Assessing the influence of harvesting intensities on structural diversity of forests in south-west Germany
Felix Storch ,Gerald Kändler and Jürgen Bauhus
Abstract
Keywords:Large-scale inventory,Structural diversity index,Harvesting intensity,Changes in structural diversity
Background
A major challenge for modern forestry is the integration of wood production with other important values such as the conservation of biodiversity and ecosystem functioning (e.g. McDermott et al. 2010). Forest management changes the structure and composition of forests (e.g.Lindenmayer et al. 2000; Parrotta et al. 2002; Kuuluvainen 2009) and there is a general concern that forest biodiversity and ecosystem functioning are negatively impacted by increasing harvesting intensity (Raison et al.2001; Bauhus et al. 2017a; Urli et al. 2017). It has been shown that the difference in key structural elements and composition between managed and natural forests(including naturally disturbed forests) increases with increasing harvesting intensity (e.g. Parrotta et al. 2002; de Avila et al. 2015; Urli et al. 2017). Yet, in many studies on the impact of forest management, the intensity of harvesting is actually not considered but comparisons are being made between unharvested or reserved and(recently) harvested forests (e.g. Gilliam 2002; Okland et al. 2003; Paillet et al. 2010). However, this approach has one important limitation. While the status of unharvested forest reserves can be reasonably well described and defined, harvested forests can cover a wide range of harvesting intensities, ranging from clearfelling to single tree selection. Hence, these simple comparisons between unharvested and harvested forests provide little information on the influence of harvesting intensity on forest structure and biodiversity (Fig. 1).

Fig.1 Forested landscapes typically represent a wide range in harvesting intensities,from strictly protected reserves to intensively managed plantations harvested on short rotation.However,harvested forests include also forests that are selectively logged,where the majority of the stand remains intact.Hence comparisons between protected or unharvested forests and harvested forests(e.g.Paillet et al.2010)can only be regarded as a rough analytical approach,unless the type and intensity of management is comparable in the harvested forests.In addition,in this type of comparison,much valuable information related to the impact of different degrees of harvesting intensity is lost.Alternative analytical approaches quantify management intensity across the whole range of forest types from strictly protected reserves to intensively managed stands,e.g.Kahl and Bauhus(2014)or Schall and Ammer(2013).These approaches consider that management intensity is different from harvesting intensity and that therefore currently unharvested areas may be still influenced by a legacy of management in the past
While there have been some studies that explicitly considered the influence of harvesting intensity or forest use intensity as a continuous variable, these typically focused on selected aspects of forest biodiversity such structural variables, tree diversity, forest understorey or particular taxonomic groups or were spatially restricted to small areas or regions (e.g. Fredericksen et al. 1999;Parrotta et al. 2002; González-Alday et al. 2008; Sullivan et al. 2008; Michel and Winter 2009; de Avila et al.2015). Depending on the respective focus of these studies, impacts of harvesting intensity ranged from positive(e.g. for the diversity of forest understorey plant communities or tree species richness (Kern et al. 2006))to negative (e.g. volume or dimension of downed deadwood and species depending on large dimensioned trees and dead wood (e.g. Müller et al. 2008; Gossner et al. 2013). The lack of broad-scale assessments of the relationship between harvesting intensity and biodiversity or biodiversity surrogates limits the generalization of these findings for larger areas (Gilliam 2002; Roberts and Zhu 2002; Vandekerkhove et al. 2016).
Management of semi-natural forests, in particular on public land, increasingly aims at maintaining or increasing compositional and structural diversity for a number of reasons (e.g. Franklin et al. 2002; Puettmann et al.2015). Structurally and compositionally diverse forests may have a higher ecological stability than monocultures or one-layered stands in relation to biotic and abiotic stress and disturbances (Neuner et al. 2015; Thurm et al.2016; Penone et al. 2019). According to the habitat heterogeneity hypothesis, the provision of many different niches and structural elements in diverse forests is assumed to support also a higher species richness(Simpson 1949; Tews et al. 2004; Jung et al. 2012). At the stand scale, this can be achieved, for example, by implementing management strategies such as ‘retention forestry', where the intentional protection and development of forest structural elements across harvesting cycles can maintain habitats and organisms (Abrahamsson and Lindbladh 2006; Bauhus et al. 2009; Gustafsson et al. 2012). At the landscape scale, higher species richness may be achieved with increased beta-diversity through increasing variation among patches owing to differences in management or successional status (e.g.Hilmers et al. 2018; Schall et al. 2018a).
One of the major challenges in the assessment of forest harvesting intensity on biodiversity is that it is not possible to monitor biodiversity in all forests directly.Presence or diversity of species from different taxonomic groups can commonly be sampled only in case studies and for small areas (e.g. Laiolo 2002;Glaser 2006),which likely provides an incomplete and potentially unrepresentative picture. Therefore, the presence and the expression of different structural elements of forests have been suggested as surrogates for information about the abundance and richness of forest-dwelling species (e.g.McElhinny et al. 2006a; Jung et al. 2012). By analysing changes in structural diversity of forests, possible changes in communities of forest-dwelling species may be assessed too. Here we aim at assessing impacts on forest harvesting intensity based on large-scale forest inventory data. National forest inventory (NFI) data of Baden-Württemberg were used to include all different types of forests and an inventory period of 10 years to analyse these impacts in a comprehensive and representative way across the whole forest area and for a broad range of harvesting intensities from unharvested forest stands to clear-fellings.
Previous studies like those of Kahl and Bauhus (2014)or Schall and Ammer (2013) developed approaches to quantify forest management intensity along a continuum of human interference. For example, the index of forest management intensity (FORMI) developed by Kahl and Bauhus (2014) includes three criteria, the proportion of the total tree volume that has recently been harvested,the proportion of cultivated tree species that are not part of the natural forest community, and the proportion of the total dead wood volume that has originated from management activities. In other approaches that used a set of variables to describe management intensity by the level of naturalness (Bartha 2004; Winter et al. 2010), it is difficult to distinguish between independent and dependent variables. These types of approaches have been typically applied to relatively small areas, where detailed information about the necessary variables could be collected. If the influence of harvesting intensity on forest biodiversity is to be assessed on a large scale, it may be determined more directly on the basis of inventory data.
Here, we used harvesting intensity calculated on the basis of NFI data as the main predictor variable to analyse changes in structural diversity of forests over large areas. Based on data of the German National Forest Inventory for the state of Baden-Württemberg (SWGermany,NFI2002and NFI2012),an index has been developed to assess the level of structural diversity of forests comprehensively (Storch et al. 2018). This index (‘FSI' -Forest Structure Index) combines many forest attributes that cover important habitat components of forest dwelling species; see 2.2. Results showed an increase of structural diversity (FSI-score) for most of the analysed types of forests in Baden-Württemberg (Storch et al. 2018).
We employed this index for our analysis because a) it describes structural diversity of forests in an objective manner b) it can be applied to large forest areas and to the whole range for forest types and site conditions and c) it can be directly related to harvesting intensity that is quantified for the same set of inventory plots. Thus, the influence of harvesting intensity on changes of structural diversity in different forest types can be assessed for the period between the two national inventories from 2002 and 2012 (NFI2002-NFI2012).
Our hypothesis was that increasing harvesting intensity leads to a decrease in structural diversity of forests(e.g. Parrotta et al. 2002; Hartsough 2003; Cazzolla Gatti et al. 2015; Urli et al. 2017). However, no hypothesis was formulated regarding the shape of this relationship, i.e.whether the decrease followed a linear or non-linear function or at which harvesting intensity structural diversity of forests starts to decrease.
Material & methods
Data basis
This study was based on 12,918 NFI plots in the state of Baden-Württemberg, Germany, covering approximately 1371 million ha (hectares) of forest area and including a broad range of different types of forests,management intensities, stand development phases and structural diversities. These plots were marked as ‘forest' in NFI2002and NFI2012and were accessible at both inventories. An additional criterion for selection of NFI plots for our analysis was the presence of merchantable trees with a diameter at breast height (DBH) larger than 7 cm at NFI2002. The selected plots were distributed over the whole state of Baden-Württemberg and included 97.7%of all sampled plots in NFI2002(Storch et al. 2018). Further information about the inventory (systematic grid,sampling design and background of this inventory) can be found at BMEL (2013) (https://www.bundeswaldinventur.de).
Structural diversity index (FSI)
Based on data of NFI2002and NFI2012for the state of Baden-Württemberg, an index to assess the level of structural diversity of forests was developed (Storch et al. 2018) following the approach described by McElhinny et al. (2006b) and modifications suggested by Sabatini et al. (2015). Eleven variables, each representing different aspects of structural diversity, were applied to calculate the FSI in a simple additive way, without weightings of individual variables (Storch et al. 2018). These variables representing important habitat components, comprising the presence of standing and downed deadwood, different decay classes, the occurrence of large living trees,species richness of trees in the stand and the regeneration layer, quadratic mean diameter at breast height(DBH), standard deviation of DBH and stand height, as well as the diversity of bark types and flowering trees.Each variable is scaled in relation to the minimal and maximal value that can be assumed for this aspect of structural diversity to yield values between 0 and 1. This index, as the sum of the values of structural variables,was calculated at the plot-level and subsequently aggregated to forest types to obtain reliable estimates of structural diversity and its change between forest inventories.Index-values range between 0 and 1, where 0 implies‘lowest level of structural diversity' and 1‘highest level of structural diversity'. Further information about the development of this index can be found in Storch et al.(2018).
Calculation of harvesting intensities and relation to changes in structural diversity
Based on the same data-set of subsequent national forest inventories (NFI2002and NFI2012), harvesting intensity(HI) was calculated for each inventory plot. In the NFI,the trees removed during the previous inventory period are identified from stumps and recorded for each plot.Since there is no precise information on the date of harvest, the volume of harvested trees is based on the assumption that trees are removed at the middle of the inventory period and their volume increment until this point in time is modelled. We decided to represent HI as percentage of the standing timber volume (m3·ha-1)at the time of the NFI2002. In this way, harvesting intensity is related to actual timber stocks and the actual intensity is described more accurately than by just volume per ha of harvested timber, which provides no information on relative changes to the growing stocks.Harvesting intensity was calculated for the inventory period between NFI2002and NFI2012. For this period, a mean value of annual harvesting intensity (harvested timber volume divided by the duration of the inventory period (10 years)) was determined because no information about actual time of harvesting was available. These values were calculated on the basis of 12.918 forest plots,which were used to develop the FSI and its changes over a period of 10 years. This number of sampling plots is smaller than the total number of NFI plots in the state and hence the results may differ slightly from official NFI-analysis for Baden-Württemberg. Changes in the structural diversity of forests (FSI-changes) were also expressed as percentage of the FSI-values in 2002 at the beginning of the inventory.
To analyse influences of harvesting intensities on changes in structural diversity of forests, different models were calculated and compared to each other. For statistical analysis, the statistic-software ‘R' (Version 3.1.2)and package mgcv for Generalized Additive Models were used (Wood 2006), as these models can be used in a flexible way, including different numbers and functions for the applied predictor variables. Additionally, the packages randomForest and lmer were used to calculate and compare different types of models to identify the model that describes best the relation between harvesting intensity and changes in structural diversity(Table 1).Plot-level HI data were aggregated to harvesting classes(10%-intervals referred to standing timber volume of NFI2002), as the calculation of FSI and HI at the plotlevel includes a certain variability, caused by the sampling method for trees with a DBH ≥7 cm which is based on the ‘angle count method' - ‘probability proportional to size'. This method is characterized by unequal probabilities for trees to be included in the sample in successive inventories as well as small sample areas for trees of small dimension (see also Bitterlich 1952; Motz et al.2010; Storch et al. 2018). This variability can be reduced by aggregation of single plots into strata, here harvesting classes, containing at least 15 sampling plots. Therefore,results on the interactions of harvesting intensity and changes in structural diversity (FSI) were expressed for classes of HI, using 10%-intervals, and not for individual plots.
Results
Mean harvesting intensity for the inventory period 2002-2012 varied surprisingly little for the different forest types analysed and was between 30% and 40% of the standing timber volume of NFI2002(see also supplementary material). For that particular period, mean FSIscores increased in all analysed types of forests, except for young stand development phases(Storch et al. 2018).
Surprisingly, structural diversity increased for the entire population of NFI plots for a wide range of harvesting intensities (Fig. 2). A decrease in structural diversity was observed only for harvesting intensities greater than 90% (similar graphs for different types of forests are provided in the Additional file 1). When compared to the average harvesting intensity of this inventory period,which was about 30%, considerably more woody biomass could theoretically be harvested without a reduction in structural diversity. To understand the underlying patterns for the response of the FSI to harvesting intensity,the response of all individual variables of forest structure contributing to the FSI were analysed in relation to the gradient in harvesting intensity (Figs. 3 and 4 and Additional files 2 and 3).
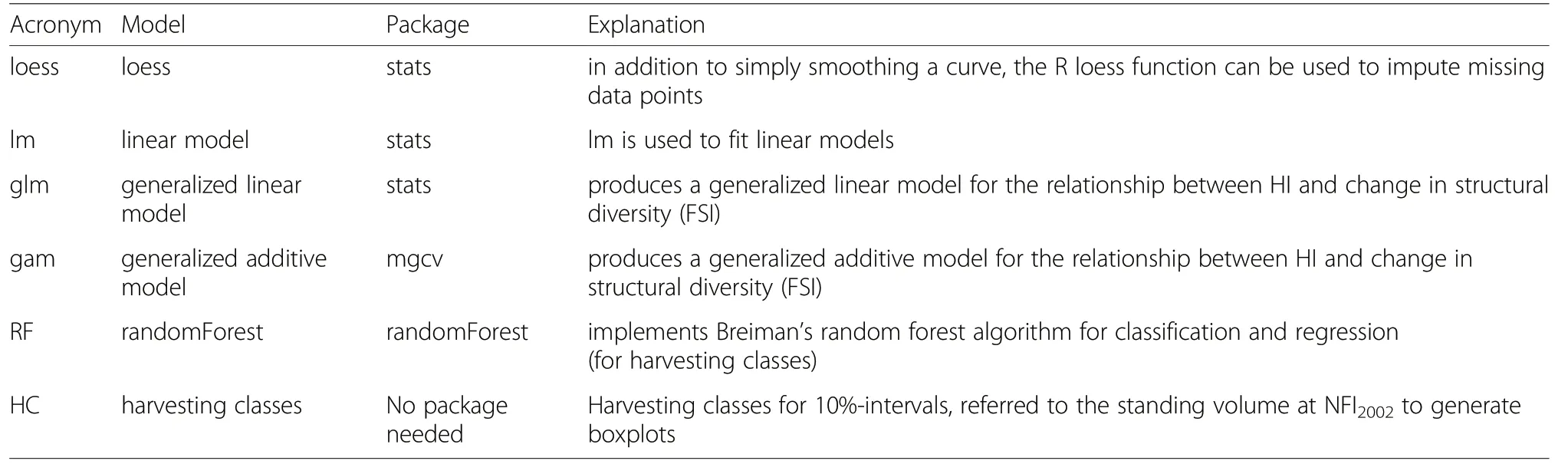
Table 1 List of models tested to explain the relationship between harvesting intensity and changes in structural diversity (FSIchange)
As was the case for the full index, the influence of harvesting intensity on most individual variables used in the FSI was quite small for the entire inventoried forest area of Baden-Württemberg, as well as for the different types of forests (Fig. 3 and Additional files 2 and 3). A reduction in the score of some structural variables like ‘DBHq'or ‘Vol40' was found only at harvesting intensities higher than 60%. Hence, the small influence of HI on structural diversity can be attributed to small changes in the individual variables and not to contrasting responses of different variables that outweigh each other. For forest inventory plots with no recorded harvest in the latest inventory period (4411 plots out of 12,918 sampled plots),changes in individual variables over the period of 10 years tended to be positive or neutral, but were quite variable. As shown in Fig. 4, relatively small changes were found for structural variables such as ‘number of decay classes' and ‘mean diameter of downed deadwood',indicating a limited dynamic in these aspects of structure; e.g. changes in decay classes need several years to decades to occur. Other variables like ‘volume ha-1of large living trees with a DHB ≥40 cm' showed relatively strong changes, indicating that these variables might change relatively fast or at least within a period of 10 years (e.g. after storm or drought). Changes in all individual variables used in the FSI for different forest types can be found in Additional file 3.
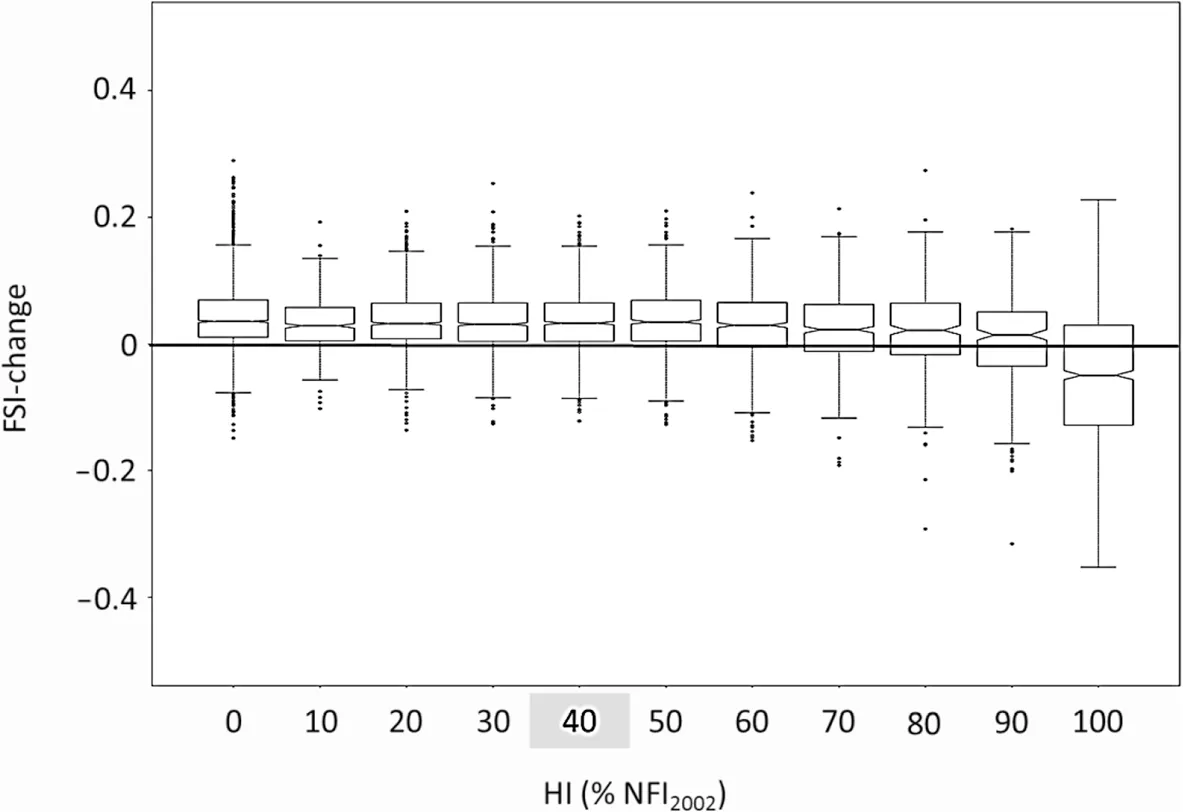
Fig.2 Boxplots for changes in the Forest Structure Index in relation to harvesting intensity(in%of standing timber volume per ha of NFI2002,depicted in 10%-intervals). The grey square on the x-axis indicates average harvesting intensity for the entire forest area of Baden-Württemberg for the 10-year period between NFI2002 and NFI2012(30.8%);black horizontal line indicates no change in the FSI

Fig.3 Changes in ranges of individual variables of forest structure with harvesting intensity for national forest inventory plots from Baden-Württemberg; DBHq:quadratic mean diameter at breast height,DBH sd:standard deviation of diameter at breast height,Height sd:standard deviation of stand height,SR:species richness,SR Reg:species richness in regeneration layer,Bark:diversity of bark types,Flower:diversity of flowering trees,Vol40:occurrence of large living trees with a DBH ≥40 cm,DW s:mean DBH of standing deadwood, DW d:mean diameter of downed deadwood and N DC:number of decay classes
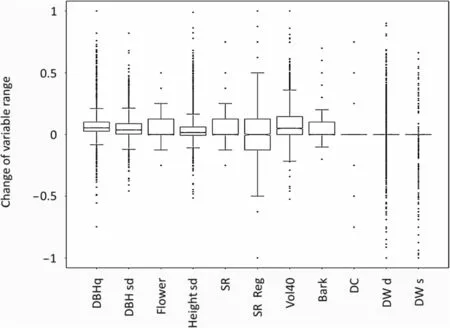
Fig.4 Relative changes in values for individual variables of forest structure for plots with no harvesting activity(HI=0)over the most recent inventory period(2002-2012)for Baden-Württemberg;DBHq:quadratic mean diameter at breast height,DBH sd:standard deviation of diameter at breast height,Height sd: standard deviation of stand height,SR:species richness,SR Reg:species richness in regeneration layer,Bark:diversity of bark types,Flower:diversity of flowering trees,Vol40:occurrence of large living trees with a DBH ≥40 cm, DW s:mean DBH of standing deadwood, DW d:mean diameter of downed deadwood and N DC:number of decay classes
The influence of HI on structural diversity differs with stand development phases, as can be seen for the comparison between the first (mean DBH ≤20 cm) and second stand development phase (mean DBH between 20 and 50 cm) (Fig. 5). In young stands, structural diversity responds considerably more sensitive and negative to harvesting intensity than in middle-aged stands, mainly caused by decreasing values for the variables ‘quadratic mean diameter at breast height' and ‘volume of trees with a DBH ≥40 cm'.
Except for young stands (SDP1 - mean DBH <30 cm), HI could be theoretically increased without a loss in structural diversity. In addition, structural diversity in conifer-dominated stands seems to be less influenced by HI than in broadleaf-dominated forest stands. For coniferous stands, especially spruce-dominated stands in the second stand development phase(SDP2 - mean DBH between 30 and 50 cm), HI may be doubled before a loss in structural diversity occurs.
Discussion
Assessing changes in structural diversity based on NFI data
Using the NFI-based index of structural diversity (FSI)(Storch et al. 2018), an assessment of the influence of previous harvesting intensities on forest structural diversity can be performed. Theoretically, this approach may be used to identify for different types of forests thresholds of harvesting intensity before a reduction in the overall structural diversity occurs. Our results show, that structural diversity, as it was quantified here, is not very sensitive to harvesting intensity. Hence, in a sustainable forest management framework, other indicators that are more directly linked to the sustained provision of ecosystem services would be considered more important for determining upper limits of harvesting intensity. At a local level, limits to harvesting intensities may be set by changes in more sensitive variables such as the volume of large trees,if they present habitats for rare and endangered species like species such as those depending on deadwood of large dimensions or large trees including habitat characteristics (Bütler and Schlaepfer 2004; Bußler et al. 2007).
One major outcome of our analysis is that harvesting does not automatically reduce the level of structural diversity. Especially harvesting intensities lower than 20%-30% of the standing volume in NFI2002have led to a slight increase or maintenance of structural diversity in most types of forest ecosystems (Additional file 1). This perhaps surprising observation may be explained by a number of points.
First, the index of structural diversity aims at a comprehensive cover of structural attributes that provide important habitat components of forest-dwelling species(Storch 2018; Penone et al. 2019). Unlike other indices(e.g. the ‘Old-Growth Index' developed by Acker et al.(1998)) it is therefore not focussed on structural attributes of old forests, which are often in the focus of biodiversity conservation approaches. An index focussing on such old-growth attributes may be more sensitive to harvesting intensity.
Secondly, in our index, some aspects of structure clearly benefit from harvesting, e.g. tree species richness in the regeneration layer,which increases with additional light demanding species (Hooper et al. 2005; Bauhus et al. 2017b).
Third, we have only analysed the short-term changes(10 years) in forest structural diversity in relation to harvesting intensity. There may be contrasting patterns for short-term and long-term responses to certain harvesting-related impacts. For example, in the short term, harvesting may provide larger amounts of deadwood in the form of tree parts that are not removed than would be found in otherwise undisturbed forests (Meyer 1999).However, in the long run, more deadwood of larger dimension would accumulate in forests with little or no harvesting, where deadwood would be generated by natural disturbances (Christensen et al. 2005). While unharvested, primary forests may have a high degree of structural diversity (Stiers et al. 2018), it may also be high following natural disturbances (e.g. Nagel et al.2006; Thom and Seidl 2016). For example, windstorms or fires create deadwood, increase light conditions near the ground that influence species richness in the regeneration layer and lead to establishment of pioneer tree species that increase tree species richness and the diversity of pollen and fruit production (Hooper et al. 2005;Bauhus et al. 2017b; Hilmers et al. 2018). However,many of the inventory plots without harvesting in the most recent inventory period do not represent strictly protected forest reserves, which might develop into structurally diverse stands in the long term, but merely patches that were excluded from harvesting for that period. In the state of Baden-Württemberg, the number of forest inventory plots where no trees were harvested amounted to 34% of the total number of plots. This contrasts with an area of strictly protected reserves of only 2.8% in the state for 2012. Therefore, many of the plots without harvesting in the most recent inventory period might have been harvested in the previous period (about 76% of unharvested plots for inventory period 2002-2012). In addition, in the majority of strictly protected forest reserves, which cover only a small proportion of Germany's total forest area (Engel et al. 2016), management has ceased only recently or a few decades ago and therefore there has been little time to develop high levels of structural diversity. Hence, we do not necessarily have high levels of those structural variables indicative of old and unharvested forests at zero or low levels of harvesting intensity. This also indicates that long-term monitoring is required to facilitate meaningful interpretation of the relationship between harvesting intensity and structural diversity. Any short-term changes to structural diversity from harvesting will be more pronounced in plots harvested recently before the last inventory,whereas they will be at least partly compensated in plots harvested in the beginning of the last inventory period(see also further below).
Fourth, new management strategies such as ‘retention forestry' have been employed to reduce the influence of harvesting on biodiversity through the deliberate maintenance of important structural elements such as habitat trees and dead wood to sustain structural diversity of forests (Hartsough 2003; Gustafsson et al. 2012). However, as mentioned above, it is not clear to what extent these relatively recent changes have already influenced structural diversity in the last forest inventory.
The assessment of structural diversity in forests using data sampled by the angle count method contains a certain inaccuracy, which is caused by the selective sampling of trees ≥7 cm DBH (Storch et al. 2018). This can be seen in the changes in forest structural diversity that were recorded in individual inventory plots, where actually no harvesting took place (Fig. 5). Here, the changes in FSI at the plot level were partially the result of the applied sampling method (angle count sampling). For example, the highest increase in structural diversity for a single plot without recorded harvesting was 2186% (FSI_NFI2002: 0.01, FSI_NFI2012: 0.26), which was caused by the sampling method and not by substantial changes in natural conditions. In this concrete case, the FSI score increased dramatically as a result of ingrowth of several trees into the collective of trees sampled by the angle count method.
This expected limitation at the plot-level is accounted for by aggregation to forest types using mean values of the developed index and the included structural variables to assess the level of structural diversity and the changes over inventory periods. In addition, the calculated HI can include an inaccuracy that is caused by extrapolating volumes of sampled trees to hectare-values,which is necessary when using angle-count sampling. In one extreme case, the calculated tree volume ha-1at one inventory plot at the time of NFI2002was 142 m3·ha-1, while the harvested volume at this plot in the following inventory period was 258 m3·ha-1, leading to a harvesting intensity of 181% although the volume ha-1at the time of NFI2012was 231 m3·ha-1. The high standing volume of NFI2012at this particular sampling point was attributable to seven newly sampled trees, which were not sampled at NFI2002. The addition of these trees was the result of tree selection by the angle count method, which is based on tree distance and diameter.This extreme example shows the inherent issue of angle count sampling that plot-level information is not representative of the forest stand. This further underlines the need for aggregation of inventory plots to strata.
Another reason for the low impacts of HI on structural diversity might be the variable time span between the time of harvesting and the second assessment of structural variables at the end of the inventory period.For example, harvesting could have taken place directly before the inventory sampling of NFI2012, and thus have an immediate impact on certain variables, or shortly after the previous sampling close to 10 years earlier. In the latter case, the impact of harvesting could have been partially compensated by processes such as tree growth or it may have only become apparent in the form of a more species rich tree regeneration layer. Variables such as ‘quadratic mean diameter at breast height' and ‘volume of trees with a diameter at breast height ≥40 cm'could change immediately after harvesting, while variables such as the ‘number of decay classes' and ‘mean diameter of downed deadwood' might require years to decades to change in their expression. This uncertainty at the level of individual plots can however be overcome by aggregation to different types of forests, and thus increase the reliability of the results. This might partly explain the fact, that inventory plots with a HI of 100%can show a positive change of the FSI. In addition, one limitation of the sampling approach of the NFI and thus the FSI is that it does not account for spatial patterns of structural elements such as trees, although these can contribute considerably to structural diversity in forests(Schall et al. 2018b).
Assessment of harvesting intensities for period NFI2002 -NFI2012 in different types of forests
Our results show that HI could theoretically be increased without a loss in structural diversity for all analysed types of forests, except for young stands (SDP1).Additionally, harvesting intensity for most types of forests was below the annual increment level. Thus, a certain level of intensification would also not exceed the sustainable yield. Standing tree volume increased from 486 million m3at NFI2002(365 m3·ha-1) to 499 million m3at NFI2012(377 m3·ha-1) for Baden-Württemberg(Eltrop et al. 2006; Kändler and Cullmann 2014). Some forest types can theoretically be harvested more intensely than others before a loss in structural diversity sets in. As shown in Additional file 1, broadleaf-dominated forest stands have less potential in this regard than conifer-dominated stands. Structural diversity in beech(Fagus sylvatica L.) and oak-dominated (Quercus spp.)stands appeared to be more sensitive towards HI than spruce-dominated (Picea abies L.) stands (Additional file 1). These differences are mainly caused by different stand characteristics and therefore by different responses of individual variables to harvesting intensity. For example, the variables ‘species richness in the regeneration layer' (caused by a higher species richness of the regeneration layer in broadleaf-dominated stands), ‘diameter at breast height of standing deadwood', ‘standard deviation of stand height' (caused by the fact that broadleafdominated stands are more often multi-layered stands and therefore show a higher expression of this variable as conifer-dominated stands, which are often one-layered stands) and ‘diversity of bark types' all responded more sensitively to harvesting intensity. All other structural variables had more positive changes with increasing HI in broadleaf-dominated stands than in coniferousdominated stands (Additional file 4). Some individual structural variables seem to be not affected by harvesting at all (e.g. standing deadwood or flower diversity). One could argue that these variables should therefore be dropped from the index. Keeping insensitive variables in the index could blur the impact of harvesting on the FSI.That would be true for percent changes but not for absolute changes. However, a reduction of variables to the ones being sensitive to HI was not performed, because all included variables cover an important aspect of structural and taxonomic diversity and should be included in a comprehensive assessment of diversity, even if at this stage, no change was recorded over one inventory period.
Harvesting intensity was derived in our inventory-based study from the volume of trees that were removed. However, this variable does not indicate how much biomass was actually taken from the forest, since variable proportions of residues may remain in the forest. Woody material smaller than 10 cm diameter is not captured as dead wood in the inventory. Hence, the influence of harvesting forest residues smaller than 10 cm on structural diversity and biodiversity,which can be very important(e.g.Ranius et al.2018)cannot be captured by our approach.
Influence of harvests on individual structural variables
Impacts of harvests on individual structural variables included in the Forest Structure Index are quite variable and should be assessed separately. For example, the amount of downed deadwood might increase after harvests, which favours the deadwood depending flora and fauna. As timber harvests produce mainly small-dimensioned deadwood such as in crowns left on site, especially taxonomic groups depending on these small diameters and early decay classes of deadwood will be supported,as e.g.Brin et al.(2011)showed for saproxylic beetle assemblages. Other saproxylic species relying on large dimension deadwood are likely disadvantaged in the long-term by increasing harvesting intensity, if no special provision is made (e.g. Lachat et al. 2013).
Species richness in the regeneration layer responded positively to harvesting, although there was no further increase beyond 20% harvesting intensity. Obviously,given the high average growing stock in the forests, harvesting improves light conditions near the ground and thus facilitates regeneration and establishment of more light demanding species (Boch et al. 2013). This finding is in agreement with those of a recent study that showed that there is no positive effect of aboveground carbon stocks of European broadleaved forests on multi-taxa biodiversity (Sabatini et al. 2019). This study showed that in particular among plant species, more species are lost than gained with increasing aboveground carbon stocks;including those shade-intolerant and drought-tolerant species which may be required for adaptation of forests to climate change (Kunz et al. 2018).
Other variables like the occurrence of large trees with a diameter at breast height ≥40 cm will inevitably be reduced at high levels of harvesting intensity, so populations of species depending on these habitats (e.g.epiphytes)might also be reduced. An overview of all variables applied in the FSI and their changes with increasing harvesting intensity is provided in Additional file 4.Therefore, influences of harvests or harvesting intensity can have totally different impacts on single taxonomic groups or individual species within taxonomic groups.As has been shown by Sabatini et al. (2019) within the taxonomic groups of vascular plants, lichens, fungi,bryophytes, beetles, and birds found in oak and beech dominated forests, the majority of species are not influenced by aboveground biomass C stocks, yet within each of these groups there are some winners and losers of increasing or decreasing stocks.
Regarding the beta- and gamma-diversity of forestdwelling species across different taxonomic groups, a mixture of patches with high and low FSI-values at the landscape-level may be recommended, as is suggested by the analysis of Schall et al. (2018a) for a beech forest landscape in the German Biodiversity Exploratories. Leston et al.(2018)showed that long-term changes of structural elements of forests after different harvesting approaches can provide habitats for different bird species and thus increase the overall species richness when compared to unharvested stands. Therefore, to maintain high-levels of species diversity, a broad spectrum of structural elements and structural diversity should be present at the landscape-level (Okland 1996; Sullivan and Sullivan 2001; Schall et al. 2018a).
The main focus of this study was on the influence of harvesting intensity on forest structural diversity,whereas harvesting methods were not considered because there is no relevant information available in the NFI-data of Germany. Different harvesting methods such as selection cutting (e.g. single tree or group selection) lead to differences in structural diversity of forests too (Siira-Pietikäinen et al. 2001; Rosenvald and Lohmus 2008; Kuuluvainen 2009). Retention forestry can support structural diversity by maintaining certain structural elements or creating them artificially (e.g. standing dead trees or high stumps; Abrahamsson and Lindbladh 2006;Bauhus et al. 2009; Gustafsson et al. 2012). It would be very valuable, if inventories provided information about the influences of different harvesting and regeneration methods on forest structural diversity since this information could be used to evaluate these management systems on a large scale.
Conclusion
Our results show the general possibility to use largescale inventory data like the NFI of Germany to analyse the influence of harvesting intensity on structural diversity of different forest stands.The small influence of harvesting intensity on forest structural diversity and individual structural variables over a period of 10 years was surprising. At the scale of forest types or the entire forest area, the results suggest that harvesting as practiced in the forests of south-western Germany has no negative impact on structural diversity and thus the diversity of habitats. However,at very high harvesting intensities of greater than 70% of the growing stock, structural diversity can be impaired. The monitoring of the influence of harvesting intensity on structural diversity should be continued, considering the medium-to longterm influence, which may be different from short-term responses. The observed trends in the development of aspects of forest structural elements may help to understand changes in the occurrence and abundance of forest-dwelling species.
Additional files
Additional file 1: Boxplots for analysed types of forests in SW-Germany illustrating the influences of harvesting intensities (10%-intervals of standing volume at NFI2002,x-axis) on changes in structural diversity of forests (FSI-Change, y-axis). Black line indicates no change in structural diversity; highlighted number (x-axis) indicates mean harvesting intensity of period 2002-2012. (PDF 490 kb)
Additional file 2: Changes of individual variable ranges (‘variablerange_NFI2012' - ‘variable-range_NFI2002') with increasing HI (HI-classes of 10% referred to NFI2002-value) for forests of whole Baden-Württemberg;mean harvesting intensity of 31% (box ‘40'). (PDF 419 kb)
Additional file 3: Changes of individual variables applied in the FSI(yaxis: change of variable ranges; x-axis: classes for HI of 10%-intervals (0-100%), referred to standing volume at NFI2002),for all analysed types of forests. (PDF 1349 kb)
Additional file 4: Changes of applied variables in the FSI with increasing HI for all analysed forest types; x-axis: increasing HI in 10%intervals of NFI2002standing volume; y-axis: change of variable range.(PDF 1249 kb)
Abbreviations
BL: Broadleaf-dominated forest stand; BW: State of Baden-Württemberg,SWGermany; CF: Conifer-dominated forest stand; DBH: Diameter at breast height (130 cm); DBHq: Quadratic diameter at breast height (130 cm);FORMI: Forest management index;FSI: Forest Structure Index; HI: Harvesting intensity; m3·ha-1: Cubic metre per hectare; NFI2002/2012: German National Forest Inventory of 2002 and 2012;SDP: Stand development phase;SDP1: mean DBH <20 cm (taken from NFI classification);SDP2: mean DBH between 20 and 50 cm (taken from NFI classification); SDP3: mean DBH >50 cm (taken from NFI classification); SW: South-west Germany; Vol40: Volume ha-1of trees with a DBH ≥40 cm
Acknowledgements
Not applicable.
Authors'contributions
FS planned and conducted the study including data management and analysis and wrote the majority of the manuscript; GK provided support in inventory analysis and contributed to the manuscript; JB conceived and guided the study and co-wrote the manuscript.
Authors'information
Not applicable.
Funding
This work was supported by a grant from the Ministry of Science, Research and the Arts of Baden-Württemberg(7533-10-5-78) to Jürgen Bauhus. Felix Storch received additional support through the BBW ForWerts Graduate Program.
Availability of data and materials
The datasets that form the basis of all analyses in our study are free available at https://bwi.info/Download/de/BWI-Basisdaten/ACCESS2003/.The datasets supporting the conclusions of this article are included in the additional files 1-4.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Author details
1Chair of Silviculture, University of Freiburg, D-79085 Freiburg,Germany.
2Department of Biometry, Forest Research Institute of Baden-Württemberg,D-79100 Freiburg, Germany.
Received: 17 April 2019 Accepted: 19 August 2019
- Forest Ecosystems的其它文章
- Effects of forest management on biomass stocks in Romanian beech forests
- The functional complex network approach to foster forest resilience to global changes
- Effects of prescribed burning on carbon accumulation in two paired vegetation sites in subtropical China
- The Chapman-Richards Distribution and its Relationship to the Generalized Beta
- Designing near-natural planting patterns for plantation forests in China
- Editorial Board

