Diabetic cardiomyopathy:Pathophysiology,theories and evidence to date
Lavanya Athithan,Gaurav S Gulsin,Gerald P McCann,Eylem Levelt
Lavanya Athithan,Gaurav S Gulsin,Gerald P McCann,Department of Cardiovascular Sciences,University of Leicester and NIHR Leicester Cardiovascular Biomedical Research Centre,Glenfield Hospital,Leicester LE3 9QP,United Kingdom
Eylem Levelt,Multidisciplinary Cardiovascular Research Centre and Biomedical Imaging Science Department,Leeds Institute of Cardiovascular and Metabolic Medicine,University of Leeds,Leeds LF9 7TF,United Kingdom
Abstract
Key words: Diabetic cardiomyopathy; Cardiac metabolism; Myocardial steatosis;Myocardial strain
INTRODUCTION
Type 2 diabetes (T2D) is now a global pandemic.The disease is characterised by insulin resistance,relative impairment of insulin secretion and increased hepatic glucose output resulting in high blood glucose levels.It is now among the top 10 causes of death and represents a major cause of mortality and morbidity in the world[1].Globally there were 422 million patients with diabetes in 2014,with growing prevalence from 4.7% in 1980 to 8.5% in 2014[2].Heart failure (HF) has emerged as the most common initial cardiovascular complication of diabetes[2,3].T2D is likely to contribute to the development of HF through a variety of mechanisms,including disease specific myocardial structural,functional and metabolic changes.In the 2015-16 England and Wales National Diabetes Audit,there were 115695 emergency admissions for patients with diabetes and HF compared to 21399 with myocardial infarction and 29392 with stroke[4].Once HF diagnosis is established in T2D patients over the age of 65,mortality risk increases tenfold,and five-year survival reduces to 12.5%[5].
The diabetic population has been shown to pose a marked preponderance to developing HF following a myocardial infarction[6-8].In addition to this,systolic and diastolic left ventricular dysfunction has also been described unrelated to the presence of macrovascular coronary disease[9-11].The prevalence of HF in the general population has been estimated to be 11.8%[12],whereas in clinical trials of cardiovascular outcomes in T2D patients,the prevalence of HF at baseline has varied between approximately 10% and 30% encompassing both HF with reduced ejection fraction (HFrEF) and HF with preserved ejection fraction (HFpEF) with a doubling of the risk of developing HF in those aged 75-84[13-23].
Diabetic cardiomyopathy is defined as cardiac dysfunction encompassing structural,functional and metabolic changes in the absence of coronary artery disease(CAD)[24-26].This distinct clinical entity was first proposed by Lundbaek[27]in 1954 as diabetic heart disease independent of hypertension and CAD that commonly co-exist with T2D.Rubleret al[9]in 1972 went on to confirm these observations,describing diabetic-related post-mortem findings in four patients with T2D,glomerulosclerosis and HFrEF with normal epicardial coronary arteries the absence of hypertension,CAD,valvular or congenital heart disease.A large United States nationwide casecontrol study by Bertoniet al[28]in 1995 further confirmed an association between nonischaemic idiopathic cardiomyopathy and diabetes.Another recent large population study showed that despite good control of all cardiovascular risk factors,the risk of HF hospitalisation in T2D was still consistently higher although there was little or no increase in risk of mortality,myocardial infarction,or stroke in comparison to the general population[29].
Experimental studies have shown that functional and structural alterations within the diabetic heart may be caused by a significant difference in underlying metabolic mechanisms and substrate utilisation.These have been supported by multiple hypotheses on how T2D disease processes affect the structure and function of the myocardium resulting in a HF-like phenotype[30].The aetiology of diabetic cardiomyopathy is multifactorial.Despite decades of research aiming to determine the causes of this distinct pathology,more research is needed.Current hypotheses include insulin resistance,endothelial dysfunction,fibrosis,cardiac lipotoxicity and energetic impairment.In particular,cardiac metabolism may play a central role in diabetic cardiomyopathy.
Type 1 diabetes (T1D) is a condition of absolute insulin deficiency due to Tcell-mediated autoimmune destruction of pancreatic β-cells.Cardiovascular disease is again a major long-term sequelae of the disease with an impact on healthcare resources.This encompasses coronary artery disease,cerebrovascular disease,peripheral artery disease,heart failure and cardiomyopathy.The pathophysiology of these processes vary and most of the data from population studies and large databases are focused on T2D.Studies looking at atherectomy samples have previously shown that the pathology of atherosclerosis did not differ between diabetes type[31].Angiographic evidence showed that T1D caused more multivessel,distal and severe stenosis[32].T1D appear to be affected more by hypoglycaemia and inflammation.Inflammatory markers such as C reactive protein,interleukin receptors and CD4 ligands are higher in T1D[33,34].Excess adiposity and altered fat distribution have been shown to contribute to diabetic cardiomyopathy in T1D similar to T2D.For the purposes of this review,we will be focusing on the evidence base behind diabetic cardiomyopathy in T2D.
MYOCARDIAL ENERGY METABOLISM
Normal vs the diabetic heart
The heart converts chemical energy present in the form of substrates and oxygen to mechanical energy and heat[35,36].Maintenance of adequate levels of cardiac highenergy phosphate metabolites,adenosine triphosphate (ATP),the energy source for contraction,and phosphocreatine (PCr),the major energy storage compound,are of vital importance for normal heart function[37].A healthy heart is capable of metabolising a range of substrates,including fatty acid (FA),glucose,amino acids,ketones and lactate,to produce ATP[38,39].In the normal heart,the main substrates for acetyl-CoA and therefore ATP formation are long chain FAs (60%-90%) and glucose and pyruvate oxidation (10%-40%,formed from glycolysis).The normal cardiac metabolic process involves energy transfer initially from substrate to the high energy phosphate metabolite ATP.This occurs through the generation of acetyl-CoA,that then enters the tricarboxylic acid cycle (TCA),also known as Krebs or citric acid cycle,followed by oxidative phosphorylation[40]resulting in the production of ATP (Figure 1).This is then transferred through facilitated diffusion to the areas requiring the high energy released through ATP hydrolysis,i.e.,myosin and sarcoplasmic reticulum[37,41-43].Figure 2 shows the role of carnitine in the mitochondrial oxidation of fatty acids contained within Figure 1.Figure 3 details the Randle cycle,also referred to as the glucose-fatty acid cycle which helps regulate uptake and utilisation of glucose within the muscle dependent on the rate of fatty acid oxidation.
Altered myocardial substrate metabolism may play a central role in cardiac dysfunction in T2D patients[40,44,45],by affecting myocardial oxygen demand which in turn results in reduced metabolic flexibility[39,46,47].Metabolic flexibility describes the ability of an organism to respond to changes in metabolic or energy demand as well as the prevailing conditions or activity[38,39].There are no myocardial ATP reserves[35,48,49].Energy in the heart is stored in three forms.The first form of stored energy is phosphocreatine,which can rapidly donate its high energy phosphates to produce ATP from ADP.The second endogenous form of energy is glycogen.The third form of stored energy is represented by triglycerides.
Stress metabolism studies and metabolic flexibility
Myocardial substrate metabolism can be measured directly by cardiac catheterization and transmyocardial blood sampling.Cardiac metabolic flexibility can be evaluated by coronary sinus (CS) studies invasively and positron emission tomography (PET)studies.Substrate concentrations in arterial and CS blood,in combination with the measured CS flow,yield information about the myocardial use of substrates and oxygen[50].Simultaneous CS and coronary artery blood sampling allow detailed analyses of myocardial substrate metabolism obtained by measuring the arteriovenous extraction of carbohydrates,fat,ketones,and amino acids.The flow in the CS correlates with cardiac output,mean arterial pressure,and the flow through the coronary arteries and varies with myocardial oxygen demand[51].The technique has been used in healthy individuals[52],in patients with dilated cardiomyopathy[53],and syndrome X[54]during incremental pacing.These studies looked into FA,glucose and lactate metabolism in the heart as key sources of energy production as well as myocardial oxygen consumption at rest and during periods of increased cardiac work.They confirmed that in healthy individuals,cardiac metabolism relies predominantly on oxidation of FAs,but during increased cardiac workload with maximal pacing stress,glucose uptake increases by a factor of two,while FA metabolism does not change.In summary these studies confirmed that,although FAs are the predominant fuel for energy provision in the human heart in the resting state,carbohydrates are the fuel for the heart in a state of exercise or stress.In T2D,cardiomyocytes have been shown to exhibit insulin resistance,decreased glucose utilisation and increased fatty acid oxidation in response to stress,insulin or variability in FA availability confirming that cardiac metabolic reserve is impaired in diabetes[55].
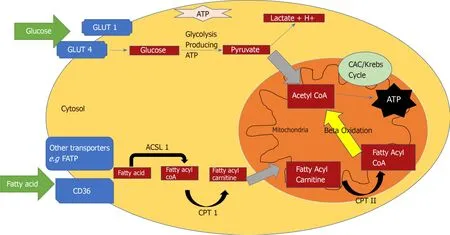
Figure1 An overview of myocardial energy substrate utilization.
Stress metabolism can also be assessed non-invasively by PET.There have been only a few studies to date using PET to assess cardiac metabolism in patients with T2D.However,there has been conflicting outcomes from these studies[56]regarding the changes in myocardial glucose uptake and insulin resistance in T2D patients.While one study showed reduced myocardial glucose uptake and myocardial insulin resistance,another study showed preserved myocardial insulin responsiveness in patients with T2D[46,57].One small study using PET imaging to characterize myocardial glucose metabolism in patients with T2D evaluated the influence of chronic hypertriglyceridemia on myocardial glucose uptake.They compared five T2D patients with high triglyceride (TG) levels to 11 T2D patients with normal TG.Patients were matched by age,gender,blood pressure and glycaemic control.They showed 40 and 65% lower myocardial glucose uptake in the high TG group compared normal TG group,respectively,and evidence of myocardial insulin resistance in T2D.Another small PET study assessing skeletal muscle and myocardial glucose uptake in 10 patients with T2D compared to nine age and weight-matched healthy male controls under normoglycaemic hyperinsulinaemic conditions,showed preserved myocardial glucose uptake[57].A third PET study compared glucose utilization in diabetic (n= 19) and nondiabetic CAD patients (n= 35) undergoing coronary artery bypass graft surgery grafting surgery (CABG) also showed lower myocardial glucose uptake in diabetic cohort,but this study did not assess myocardial insulin resistance[56].
Myocardial energetics
The phosphocreatine to ATP (PCr/ATP) ratio is a sensitive measure of myocardial energetic status.31-Phosphorus magnetic resonance spectroscopy (31P-MRS) provides a non-invasive quantification of myocardial PCr/ATP.Normally,the body is able to increase phosphotransferase reactions,glycolysis and glycogenolysis to function under higher energy demands[43,58,59].Scheuermann-Freestoneet al[60]pioneered the study of myocardial energetics in 2003 by studying cardiac and skeletal muscle energetics in T2D.Twenty one diabetics and 15 controls were studied to determine normal energy metabolism and the effect of T2D on circulating glucose and free fatty acid concentrations.They showed that,in the presence of normal cardiac structure,function and morphology,patients with T2D had 35% lower PCr/ATP ratios.There was a significant negative correlation with fasting plasma FA concentration as well as a significant positive correlation with plasma glucose concentration in diabetics compared to controls.In skeletal muscle,energetics was normal at rest,but the decrease in PCr was faster during exercise in those with diabetes and the recovery was slower after exercise.This lends credibility to the theory that changes in myocardial and skeletal muscle energetics is part of the initial disease process in T2D and this results in changes in substrate use and availability.Cardiac muscle is more affected by the energetic alterations and substrate availability[60].
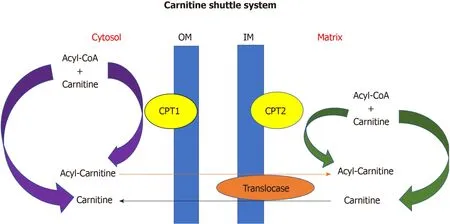
Figure2 Carnitine shuttle system.
Shivuet al[61]the looked at 25 asymptomatic type 1 diabeticsvs26 healthy controls who underwent31P-MRS and adenosine stress cardiovascular magnetic resonance(CMR) and again this showed significantly reduced PCr/ATP ratio in diabetics,both long-term and newly-diagnosed,compared to healthy volunteers (2.23 ± 0.56vs1.49 ±0.44,respectively,P< 0.001).Mean myocardial perfusion reserve index (MPRI)correlated negatively with duration of diabetes,with a significant reduction in longterm diabetes (1.7 ± 0.6) compared to newly diagnosed subjects (2.1 ± 0.2,P< 0.05)and controls (2.3 ± 0.3,P= 0.05).However,there was no significant correlation between PCr/ATP and MPRI implying the myocardial energetic alteration was independent of any coronary microvascular dysfunction primarily results from metabolic dysfunction[61].
Most recently,Leveltet al[62]have shown significant correlation between myocardial systolic strain and rest and stress PCr/ATP in 31 T2D compared to 16 healthy controls.Subjects underwent31P-MRS performed both at rest and exercise and adenosine stress CMR for assessment of perfusion by MPRI and oxygenation.The PCr/ATP was 17% lower in T2D compared to controls (1.74 ± 0.26vs2.07 ± 0.35,respectively,P= 0.001) at rest,with a further 12% reduction in PCr/ATP during exercise in T2Ds.However,there was no change in PCr/ATP in the control group during exercise.Similarly,MPRI was lower in diabetes (1.61 ± 0.43vs2.11 ± 0.68 in controls,P= 0.002).At rest,no correlation was observed between PCr/ATP and MPRI,but a significant correlation was noted during exercise.These findings support the notion that under stress microvascular dysfunction may exacerbate energetic derangement[62].
Cardiac metabolic reserve is impaired in diabetes.Lower metabolic reserve is likely to be the consequence of impaired metabolic flexibility and may be associated with increased mortality,as has been shown in a small study of patients with dilated cardiomyopathy[63].
DIABETIC CARDIOMYOPATHY AND POTENTIAL MOLECULAR MECHANISMS
Glucotoxicity
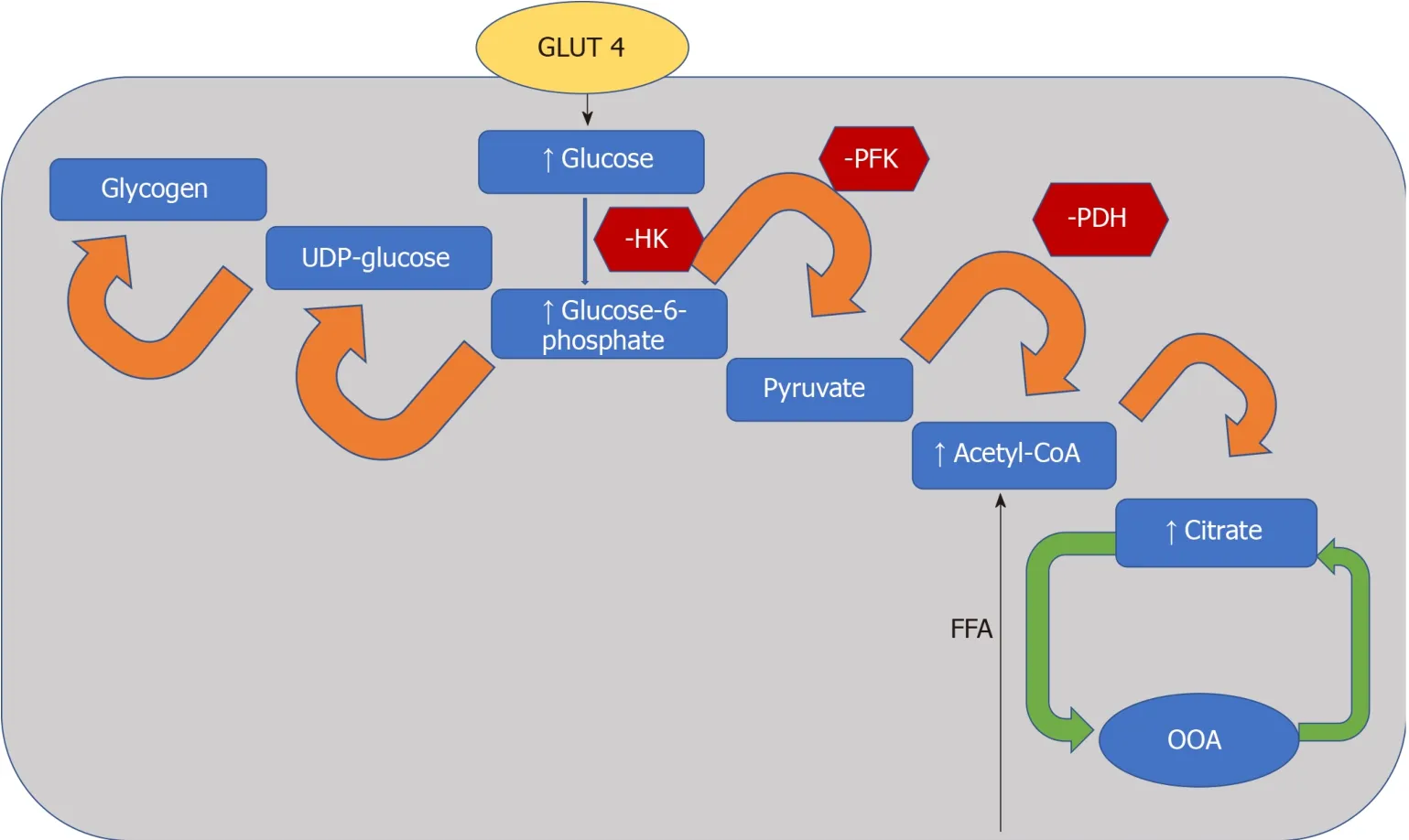
Figure3 Randle cycle.
Activity of pyruvate dehydrogenase (PDH),which is a key enzyme that regulates the balance between carbohydrate and fat metabolism in the heart,is decreased in diabetes and pyruvate oxidation impaired[64,65].Inhibition of PDH limits pyruvate oxidation[66].The dissociation of glycolysis and pyruvate oxidation in the diabetic heart results in the accumulation of glycolytic intermediates[67].Failure to adequately control intracellular glucose levels has also been implicated in the development of insulin resistance and in the generation of reactive oxygen species (ROS).These glycolytic intermediates activate glucose sensing transcription factors[68,69].It has been previously postulated that accumulation of glycolytic intermediates decreases the sarcoplasmic reticulum calcium ATPase 2a (SERCA2a) expression[70],an essential enzyme in calcium homeostasis,which results in diastolic dysfunction[71].Confirming this,SERCA2a expression in the heart was shown to be decreased in response to diabetes[71,72].
Lipotoxicity and myocardial steatosis
In diabetes,a decrease in insulin sensitivity leads to an inability to suppress lipase within adipose tissue and very low-density lipoprotein within the liver.This results in high levels of circulating FAs and as a consequence peroxisome proliferator activated receptor-α (PPARα) activation,while decreasing glucose-transporter-4 activity[47,73].PPARα is an essential component in cardiac substrate switching.Decreased PPARα expression (due to pressure overload and/or prolonged exposure to hyperglycemia and/or hyperlipidemia) will limit the FA oxidative capacity of the heart[74].The discordance between the rates of FA availability and/or uptake with that of FA oxidation results in increased intracellular long chain fatty acyl-CoA concentrations[46]Since cardiomyocytes are not specialised to store lipid,this finding suggests a deleterious effect,and cellular lipid overloading underlies the concept of“lipotoxicity” as a potential mechanism for impaired cardiac function[75-78].The excess long chain fatty acyl-CoA is then diverted towards non oxidative processes with the production of lipotoxic intermediates such as ceramide and diacyl-glycerol[47].This is postulated to be a potential mechanism for impaired cardiac structure and function[76-79].The molecular mechanisms described above that have been implicated in the pathophysiology of diabetic cardiomyopathy are detailed in Figure 4.
Imaging myocardial triglyceride
Proton (1H)-MRS offers a non-invasive method to measure cardiac triglyceride (TG)content.Using this methodology,studies have shown myocardial TG content to be significantly raised in T2D (Table 1).The studies are all consistent in reporting an increased hepatic triglyceride content in the diabetic population.Leveltet al[62]has also recently shown that myocardial lipid level is a predictor of concentric LV remodelling independent of BMI,systolic and diastolic blood pressure,and circulating FA,and is associated with subclinical contractile dysfunction in T2D.The study recruited 46 non-hypertensive T2D patients and 20 matched controls.Seventy four percent of the diabetic patients were on statin therapy resulting in lower total cholesterol and lowdensity lipoprotein cholesterol levels in patients compared to controls.This provides another avenue for potential therapy in T2D as myocardial steatosis has been found to be reversible with cholesterol and triglyceride control in addition to glucose control[80,81].
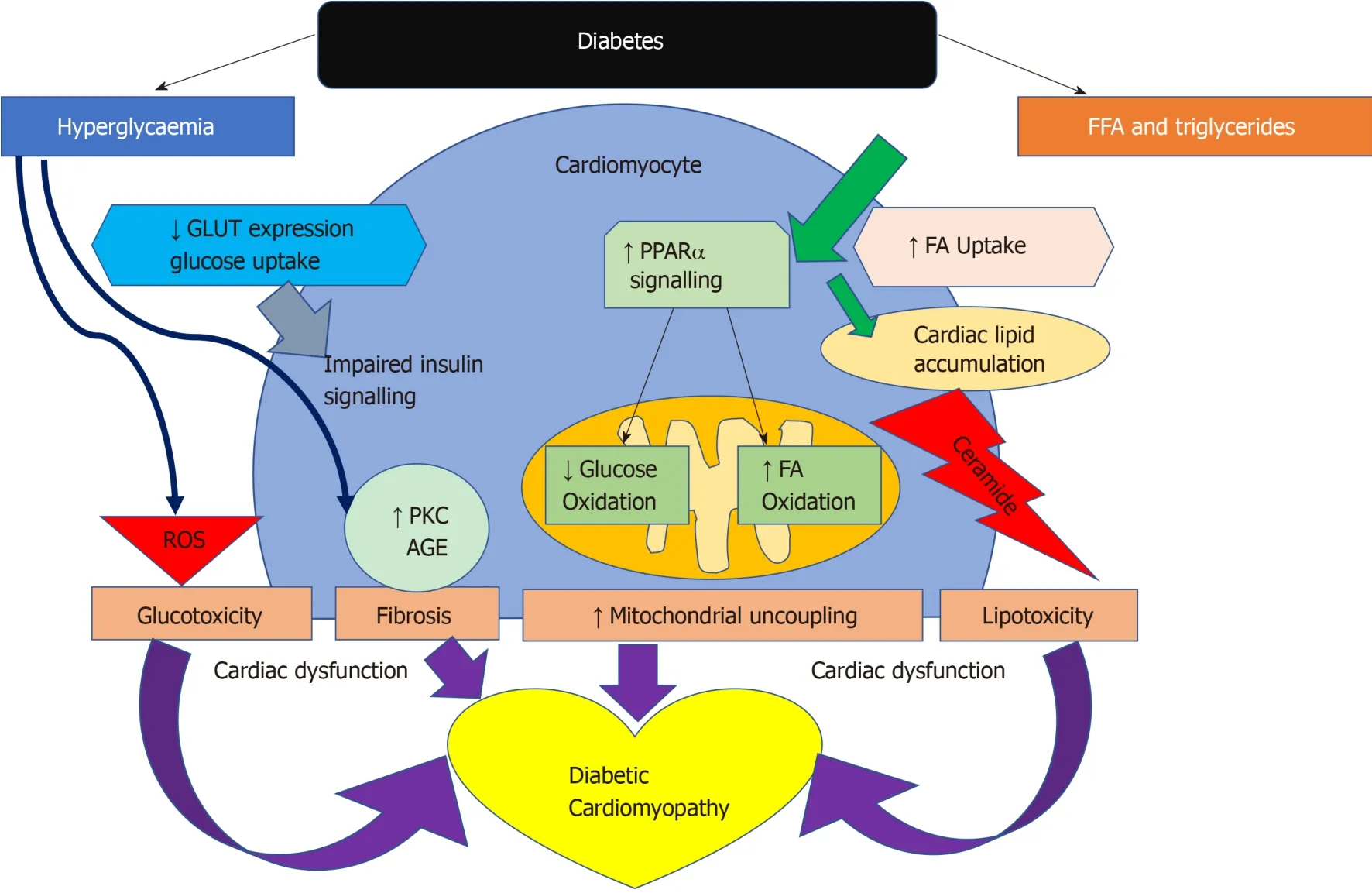
Figure4 Pathways of cardiac dysfunction leading to diabetic cardiomyopathy.
Increased myocardial TG content has been shown in T2D.Compared with lean subjects,myocardial TG content was elevated 2.3-fold in impaired glucose tolerance and 2.1-fold in T2D.This was evidenced in a cross sectional study looking at both lean and increased BMI participants with and without T2D[82].Myocardial TG has also been shown to be an independent predictor of left ventricular diastolic function in multivariable analysis accounting for BMI,heart rate,visceral fat and diastolic blood pressure.In a study of 38 T2D and 28 matched controls,myocardial TG measured by(1H)-MRS was significantly higher in T2D.Although there was no significant difference in systolic function,all indices of diastolic function were significantly impaired in T2D[83].Interestingly,calorific restriction in obese T2D patients on a very low calorie diet of 450 kcal/d was been shown to decrease myocardial TG content from 0.88 +/- 0.12% to 0.64 +/- 0.14%,respectively (P= 0.019) over a 16 wk period,and this was associated with better diastolic function (E/A ratio from 1.02 +/- 0.08 to 1.18 +/- 0.06)[84].Myocardial TG level is a predictor of concentric LV remodelling independent of BMI,systolic and diastolic BP,circulating FA and is associated with subclinical contractile dysfunction in T2D[62].
MYOCARDIAL STRUCTURAL AND FUNCTIONAL CHANGES IN DIABETES - EVIDENCE FROM HISTOLOGY,MRI AND ECHOCARDIOGRAPHY STUDIES
Structural changes
Pathological evidence characterised by myofibrillar hypertrophy with fibrotic strands extending between muscle fibres to cause diffuse myocardial fibrosis first helped distinguish cardiomyocyte damage in diabetes[9].The advancement in non-invasive imaging techniques has facilitated further delineation and phenotyping of the diabetic heart.Alterations to left ventricular geometry lead to concentric remodelling,hypertrophy and eventually increased mass.Left ventricular hypertrophy in diabetes includes both concentric and eccentric hypertrophy.Left ventricular concentric remodelling has been shown to have a higher association with cardiovascular mortality than eccentric remodelling on both echocardiographic and CMR studies[62,85-87].
Increased left ventricular mass had initially been shown to be associated with a rise
in HbA1c levels in a HF population[88].Studies using both echocardiography and MRI have been successful in showing an increase in left ventricular mass associated with diabetes independently of other factors[10,62,85,87,89-94].Table 2 details studies that have examined left ventricular mass and concentric remodelling on MRI in T2D.In summary,there is a general trend that shows an increase in left ventricular mass.However,when corrected for body surface area,this does not persist.In two studies,there remained a higher left ventricular mass/volume ratio,but due to a general increase in end diastolic volume this difference is obliterated when corrected for volume.A decrease in stroke volume is as expected in concentric remodelling.Increased left ventricular mass is a known predictor of cardiovascular mortality and morbidity[95,96].
CMR T1 mapping pre and post-contrast allows calculation of myocardial extracellular volume (ECV) which is a surrogate for diffuse interstitial fibrosis and correlates with pathology samples obtained in patients with heart failure[97].Leveltet al[62]used similar techniques and concluded that although there was concentric remodelling and evidence of diastolic dysfunction,there was no evidence of expansion of extracellular matrix in people with well-controlled T2D.However,this is contrary to most existing literature on ECV and T1 mapping to date,which has shown increased ECV and native T1 values in T2D compared to healthy controls[98,99].Swobodaet al[100]also showed increased ECV and native T1 relaxation at baseline in T2D and demonstrated that treatment with renin-angiotensin-aldosterone system inhibition led to an improvement in left ventricular ejection fraction and a decrease in myocardial ECV.The differences in literature on extracellular matrix may be related to patient selection for the controls.For example,young and healthy controls rather than age-matched non-diabetics.Sensitivity of different T1 mapping techniques may also play a small role.
Functional changes
Diabetic cardiomyopathy has mainly been linked with features of diastolic dysfunction.This is especially apparent in asymptomatic individuals as the earliest sign of HF.Most of the evidence in imaging of patients with T2D have not shown a significant decrease in ejection fraction/systolic dysfunction[88,101-103]with the exception of the Strong Heart Study where a direct correlation of ejection fraction was seen associated with HbA1c levels[87].Diastolic dysfunction is now regarded as the first functional change occurring in diabetic cardiomyopathy.
Strain is a measure of tissue deformation.As the ventricle contracts,muscle shortens longitudinally and circumferentially and thickens radially.The application of strain to measure deformation is constrained by a number of complexities when the parameter is measured by echocardiography[104].Tissue Doppler imaging (TDI) in echocardiography improved the identification of early diastolic dysfunction and recent developments in strain imaging on CMR has very much improved the sensitivity and accuracy of identifying subclinical diastolic dysfunction in the T2D population.Measurement of strain using CMR is now considered the gold standard.Strain rate measures the time course of deformation and this can be derived from speckle tracking echocardiography or measured directly from MRI cine images with dedicated software programmes of which several are available.
Both longitudinal and circumferential strain have been shown to be impaired in T2D.Table 3 identifies the larger and more recent studies that have shown early changes in strain in people with diabetes[11,88,91,94,105-107].Different studies have studied and reported different measures of strain making the literature not directly comparable.Global longitudinal strain (GLS) has been shown to be decreased in the diabetic population compared to healthy volunteers in all the studies described across tissue Doppler,speckle tracking echocardiography and CMR.Additionally,some studies have shown a decrease in radial strain.One study has shown that peak early diastolic strain rate (PEDSR) and peak systolic strain rate are both decreased in diabetes even when compared to obese/overweight non-diabetic controls[11].Strain and strain rate,especially PEDSR is a good measure contractility and contractile reserve.Its application in clinical practice remains in infancy to be used to predict potential development of heart failure with preserved ejection fraction in the form of a contractility index.It is widely regarded as a precursor to the onset of heart failure in T2D and a predictor of cardiovascular mortality and morbidity even in the absence of symptoms[108,109].
Coronary microvascular dysfunction
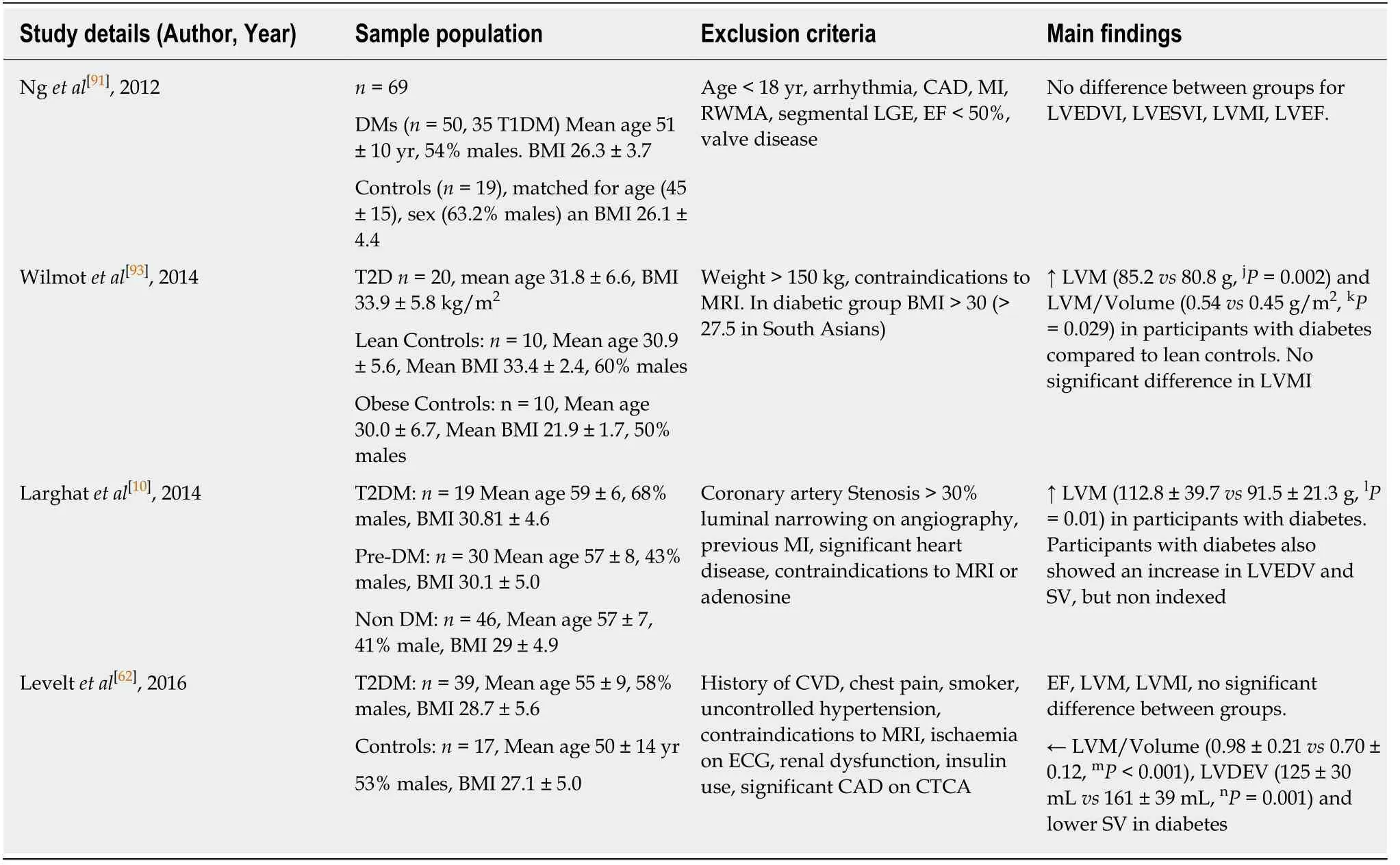
Table2 Magnetic resonance imaging studies looking at left ventricular mass and concentric remodelling in diabetes
Coronary microvascular dysfunction in diabetes is a complex pathophysiological process,which involves structural,functional and metabolic alterations.It is likely to be a multifactorial phenomenon,related to changes in perivascular and interstitial fibrosis[110],myocardial hypertrophy[111],reduced capillary density,and autonomic neuropathy[112].Coronary microvascular dysfunction has emerged among the potential mechanisms leading to increased incidence of heart failure[113]and risk of cardiovascular mortality[3,114]in patients with diabetes.Both hyperglycaemia and dyslipidaemia have been shown to cause abnormal structure and function within the endothelium.High circulating glucose concentration causes downregulation of nitric oxide resulting in increased vasoconstriction[115].This in turn results in increased vascular tone,permeability,thinning of vascular endothelium,weakening of intercellular junctions,altered protein synthesis and ultimately causes remodelling[115].
Using CMR[10,94,116]and PET[114,117]myocardial perfusion during vasodilator stress has been shown to be impaired in patients with diabetes[10,94,116]and in the absence of epicardial coronary artery stenosis,this finding is indicative of coronary microvascular dysfunction.Importantly,coronary microvascular dysfunction is an early precursor of cardiovascular events and was shown to be associated with a 2.5%annual major adverse event rate that includes cardiovascular mortality,nonfatal myocardial infarction,nonfatal stroke,and congestive HF even among patients without epicardial coronary artery stenosis[118].Consequently,early identification of coronary microvascular disease may be beneficial in prognosis evaluation and patient stratification for optimal medical therapy
Using adenosine stress perfusion CMR Larghatet al[10]have assessed myocardial perfusion reserve in 65 patients with no significant epicardial coronary artery stenosis on angiography (< 30% coronary luminal stenosis).Left ventricular mass was shown to be higher in diabetics than non-diabetics (112.8 ± 39.7 gvs91.5 ± 21.3 g,P =0.01)and in diabetics than prediabetics (112.8 ± 39.7 gvs90.3 ± 18.7 g,P =0.02).MPR was lower in diabetics than non-diabetics (2.10 ± 0.76vs2.84 ± 1.25,respectively,P =0.01)[10].Khanet al[11]have also studied 40 young patients (mean age 30 years),including 20 diabetics and 10 lean and 10 obese controls who underwent CMR.This study,however,did not show any significant correlation between left ventricular intracardiac measures,including mass,MPR and perfusion and the presence of T2D[11].Diastolic dysfunction was found to be associated with TG content but not withimpaired myocardial perfusion reserve in another study with patients with T2D with preserved systolic function[119].
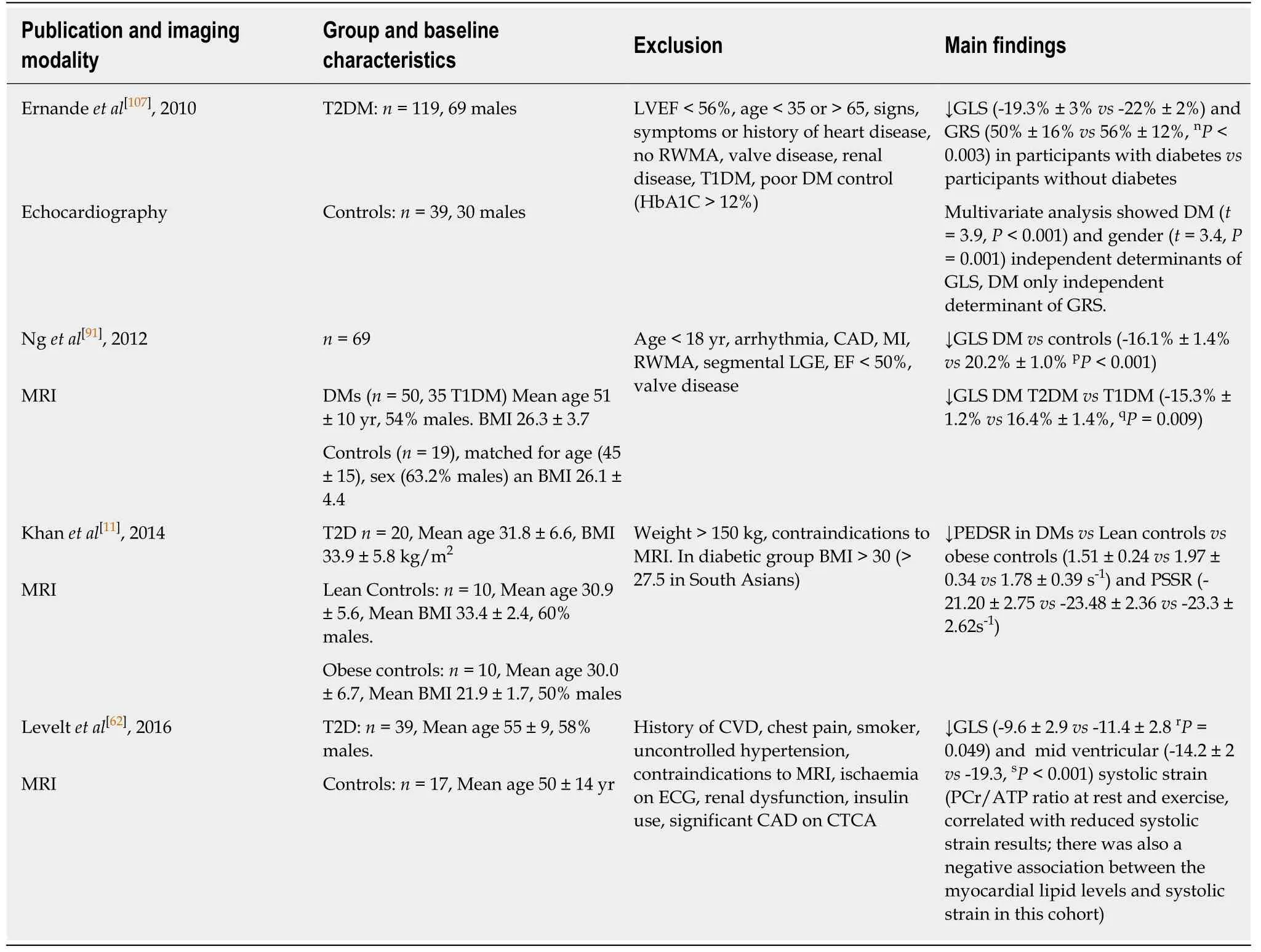
Table3 Studies on left ventricular function and myocardial strain in diabetes
Another study looking at the interplay between metabolic and ischaemic changes in the hearts of people with well-controlled T2D,even in the absence of arterial hypertension or significant CAD on computed tomography coronary angiography,showed there were significant reductions in MPRI and evidence of blunted oxygenation response compared with controls.These findings support the concept that hypoperfusion as a result of microvascular dysfunction plays a role in the impaired ability to increase and/or maintain myocardial oxygenation during vasodilator stress in diabetes[94].
In a large study of 2783 consecutive patients (1172 diabetics and 1611 non-diabetics)measuring coronary flow reserve (CFR) by PET[120],they showed that diabetic patients with preserved CFR have similar rates of cardiac events to nondiabetic patients,whereas impaired CFR was associated with an adjusted 3.2- and 4.9-fold increase in the rate of cardiac death for both diabetics and nondiabetics.This raises the question of whether CFR should be included in a cardiac risk model for patients with diabetes.However,there is selection bias due to patients recruited consecutively,some had regional perfusion defects and the results of angiography were not known therefore the impaired flow reserve cannot be attributed directly to microvascular dysfunction.
In a small (n= 64) randomised trial comparing the effects of spironolactone,hydrochlorothiazide or placebo on coronary flow reserve in T2D assessed by cardiac positron emission tomography,spironolactone was associated with modest improvements in coronary flow reserve (from 2.77 ± 0.82 to 3.10 ± 0.74) compared to the other two treatment arms (P =0.01) after six months[117].The mechanism for this is thought to be due to expression of the mineralocorticoid receptor in endothelium,vascular smooth muscle cells,cardiomyocytes and circulating leukocytes[117].Mineralocorticoid receptor activation causes vascular inflammation with increased ROS production and increased vascular damage,vascular dysfunction,and perivascular fibrosis.The findings of this study support the potential role of mineralocorticoid use in reversing coronary microvascular dysfunction in T2D.However,there was no reporting of cardiovascular outcomes,relatively small sample size and limited follow up period.
The evidence of the role of coronary microvascular dysfunction in T2D is conflicting.This suggests that the metabolic dysfunction caused by diabetes likely may predispose to some degree of microvascular dysfunction however these may not be the main mechanisms contributing to cardiac dysfunction in diabetes.
Therapeutic developments and cardiovascular outcomes
The evidence for pharmacological therapy for heart failure targeted at the diabetic population thus far has been limited to studies of both patients with and without diabetes.ACE-inhibitors are recommended within the ESC/EASD guidance for diabetes and impaired glucose tolerance.The CHARM,ATLAS and HEAAL trials have all shown the beneficial effects of ACE in HF in terms of morbidity and mortality,however subgroup analyses showed no difference with and without diabetes[121-124].This is similarly the case with the use of mineralocorticoid antagonists[125,126],nitrates and hydralazine[127],ivabradine[128]as well as more recently sacubitril/valsartan[129].
The impact of better glycaemic control on left ventricular systolic and[130]diastolic function remains debatable due to varying evidence.Previous large studies including United KingdomPDS[131],ADVANCE[130],ACCORD[132]and VADT[15]have not shown an improvement in cardiovascular outcomes with good glycaemic control.A metaanalysis of 37229 people with T2D data from multiple randomized trials concluded there was no observed benefit of intensive glycaemic control on HF related outcomes[133].Patients with T2D who received intensive glucose lowering therapy had a reduced risk of major adverse cardiovascular events,but there was no effect on total mortality,cardiac death,stroke,and heart failure[133].
There is also a large body of evidence to suggest T2D therapies may increase the risk of developing heart failure.This has been particularly the case with dipeptidyl peptidase-4 (DPP4) inhibitor saxagliptin and thiazolidinediones (TZDs).In SAVORTIMI 53 looking at saxagliptinvsplacebo there was a significantly increased risk of heart failure hospitalisation[134,135].These patients then went on to have a high rate of subsequent death.The latter result was also found in the RECORD trial[136].The TECOs trial looking at sitagliptin[17]and EXAMINE for alogliptin[18],both against placebo there was no increase in heart failure.Further studies are required to consolidate the effects of DPP4 inhibitors on heart failure in T2D.There are current ongoing studies,CARMELINA (Cardiovascular and Renal Microvascular Outcome Study With Linagliptin in Patients with Type 2 Diabetes Mellitus; NCT01897532) and CAROLINA (Cardiovascular Outcome Study of Linagliptin Versus Glimepiride in Patients With Type 2 Diabetes; NCT01243424) ,looking at Linagliptin in T2D that may provide better insight.
More recent studies have shown favourable cardiovascular outcomes with newer glucose lowering agents.There have recently been numerous non-inferiority cardiovascular outcome trials using newer glucose lowering therapies in T2D,namely GLP1 - Receptor Agonists and sodium-glucose cotransporter 2 (SGLT2) inhibitors.
Glucagon like peptide 1-receptor agonists
Multiple glucagon-like peptide-1 (GLP-1) receptor agonists in the form of once weekly exenatide,dulaglutide,albiglutide and semaglutide have been recently developed.GLP-1 agonists exert their effects by stimulating insulin secretion,suppressing appetite,decreasing circulating glucagon levels and gastric emptying[137].They have also been associated with weight loss.The cardiovascular benefits of GLP-1 is multifaceted.The first is glycaemic control.Long acting agonists have been shown to be better at reducing HBA1c than short term[138].Despite the failure to show an improvement in cardiovascular outcomes with some large studies in the past[15,139,140],the United Kingdom PDS study that looked at tight glycaemic control at diagnosis showed a reduction in cardiovascular events after a decade even with similar HbA1c levels[131].The second is through blood pressure control.None of the GLP-1 studies conducted thus far have been specifically to look at its’ effects on blood pressure however both studies as well as reviews and meta analyses have shown a decrease in BP of both exenatide and liraglutide compared with placebo and other antidiabetic drugs[141].It has also been shown to have favourable effects on the vascular endothelium in that Liraglutide has been shown to improve nitric oxide synthase(NOS) activity as well as attenuates plasminogen activator inhibitor type 1 (PAI-1)and vascular adhesion molecule (VAM) within the vascular endothelium.These apply a protective effect against endothelial dysfunction[142,143].GLP-1 agonists also appear to have a cardioprotective effect on atherosclerosis progression and myocardial ischaemia,and this has been shown with the use of exenatide as an adjunct therapy in STEMI and thrombolysis[144,145].GLP-1 agonist use has not been associated with a significant effect on heart failure hospitalisation.
Large studies examining the impact of GLP-1 agonists on cardiovascular outcomes in T2D have been summarised in Figure 5.In the LEADER trial,patients with T2D on Liraglutide had lower rates of cardiovascular death[146].Similarly SUSTAIN-6 also looked at GLP-1 agonists with Semaglutide being an injectable version with longer half-life have also shown improved cardiovascular outcomes.However,in the EXSCEL[23],Exenatide did not show overall cardiovascular risk benefit compared to placebo.Most recently,the PIONEER 6 trial has studied the non-inferiority of oral daily semaglutide against placebo for cardiovascular outcomes[147].It has been shown to have a significant reduction in the composite primary outcome of first occurrence of death from cardiovascular causes,nonfatal myocardial infarction (including silent),or nonfatal stroke,i.e.,8.6% in the semaglutide groupvs6.6% in placebo,with a hazard ratio of 0.79,95%CI:0.57-1.11,P <0.001 for non-inferiority,P= 0.02 for superiority.However,when the components of the primary outcome are looked at,non-fatal MI and death from cardiovascular causes comes up non-significant.However,this study was relatively underpowered compared to the other cardiovascular outcome trials and this may explain why the reduction was not statistically significant.Increasing evidence for oral GLP-1 agonists has marked benefits for those who have reservations regarding all previous GLP-1 agonists that have been in the form of a subcutaneous injection and will allow for better compliance with the new ADA/EASD guidance to use a GLP-1 agonist as second line therapy in diabetes.There is a larger phase three cardiovascular outcome trial underway,the Heart Disease Study of Semaglutide in patients with Type 2 Diabetes (SOUL;NCT03914326),looking at oral semaglutide against placebo and its effects on lowering MACE in over 9000 patients.The results of this is eagerly awaited.
Sodium glucose co-transporter 2 inhibitors
In both the EMPA-REG OUTCOME[19]and CANVAS[20]studies (Figure 6) there was a relative risk reduction in cardiovascular mortality and hospitalisation for HF in patients with T2D.The hazard ratio for HF hospitalisation in the EMPA-REG OUTCOME trial was 0.65,(0.57,0.82,P= 0.002) for empagliflozinvsplacebo[21].A reduction in hospitalization for HF was also observed with Sodium glucose cotransporter 2 (SGLT2) inhibitor Dapagliflozin in DECLARE-TIMI 58 trial[148].An important consideration in interpretation of these results is that the patients in these studies either had established cardiovascular disease or multiple risk factors for atherosclerotic cardiovascular disease risk factors and it is uncertain whether the results can be translated in to lower risk groups with prevention of the development of HF.
The mechanisms by which SGLT2 inhibitors lower HF hospitalisation rates in T2D are still under scrutiny.However,it is hypothesised to be related to the urinary sodium and glucose excretion that leads to increased fluid losses resulting in reduced pre-load and afterload[149].Secondary effects include fluid loss and blood pressure lowering.There is also suggestion that SGLT2 inhibitors shift myocardial metabolism towards ketones,resulting in this having a favourable effect on cardiac energetics[150].
CONCLUSION
Diabetic heart disease is multi-faceted and spans metabolic,structural and functional changes.Recent advancements in imaging has aided significantly in understanding the pathophysiology as well as the remodelling and functional changes within the heart.Further research into the degree of dependence on fatty acid metabolism instead of glucose in the presence of diabetes is required.The relationship between the metabolic changes within the heart and functional measures such as myocardial strain rates as well as triglyceride content will help us better understand how to treat this disease process.This will also add towards increasing the mechanistic accuracy of new therapeutic targets.
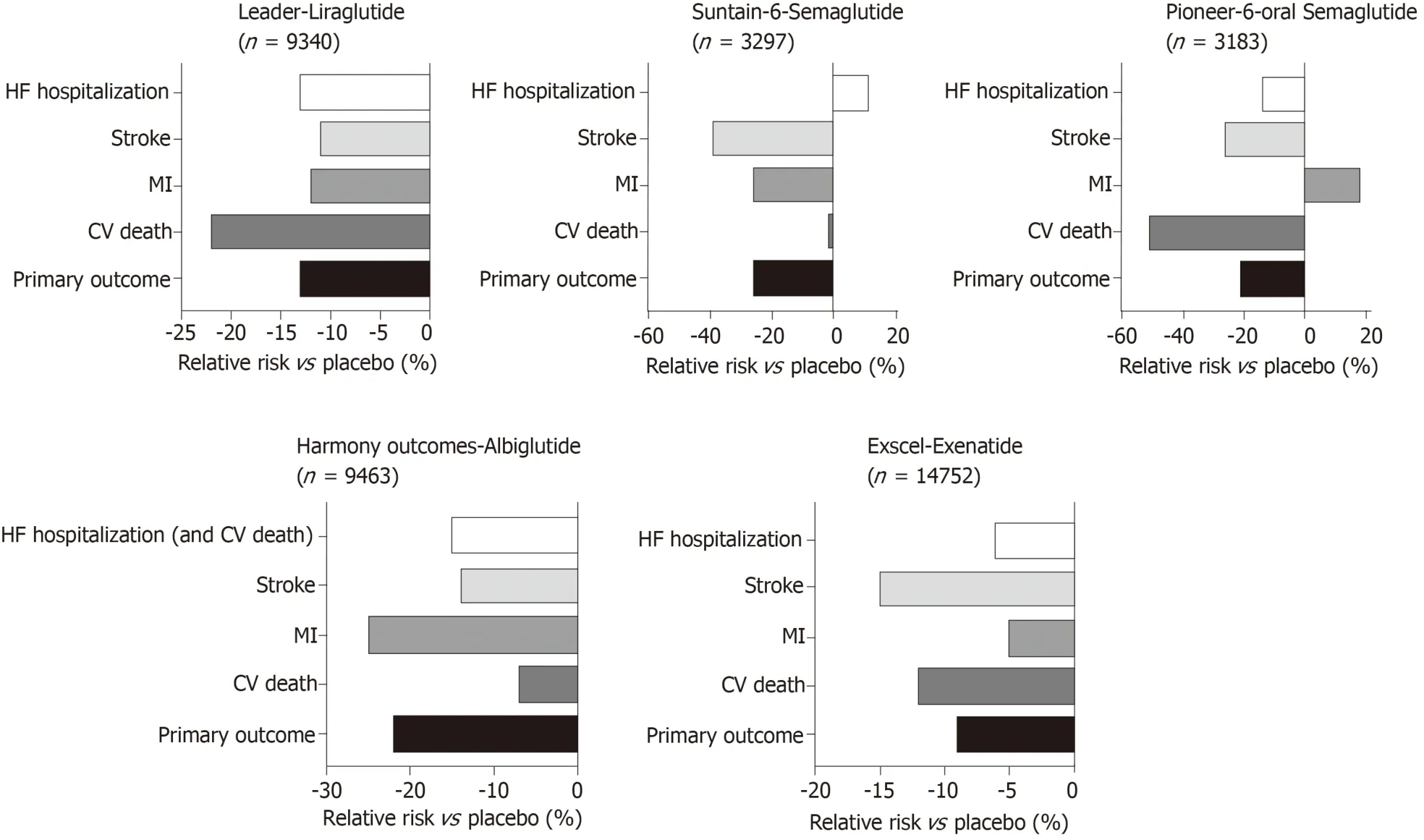
Figure5 Major cardiovascular outcome trials using GLP1 receptor antagonists.
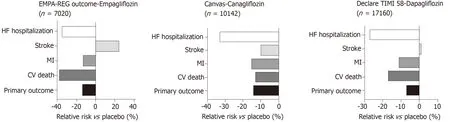
Figure6 Major cardiovascular outcome trials examining SGLT2 inhibitors.

