial VI from orbit; six postsupraoculars(pretemporals); one primary temporal; two secondary temporals, dorsal largest; two postsupralabials; lower eyelid bearing large transparent spectacle; scales on upper row of lower eyelid small, 12 in number; mental wider than long; one postmental, in contact with first infralabial and anterior portion of second infralabial; seven infralabials; three pairs of chinshields, first pair in contact medially, second pair separated by one scale, third pair separated by three scales;external ear opening visible and subcircular(Figure 7I).
Body slender (Figure 15A-B), TrunkL 18.7 mm; head slightly distinct from neck and body; 56 middorsal scales from parietal to point above vent, scales in four median longitudinal rows enlarged; ventrals in 66 rows, counted from first postgular to preanal scales; body scales smooth, subcycloid;28 scales around midbody; four slightly enlarged preanals;Tail relatively long, TaL/SVL ratio 1.0, tip rounded; tail gradually tapering to point; median row of subcaudals enlarged; Limbs slender, pentadactyl, and clawed; forelimb and hindlimb meeting when adpressed; subdigital lamellae under finger IV:15/14 (Figure 15F); subdigital lamellae under toe IV: 21/19(Figure 15G);all subdigital lamellae enlarged.
Coloration in preservative:MDLS whitish, from head to tail base, widening at point above vent and merging into light dorsal coloration of tail (Figure 15B). MDLS on midbody about two scales wide. Two black PVDS from supraoculars to tail base, about one scale wide at midbody, merging into brown lateral tail coloration. Outer margin of PVDS frayed. Two grayish brown DLLS, from supraoculars to tail base, margins frayed. Two LDS, dissolving into series of irregular black blotches and markings, starting on loreals, running down to groin. LLS grayish white, separated from light cream ventral trunk surface by row of smaller irregular black spots (VLDS)between gular region and groin. DTM as narrow black stripe,about same width as DLLS above, running from posterior margin of eye to temporal region and terminating on neck sides (Figure 15E). Labials and surrounding of outer ear opening fawn white. Dorsal surface of tail cream, becoming darker on lateral sides (Figure 15A). Dorsal surface of limbs grayish brown scattered with irregular light and dark spots.Toes and fingers light fawn with dark brown blotches on joints.Ventral surface of throat,trunk,limbs,and tail whitish.
Coloration in life:MDLS whitish beige on head and neck,gradually turning fawn orange over trunk toward tail (Figure 6E); PVDS, LDS, and VLDS spots brownish black; DLLS light brown; LLS grayish white; limbs and digits dark brown,scattered with lighter beige and dark brown spots; digits fawn with dark brown spots on joints; labials fawn white (Figure 6F);throat and belly white; tail fawn orange, with increasing intensity toward tail tip;lateral surfaces of tail orange brown.
Distribution:Figure 1 shows the known distribution of the new species. The type locality of Lipinia vassilievi sp. nov. is located in Chu Mom Ray NP in Kon Tum Province of Vietnam,a southern outcrop of the Central (or Kon Tum) Plateau of the central Annamites (=Truong Son mountains). Sequences of tissue samples from two non-collected specimens (tail tips)originating from Virachey NP, Ratanakiri Province in eastern Cambodia, confirm the presence of Lipinia vassilievi sp. nov.in the country (Figure 1, locality 19); the Cambodian locality is situated about 50 km SW from the type locality. It is likely that the new species has a wider distribution extending to other areas of the Central Plateau foothills in central Vietnam,northern Cambodia, and, possibly, southern Laos(Champasak and Attapeu Provinces). Further research is needed to clarify the distribution range of Lipinia vassilievisp. nov.
Natural history:The holotype was collected under bark of a large tree close to a stream in a mixed montane polydominant forest at an elevation of 810 m a.s.l. Specimens from Virachey NP in Ratanakiri Province of Cambodia were recorded on the ground crossing a road.
Comparisons:The only specimen of Lipinia vassilievi sp.nov. we were able to examine morphologically was the holotype; the two other samples of this species from Ratanakiri Province of Cambodia included in the phylogenetic analyses were tissue samples (tail tips), the respective voucher specimens were not collected. Comparisons are based on original descriptions or descriptions provided in broader faunal and taxonomic publications (citations in the Materials and Methods section above). For distribution notes of each of the compared congeners, see the comparison section within the L. microcercus stat. nov. account above as well as Figure 1.
Morphological comparisons between the four members of the L. vittigera species complex from mainland Southeast Asia are summarized in Table 1. The members can be differentiated by the following opposing combination of traits:L. vittigera sensu stricto (Boulenger, 1894): PVDS with straight margins on both sides; flanks with dark and pale spots(LDS, LLS absent or indistinct); Lipinia trivittata sp. nov.: 20-26 subdigital lamellae under finger IV; PVDS with straight margins on both sides, not continuing on tail; LDS only present on anterior third of trunk; L. microcercus (Boettger,1901) stat. nov.: MDLS narrow (one scale wide at midbody),PVDS with straight margins on both sides, continuing on tail;LDS continuous, present from temporal region to anterior parts of tail.
Further, those congeners bearing a middorsal light stripe can be distinguished from Lipinia vassilievi sp. nov. by the following opposing traits: L. albodorsalis: SVL to 54 mm; 22-25 midbody scale rows; dorsum pale yellow with dark lateral stripe; L. infralineolata: SVL to 49 mm; 22 midbody scale rows; L. leptosoma: 7-10 lamellae under toe IV; L. longiceps:24 midbody scale rows; snout strongly elongated; dorsum light brown with two dorsolateral stripes converging on tail base; tail golden yellow; L. macrotympanum: 21-23 midbody scale rows; prefrontals separated; hindlimbs and forelimbs not meeting when adpressed; L. miangensis: 24 midbody scale rows; back golden with two dark brown longitudinal stripes from snout to tail; L. nitens: SVL to 33.6 mm; external ear opening replaced by scaly dimple; back metallic green with pale yellow vertebral stripe; L. noctua: dorsum brown with light vertebral stripe, starting with wide and contrasting light spot on head; dark paravertebral stripes with irregular borders,often broken into row of irregular dark blotches; L. pulchella:22-26 midbody scale rows; 24-31 lamellae under toe IV; only one light whitish stripe present on dorsum, from snout to tail tip, becoming yellowish from midbody toward tail; L. pulchra:dorsum black with five greenish light stripes; L. rabori: SVL to 51.0 mm in males and 54.8 mm in females; 22 midbody scale rows; L. relicta: SVL to 56 mm; 20 midbody scale rows; tail thick; light vertebral line present and labials each bearing small white spot; lateral dark stripe present from eye to insertion of hindlimbs; L. rouxi: dorsum brown, dark paravertebral stripes, with irregular borders, often broken into row of irregular dark blotches; L. septentrionalis: 24-26 midbody scale rows; one cream vertebral stripe, broadening toward tail; L. subvittata: SVL to 56 mm; dorsal longitudinal stripes extending to forearm region; 22 midbody scale rows; L.vulcania from the Philippines (Mindanao and Luzon Islands):17 lamellae under toe IV; limbs not meeting when adpressed;dorsum brownish, scattered with dark brown spots; dark lateral stripe,scattered with whitish or yellowish spots.
The remaining congeners can be distinguished from Lipinia vassilievi sp. nov. by absence of light middorsal stripe: L.auriculata; L. cheesmanae; L. inexpectata; L. miotis; L.nototaenia; L. occidentalis; L. sekayuensis; L. semperi; L.surda;L.venemai;and L.zamboangensis.
DISCUSSION
Phylogenetic studies of the genus Lipinia have been long neglected; an unpublished PhD thesis of Linkem (2013)indicates that the genus represents a non-monophyletic assemblage of several distantly related lineages of sphenomorphine skinks. Grismer et al. (2016) approached the phylogenetic relationships of Lipinia skinks by analyzing 1 035 bp of ND2 mtDNA gene. Among other samples, they included three samples of "L. vittigera" in their analysis. Their study strongly suggested monophyly of the L. vittigera species complex and deep genetic divergence between the specimens examined, indicating that its taxonomy is likely incomplete. In the phylogeny of Grismer et al. (2016), the specimen of L. vittigera from Pinang (Malaysia) was more closely related to a specimen from Pursat (Cambodia) than to a specimen from Nan (Thailand). Thus, taxonomic affinities of these specimens require clarification.
In the present paper, we applied COI DNA-barcoding to assess the taxonomic diversity of members of the L. vittigera species complex from Indochina. The resulting phylogenetic tree is not resolved in a number of major basal nodes (Figure 3) and cannot be used to discuss phylogenetic relationships within Lipinia. However, our data clearly support the presence of at least four distinct and highly divergent mtDNA lineages within Lipinia in mainland Southeast Asia. Our study demonstrates that these lineages are also different in external morphology and coloration pattern and thus warrant recognition as separate species, two of which are new to science. It is remarkable that the presence of one of these new species was predicted almost 100 years ago (Smith,1922).
Our study also raises many questions on the taxonomy and distribution of the L. vittigera species complex members in mainland Southeast Asia. Molecular and morphological differentiation of L. vittigera sensu stricto from Mentawai,Sumatra, Malaysia, southern Thailand, and Borneo need to be examined in greater detail; our preliminary data and previously published observations (Grismer, 2011b) suggest the presence of at least two morphologically distinct lineages within L. vittigera sensu stricto. Morphological examination of the type series of Leiolopisma pranensis Cochran, 1930 suggests that this taxon is more closely related to L.microcercus stat. nov. rather than to L. vittigera sensu stricto,as suggested earlier (Smith, 1935; Taylor, 1963). However,samples from western and northern Thailand were lacking in our analysis. The phylogenetic position and taxonomic status of Lipinia populations from mainland Thailand and Myanmar require further molecular and morphological studies. We assume that the geographic border between the ranges of L.vittigera sensu stricto and other members of the species complex in Indochina probably coincides with the Isthmus of Kra; however, this assumption needs to be verified by further studies. Lipinia microcercus stat. nov. is assumed to be the most widespread Lipinia species in Indochina. However, while its range in Vietnam and Cambodia is well documented, its distribution in Thailand and Laos requires further study.
Our preliminary research revealed the presence of three distinct mtDNA lineages within Lipinia trivittata sp. nov.,corresponding to populations from Cambodia, Phu Quoc, and mainland Vietnam (Figure 3). Though the ranges of the three species in the L. vittigera species complex recorded in Indochina are largely overlapping, none have yet been found in sympatry. In KPWS in Cambodia, L. microcercus stat. nov.and Lipinia trivittata sp. nov. were recorded in different sectors of the WS, ca. 60 km from each other. In the Langbian Plateau, records of L. microcercus stat. nov. (from Bao Loc)and Lipinia trivittata sp. nov. (from Loc Bao) are separated by a distance of ca. 45 km. Future research is needed to clarify the actual distribution patterns and habitat preferences of Indochinese Lipinia species as well as possible sympatry.
Our study raises the total number of Lipinia species known for mainland Southeast Asia to six. This underscores that many widely distributed lizard species in Indochina actually represent species complexes (Grismer et al., 2012, 2018;Murdoch et al., 2019 and references therein). Our work provides further evidence that the herpetofaunal diversity of Indochina is currently underestimated. As deforestation and habitat destruction are progressing at an increasing rate in Southeast Asia (Meyfroidt & Lambin, 2008), further herpetological surveys and taxonomic expertise are urgently needed to catalog its biodiversity before elimination.
Key to species of Lipinia Gray, 1845 from mainland Southeast Asia
The following preliminary key can be used as a guide for the identification of Lipinia species occurring in mainland Southeast Asia and its offshore islands.
1a) Dorsum uniformly brown, outer ear opening covered with scales ……………………………………………………… 2
1b) Dorsum bearing light middorsal stripe, flanked by two dark paravertebral stripes ………………………………… 3
2a) 56 paravertebral scale rows; 65 ventral scale rows; 10 subdigital lamellae under finger IV; 15 subdigital lamellae under toe IV;subcaudals slightly enlarged ………………………………… L.sekayuensis from Peninsular Malaysia
2b) 64-65 paravertebral scale rows; 75 ventral scale rows;13-14 subdigital lamellae under finger IV; 17-20 subdigital lamellae under toe IV; subcaudals not enlarged……… L.surda from Peninsular Malaysia and islands of Seribuat Archipelago
3a) Lateral dark stripe on trunk continuous line or row of larger dark sports…………………………………………… 4
3b) Lateral dark stripe on trunk absent; middorsal light stripe broad (two scales wide at midbody); 15-16 subdigital lamellae under finger IV; no distinct dark or light stripes present on trunk…………………………………………………… L.vittigera sensu stricto from Peninsular Malaysia,Southern Thailand (south of Isthmus of Kra), Mentawai Islands,Sumatra,and Borneo
4a) Width of paravertebral dark stripes subequal to middorsal light stripe…………………………………………………… 5
4b) Paravertebral dark stripes broader than middorsal light stripe ………………………………………………………………… L.microcercus stat.nov.from southern Vietnam,Cambodia,and Thailand
5a) Lateral dark stripe and ventrolateral dark stripe dissolving into series of distinct dark spots, present between axilla and groin;19-21 subdigital lamellae under toe IV ………………… Lipinia vassilievi sp.nov.from central Vietnam.
5b) Lateral dark stripe on trunk not reaching groin, distinct on anterior third of trunk, dissolving into faint, indistinct spots on second third of trunk; 29-33 subdigital lamellae under toe IV ………………………………………………………………… Lipinia trivittata sp.nov.from southern Vietnam,Cambodia and eastern Thailand
NOMENCLATURAL ACTS REGISTRATION
The electronic version of this article in portable document format represents a published work according to the International Commission on Zoological Nomenclature (ICZN),and hence the new names contained in the electronic version are effectively published under that Code from the electronic edition alone (see Articles 8.5-8.6 of the Code). This published work and the nomenclatural acts it contains have been registered in ZooBank, the online registration system for the ICZN. The ZooBank LSIDs (Life Science Identifiers) can be resolved and the associated information can be viewed through any standard web browser by appending the LSID to the prefixhttp://zoobank.org/.
Publication LSID:
urn: lsid: zoobank. org: pub: 912E2BBD-C51D-4B07-88CFFAD604E38DD3.
Lipinia trivittata LSID:
urn: lsid: zoobank. org: act: 85F0D2A2-1794-4AA5-98F2-14EEC8DDEE9D.
Lipinia vassilievi LSID:
urn: lsid: zoobank. org: act: C57629D5-D4C5-4FF9-BD0AD4006499F1B5.
SCIENTIFIC FIELD SURVEY PERMISSION INFORMATION
Fieldwork in Vietnam was funded by the Joint Russian-Vietnamese Tropical and Technological Center (JRVTTC) and was conducted under permission of the Department of Forestry, Ministry of Agriculture and Rural Development of Vietnam (permit numbers 170/TCLN-BTTN, issued 7 February 2013; 831/TCLN-BTTN, issued 5 July 2013; 400/TCLN-BTTN, issued 26 March 2014; 547/TCLN-BTTN, issued 21 April 2016, 432/TCLN-BTTN, issued 30 March 2017).Forest Protection Departments of the Peoples' Committee of Local Administration provided permits for fieldwork and sample collection (Gia Lai Province: permit numbers 1951/UBND-NV, issued 4 May 2016, and 142/SNgV-VP, issued 11 April 2017; Lam Dong Prov.: No. 5832/UBND-LN, issued 22 October 2012). In Cat Tien NP, fieldwork was conducted in accordance with Agreement No. 37/HD on the scientific cooperation between Cat Tien NP and JRVTTC; in Bu Gia Map NP, fieldwork was conducted in accordance with Agreement No. 137/HD NCKH of 23 June 2010 on the scientific cooperation between Bu Gia Map NP and JRVTTC.In Thailand, specimens collection protocols were approved by the Institutional Ethical Committee of Animal Experimentation of the University of Phayao (certificate number 610104022);specimens were collected under approval from the Institute of Animal for Scientific Purposes Development (IAD), which issued fieldwork permission (No. U1-01205-2558) in Thailand and export permits. In Kulen Prum Tep WS and Phom Kulen NP, fieldwork was conducted in accordance with GDANCP/MoE no:277.
SUPPLEMENTARY DATA
Supplementary data to this article can be found online.
COMPETING INTERESTS
The authors declare that they have no competing interests.
AUTHORS'CONTRIBUTIONS
N.A.P. and P.G. designed the study; N.A.P., P.G., T.H., C.S., and E.A.D.collected materials for study; N.A.P., P.G., V.A.G., T.H., C.S., and E.A.D.discussed the results; P.G. and N.A.P. prepared the manuscript; N.A.P., P.G., and E.A.D. provided photographs and figures; P.G., V.A.G., N.A.P., and E.A.D. performed morphological analyses; V.A.G. and N.A.P. performed molecular and phylogenetic analyses; N.A.P., T.H., C.S., and P.G. provided funding and permits and revised the manuscript. All authors read and approved the final manuscript.
ACKNOWLEDGEMENTS
We are very grateful to Valentina Orlova (Zoological Museum, Moscow State University) and Gunther Köhler (Senckenberg Museum Frankfurt) for providing working facilities and permission to examine specimens under her care. We are indebted to Anna B. Vassilieva (Moscow/Ho Chi Minh City)and Eduard A. Galoyan (Moscow) for help in the field and permanent support. N.A.P. thanks Andrei N. Kuznetsov and Leonid P. Korzoun for support and organization of their work in Vietnam. We are indebted to Neang Thy for field-work support in Cambodia. P.G. is grateful to Wolfgang Böhme, Thomas Ziegler, and Nguyen Quang Truong, who provided initiation for the taxonomic study of scincid lizards in Southeast Asia.Wolfgang Böhme also contributed important comments on zoological nomenclature and etymology of Lipinia microcercus. We are extremely grateful to Parinya Pawangkhanant, Justin Lee and Olivier Pauwels for useful comments that helped improve the earlier version of this manuscript and to Giuliano Doria, Eduard A. Galoyan, Rachel Grill,André Koch, James Poindexter, David Sargeant/North Thailand Birding, and Vitaly L. Trounov for providing their photos for this publication.
APPENDIX I
Material examined morphologically.
Lipinia vittigera sensu stricto:
(1) MSNG 55855, Indonesia, West Sumatra, Mentawai Isl., male (holotype of Lygosoma vittigerum Boulenger, 1894); (2) ZFMK 48542,Malaysia,Penang,Penang Isl.,male;(3)ZMMU R14477,Thailand,Surat Thani,Phanom Dist.,male.
Lipinia microcercus stat.nov.:
(4) USNM 75591, Thailand, Prachuap Khiri Khan, Pran, male (holotype of Leiolopisma pranensis Cochran, 1930); (5) USNM 76850, Thailand, Chiang Mai, Doi Angka, male (paratype of Leiolopisma pranensis Cochran, 1930); (6) SMF 14593, Vietnam,Quang Nam, Phuoc Son, male (holotype of Lygosoma microcercum Boettger, 1901); (7) FMNH 196010, "peninsular Siam", male(paratype of Lygosoma vittigerum kronfanum Smith, 1922; field number M.A. Smith 4514); (8) ZFMK 90339, Cambodia, Siem Reap, Phnom Kulen NP, juvenile; (9) ZMMU R10962, Cambodia, Kampong Speu, Kirirom NP, male; (10) ZFMK 88968, Vietnam,Dong Nai, Cat Tien NP, male; (11) ZFMK 88869, Vietnam, Dong Nai, Cat Tien NP, female; (12) ZMMU R11475-1, Vietnam, Dong Nai, Cat Tien NP, female; (13) ZMMU R11475-2, Vietnam, Dong Nai, Cat Tien NP, juvenile; (14) ZMMU R11475-3, Vietnam, Dong Nai, Cat Tien NP, juvenile; (15) ZMMU R13599-9, Vietnam, Dong Nai, Cat Tien NP, male; (16) ZMMU R13599-74, Vietnam, Dong Nai,Cat Tien NP,male;(17)ZMMU R7528,Vietnam,Dong Nai,Ma Da(Vinh Cuu),male;(18)ZMMU R13337,Vietnam,Dong Nai,Cat Tien NP, male; (19) ZMMU R13843, Vietnam, Dong Nai, Ma Da (Vinh Cuu), female; (20) ZMMU R8319, Vietnam, Lam Dong,Bao Loc, female; (21) ZMMU R11184, Vietnam, Lam Dong, Cat Loc NR, female; (22) ZMMU R11165, Vietnam, Tay Ninh, Lo Go-Xa Mat NP, juvenile; (23) ZMMU R11178, Vietnam, Tay Ninh, Lo Go-Xa Mat NP, juvenile; (24) ZMMU R13698, Vietnam, Dak Lak,Yok Don NP, female; (25) ZMMU NAP-04318, Vietnam, Dak Lak, Yok Don NP, female; (26) ZMMU R4613-1, Vietnam, Gia Lai,Buon Luoi, female; (27) ZMMU R4613-2, Vietnam, Gia Lai, Buon Luoi, male; (28) ZMMU R4613-3, Vietnam, Gia Lai, Buon Luoi,male;(29)ZMMU R4613-4,Vietnam,Gia Lai,Buon Luoi,male.
Lipinia trivittata sp.nov.:
(30) ZMMU R13449, Vietnam, Binh Phuok, Bu Gia Map NP, male (paratype); (31) ZMMU R13934-112, Vietnam, Lam Dong, Loc Bao, male; (32) ZMMU R13934-101, Vietnam, Lam Dong, Loc Bao, juvenile; (33) ZMMU R13920-56, Vietnam, Dak Lak, Chu Yang Sin NP,male(paratype);(34)ZMMU R13920-58,Vietnam,Dak Lak,Chu Yang Sin NP,female,(holotype);(35)ZMMU R13920-57,Vietnam, Dak Lak, Chu Yang Sin NP, male (paratype); (36) ZMMU R13920-55, Vietnam, Dak Lak, Chu Yang Sin NP, male(paratype); (37) ZMMU R15199, Vietnam,An Giang, Nui Cam, male; (38) ZFMK 90419, Cambodia, Preah Vihear, Kulen Prum Tep NP,male.
Lipinia vassilievi sp.nov.:
(39)ZMMU R14604,Vietnam,Kon Tum,Chu Mom Ray NP,male(holotype).
- Zoological Research的其它文章
- Progress in in vitro culture and gene editing of porcine spermatogonial stem cells
- Synchronization between frontal eye field and area V4 during free-gaze visual search
- A soluble FcγR homolog inhibits IgM antibody production in ayu spleen cells
- Immunodetection of ephrin receptors in the regenerating tail of the lizard Podarcis muralis suggests stimulation of differentiation and muscle segmentation
- Genetic diversity and temporal changes of an endemic cyprinid fish species,Ancherythroculter nigrocauda,from the upper reaches of Yangtze River
- Non-invasive genetic analysis indicates low population connectivity in vulnerable Chinese gorals:concerns for segregated population management

