Co-pyrolysis of bituminous coal and biomass in a pressured fluidized bed☆
Yong Huang,Ningbo Wang,Qiaoxia Liu,Wusheng Wang,Xiaoxun Ma*
1 School of Chemical Engineering,Northwest University,229 North Taibai Road,Xi’an 710069,China
2 Hydrocarbon High-efficiency Utilization Technology Research Center of Yanchang Petroleum (Group) Co.Ltd.,Xi’an 710075,China
3 Chemical Engineering Research Center of the Ministry of Education (MOE) for Advanced Use Technology of Shanbei Energy,Xi’an 710069,China
4 Shaanxi Research Center of Engineering Technology for Clean Coal Conversion,Xi’an 710069,China
5 Collaborative Innovation Center for Development of Energy and Chemical Industry in Northern Shaanxi,Xi’an 710069,China
6 International Scientific and Technological Cooperation Base of the Ministry of Science and Technology (MOST) for Clean Utilization of Hydrocarbon Resources,Xi’an 710069,China
ABSTRACT An experimental study on co-pyrolysis of bituminous coal and biomass was performed in a pressured fluidized bed reactor.The blend ratio of biomass in the mixture was varied between 0 and 100 wt%,and the temperature was over a range of 550-650°C under 1.0 MPa pressure with different atmospheres.On the basis of the individual pyrolysis behavior of bituminous coal and biomass,the influences of the biomass blending ratio,temperature,pressure and atmosphere on the product distribution were investigated.The results indicated that there existed a synergetic effect in the co-pyrolysis of bituminous coal and biomass in this pressured fluidized bed reactor,especially when the condition of bituminous coal and biomass blend ratio of 70:30 (w/w),600°C,and 0.3 MPa was applied.The addition of biomass influenced the tar and char yields and gas and tar composition during co-pyrolysis.The tar yields were higher than the calculated values from individual pyrolysis of each fuel,and consequently the char yields were lower.The experimental results showed that the composition of the gaseous products was not in accordance with those of their individual fuel.The improvement of composition in tar also indicated synergistic effect in the co-pyrolysis.
Keywords:Bituminous coal Biomass Co-pyrolysis Pressured fluidized bed Synergistic effect
1.Introduction
The pyrolysis of bituminous coal is an ideal method for producing liquid fuels and other chemicals characterized by the high volatility,low content of sulfur,phosphor and ash,but the yield and quality of pyrolysis products are actually relatively poor due to the low hydrogen-to-carbon ratio[1,2].Accordingly,it is necessary to supply hydrogen for coal from other resources.The coutilization of coal with hydrogen-rich materials offers a number of advantages.Previous studies on the co-pyrolysis of coal with polymers,plastic wastes and other materials with similar characteristics had shown synergetic effects that improve the quality of the pyrolysis products and increase the yield of coal tar.
Compared with other resources,biomass is a renewable green energy and environmentally friendly resource.Besides,its high thermochemical reactivity and high content of volatile matters facilitate the pyrolysis reaction.Biomass with high hydrogen-to-carbon ratio acts as the hydrogen donor in the process of co-pyrolysis and synergetic effects might be expected.Co-pyrolysis is gradually perceived as useful in improving the tar yield and reducing emissions of NOX,SOX,and volatile organic compound [3-5].Therefore,the joint use of biomass and coal is an important practical direction for efficient,clean and sustainable use of coal in the future[6,7].
The studies on the co-pyrolysis are still a debatable field;it mainly focused on possible synergistic effects existed in the copyrolysis to improve coal thermochemical conversion.The experiments on the synergistic effect of the co-pyrolysis were carried out by using a range of operating parameters,reactor types and raw materials,formed different opinions consequently.
Evidences for synergistic behaviors in the co-pyrolysis are relatively unconvincing.Lacks of synergies have been reported with low heating rates and a small contraction of the samples.Vuthaluru [8]performed experiments on the thermal behavior during co-pyrolysis of subbituminous coal and biomass blends prepared at different ratios using a thermogravimetric analysis.No interactions were found between the coal and biomass during co-pyrolysis,and the pyrolytic characteristics of the blends followed those of the parent fuel in an additive manner.Moghtaderi[9]investigated the pyrolytic characteristic of biomass and coal at low heating rate in a horizontal tubular reactor.The experimental results indicated that there are no synergetic effects,the yields of the major pyrolysis products and the composition of the gaseous products from blended samples was linearly proportional to the percentage of biomass and coal in the mixture.Meesri [10]also confirmed the lack of synergistic effects on pyrolytic product yields from co-pyrolysis of coal/sawdust under both low heating rate in a fixed-bed reactor,maybe owing to the different temperature ranges from devolatilization of biomass and coal,the spatial segregation of samples particles and the short residence time.
However,there have been some studies concerning the rapid co-pyrolysis,and some evidences of synergistic effects are reported.Jones et al.[11]observed the synergistic effect in tar composition in 100 g scale batch pyrolysis experiments of high-volatile bituminous coal and pinewood,which was thought due to the sufficient contact time of volatiles.The synergies are observed on the tar composition analysis by GC-MS.Suelves and Moliner [12]found the synergistic effects during the co-pyrolysis of coal and petroleum residue by pyrolysis-gas chromatography in a pyroprobe,in which the aliphatic fraction of the petroleum residue played an important role in production of light olefins and aromatic compounds.Zhang [13]conducted co-pyrolysis of lignite and legume straw in a free fall reactor over a range of 500-700°C under atmospheric pressure with nitrogen as balance,observed the synergistic effect on the overall weight loss yield and characteristics of pyrolysis products.
As mentioned above,the occurrence of synergy during copyrolysis is generally not conclusive.It could be reckoned that no synergistic effect was observed between biomass and coal with low heating rate,and the fact of the pyrolysis temperature ranges are different from each other considerably indicated that two pyrolysis processes were well separated and the rich hydrogen in biomass was not utilized effectively in coal pyrolysis [14,15].Because of rapid heat transfer and uniform temperature distribution in a fluidized reactor,different devolatilization of biomass and coal could happen simultaneously at a similar temperature under rapid heating rate pyrolysis.Therefore,it can be deduced that the synergistic effect may exist if the co-pyrolysis of biomass and coal occurs in a fluidized reactor.
Previous investigations on co-pyrolysis have mostly concentrated on the impact of synergistic effects on the yields of major pyrolysis products under atmospheric pressure in fixed-bed reactors,free fall reactors and entrained reactors.Co-pyrolysis in the pressurized fluidized reactor,which is easy to couple with most types of gasifier,is researched relatively rare,especially few studies on fundamental issues such as the pressure influence of synergistic effects on the yields and composition of the pyrolysis products in the pressurized fluidized reactor have been done.The fundamental knowledge gained from pressured fluidized bed is essential for the proper understanding of co-pyrolysis behaviors of coal and biomass which can couple with the existing combustion or gasification process.
The objective of this study is to investigate the co-pyrolysis behaviors of bituminous coal and biomass in such a pressured fluidized bed reactor,especially to judge the synergism of the copyrolysis.The experimental results have shown some obvious synergies,mainly observed on the yields of the major co-pyrolysis products,the components of gas and tar from pressured copyrolysis process.
2.Experimental
2.1.Samples
Four kinds of materials,Wheat Straw (WT),Walnut Shell (WS),Wood Chip(WC)and Xiwan bituminous coal(XW),were chosen as feedstock in the study.The samples were sieved,classified,and dried for 8 h at 110°C,the particle sizes of both coal and biomass were <0.3 mm,and then stored in the hoppers for the test.The mass ratios of biomass were 0,20%,30%,40%,50%,70% and 100%,respectively.Proximate and ultimate analyses of samples were listed in Table 1.
2.2.Experimental apparatus and procedure
The co-pyrolysis was performed in a fluidized bed reactor,and its schematic diagram was shown in Fig.1.The design and material of the reactor (Incoloy Alloy 800 HT:42 mm i.d.,1500 mm long,7 mm wall thickness) can withstand pressure up to 1.0 MPa at 700°C.The reactor was heated by an electrical furnace,the heating rate was about 50°C·min-1.A set of three thermocouples detected temperatures of the different height zones in the reactor.Coal and biomass were stored with a certain blend ratio in the hopper,and continuously fed at a rate of 2.0-5.0 kg·h-1into a 300 g sand fluidized bed(approximate height:20 mm)in the reactor by conveying gas,fluidizing gas and the vibrator outside of the hopper.Copyrolysis characteristics were investigated in the co-pyrolysis temperature range from 400°C to 700°C in 1.0 MPa pressure.The conveying gas and fluidizing gas used were nitrogen/carbon dioxide with flow rates of 10-20 L·min-1and 20-50 L·min-1respectively.The residence time of the co-pyrolysis volatiles was generally estimated to be less than 2 s.
The solids used in the experiments were biomass,coal and sand mixture;their physical properties were listed in Table 2.Only the co-pyrolysis production from biomass and coal can be elutriated,but sand particles were too large to be carried over from the bed with the fluidizing gas.
For every experimental run,the whole system was purged with nitrogen for about 20 min,the flow rates of the fluidizing gas and conveying gas were fixed at 10 L·min-1and 20 L·min-1.Then the fluidized bed (reactor) was heated to a target temperature and pressurized to the specified value with the fluidizing gas by adjusting the back pressure valve.In addition,the hopper (with coal or biomass) was pressurized by the fluidizing gas and its pressure was maintained to a higher value than that of the fluidized bed so as to feed coal or biomass into the fluidized bed continuously.The gas depressurized to normal pressure by the back pressure valve and flowed into the gas sample bag or vented out.
The solid residues produced during the co-pyrolysis process were removed and then weighed from the reactor and char collectors.The yields of the liquid were calculated based on the difference between the weight of the tar tank and the scrubbing oil tank.The yield of co-pyrolysis gas was determined from the overall mass balance.The measurement errors were found to be within±1%according to the repetition experimental results for each set of conditions.

Table 1 Proximate and ultimate analysis of samples

Fig.1.Schematic diagram of pyrolysis procedure.

Table 2 Properties of particle used in the experiment
The gas composition was analyzed by gas chromatography(GC-2014,SHIMADZU).Proximate and ultimate analyses of chars were carried out with a CHNS/O Elemental Analyzer (Vario Micro cube,Germany).The chemical composition of the tar was characterized by a gas chromatography mass spectrometry detector (GC/MSQP2010,SHIMADZU).
2.3.Product yield calculation
To determine the synergetic effect during the co-pyrolysis,the calculated values were obtained through the following equation:

where BR was the blending ratio of biomass ranging from 0 to 100%,Ybiomassand Ycoalwere the experimental values from the individual pyrolysis of biomass and coal,and Yproductwas the calculated value.
3.Results and Discussion
3.1.Pyrolysis behaviors of individual
Fig.2 showed the production behaviors of tar,char and gas from each of the two fuels over the temperature ranges of 350-650°C,pressure ranges of 0.1-0.7 MPa and nitrogen/carbon dioxide atmospheres.Pyrolysis of bituminous coal and biomass demonstrated some similar variation trends on pyrolysis product distribution.The remarkable difference between the two fuels can be seen in the quantity of the product yields and the temperature range at which the bulk of devolatilization took place.More volatiles were generated from the pyrolysis of biomass than that of coal under the same condition,thus biomass could produce more gas and liquid when compared with coal.
For coal and biomass,with the increase of temperature,the yield of gas increased and the yield of char decreased,both the yields of tar/biomass oil were first increased and then decreased.The maximum values of tar and biomass oil were 10.50% at 600°C and 33.70% at 500°C,respectively.The pyrolysis reaction acted a leading role as temperatures less than 600°C for coal and 500°C for biomass and a great amount of molecule hydrocarbons from aliphatic side chains and oxygenic functional group were generated,and the condensation reactions gradually occurred with the increase of temperature [16-18].
With the increase of pressure,an improvement of the char yield and a reduction of the tar/biomass oil can also be observed.These results could be explained by the fact that an increase in the pressure promoted the formation of char due to an increase of the residence time of the vapor phase within the solid particles,promoting secondary cracking and char polymerization reaction in the solid surface,which were consistent with the results of the conclusions by Matsuoka [19],Megaritis [20]and Wall [21].

Fig.2.Influences of (a) coal-temperature (0.3 MPa,N2),(b) biomass-temperature (0.3 MPa,N2),(c) coal-pressure (600°C,N2),(d) biomass-pressure (500°C,N2),(e) coalatmosphere (600°C,0.3 MPa) and (f) biomass-atmosphere (500°C,0.3 MPa) on the product yield.
Compared with nitrogen atmosphere,both for coal and biomass,product yields of tar/biomass oil and gas were higher in carbon dioxide atmosphere with the same conditions.This was probably due to the difference in the molecular structure of two atoms,carbon dioxide molecules with higher heat capacity could carry much more energy compared to that of nitrogen under the same condition,the heat transfer was uniformly distributed and pyrolysis reaction fully occurred.More importantly,carbon dioxide molecules occupied the active points of chars and decreased the secondary reaction intensity of primary pyrolysis products.This conclusion was consistent with the observation of Gao Songping,who showed that the introduction of carbon dioxide promoted the coal pyrolysis and generation of volatile [22].
3.2.Co-pyrolysis behavior
The influences of blending ratio,temperature,pressure and atmosphere on the yields of tar,char and gas generated from the co-pyrolysis were described in Figs.3-8.The straight lines in the figures indicated the calculated values of those from pyrolysis of each individual fuel,and the discrete point values showed the experimental data obtained from the co-pyrolysis under different conditions.

Fig.3.Influence of blend ratio (600°C,0.3 MPa,N2) on the product yield.

Fig.4.Influence of temperature (BR 30%,0.3 MPa,N2) on the product yield.

Fig.5.Influence of pressure (BR 30%,600°C,N2) on the product yield.
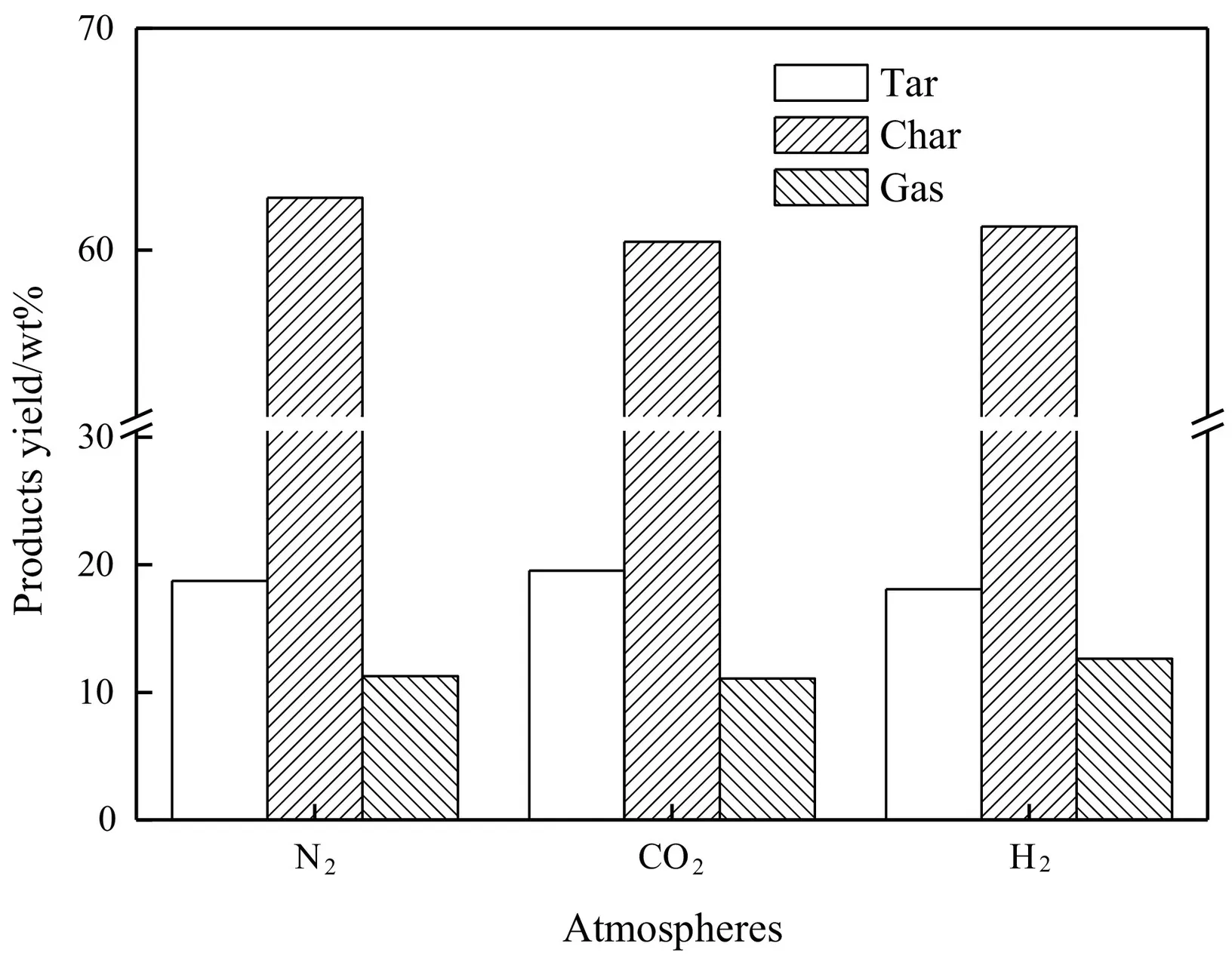
Fig.6.Influence of atmosphere (BR 30%,600°C,0.3 MPa) on the product yield.

Fig.7.Influence of biomass species (BR 30%,600°C,0.3 MPa,N2) on tar yield.
As Fig.3 showed,the yields of chars and gas were lower than the calculated values and consequently the yields of tar were higher.Moreover,the experimental condition of 600°C and BR 30%led to the lower yields of char (decreased by about 4.5%) and the higher yields of tar (increased by about 7.2%) as a consequence.It is obvious that there exist synergies during the co-pyrolysis of bituminous coal and biomass.This may be because of sufficient hydrogen donors,the alkali and alkaline earth metals (AAEM) in biomass acted hydrogenation and catalytic roles on coal pyrolysis,respectively,resulting in the remarkable synergetic effect during the co-pyrolysis [9].However,it was clear that the synergistic effect was reduced at a higher biomass blending ratio.This signified that covering the surface and pores of coal by the excess volatiles produced from a high biomass blending ratio,leading to the restraining the heat transfer rate and inhibiting the effusion of the volatiles during the coal pyrolysis.
As Fig.4 demonstrated,the yields of char and gas were lower than the calculated values,and yields of tar were relatively higher.Especially at 600°C,the yield of char decreased by more than 4%and that of tar increased by more than 7%,respectively,when compare with the calculated values.The noticeable synergies occurred under the condition of BR 30% and 0.3 MPa,this can be attributed to the fact that the proper temperature for coal would suppress secondary reaction (such as re-polymerization and cross-linking reaction)and produce plenty of free radicals,this would also guaranteed the biomass supply the hydrogen as much as possible,both hydrogen donors and free radicals achieved an optimal balance point,thus the noticeable synergies could be observed in copyrolysis [23].

Fig.8.Influence of (a) blend ratio (600°C,N2,0.3 MPa) and (b) temperature (BR 30%,N2,0.3 MPa) on relative gas components.

Table 3 Proximate and ultimate analysis of co-pyrolysis chars at different pressure (BR 30%,600°C,N2)
Fig.5 described an unobvious influence on the hydrogenation process for bituminous coal pyrolysis with low hydrogen partial pressure observed.It was indicated that increasing pressure slowed the release rates of volatiles,therefore reduced the tar yield and changed the gas yields.Pressurized pyrolysis has dual roles[24],and high hydrogen pressure was favorable for hydrogenation reaction of free radicals.Moreover,higher pressure resulted in the increase of internal mass transfer resistance and inhibited the escape of larger tar molecules that might evaporate at low pressure,so the secondary cracking and char polymerization were aggregated,and caused the tar yield reduction and char yield increment.
Fig.6 demonstrated that,the tar yield from carbon dioxide atmosphere was higher,and the char yield was lower than those in the nitrogen and hydrogen atmospheres.This can be attributed to the same fact that carbon dioxide molecules have a higher heat capacity that could carry considerable energy to achieve the pyrolysis reaction,and decrease secondary reaction intensity of primary pyrolysis products.In the hydrogenation atmosphere,excess hydrogen radicals reacted with oxygen from biomass and generated plenty of pyrolysis water by reverse water-gas shift reaction,resulting in tar yield decrease.
Fig.7 described the synergetic effects among the XW/WS,XW/WC and XW/WT blends,and tar yields were all higher than 18.5%in the co-pyrolysis.The enhancement of coal decomposition could be attributed to the acquisition of hydroxyl,hydrogen radicals,and AAEM species from the biomass [25].The amount of released hydroxyl and hydrogen radicals from WT was higher than the ones from WS and WC,and it would be due to the high hydrogen-tocarbon ratio of WT.Moreover,the relatively high AAEM content in WT would promote the coal pyrolysis,and in particular the silicon contents were higher in WT than in WS and WC.During copyrolysis,some of the silicon contents were released as a volatile and played a catalytic role in the pyrolysis through the promotion of secondary decomposition.
3.3.Gaseous components
The synergistic effects on the relative gas components during the co-pyrolysis were also investigated in Fig.8.With the increase of blend ratio,the yields of CO,CO2and H2were increased,while the yield of CH4was declined.Compared with the calculated values,the gaseous component yields of H2and CH4were lower,while the yields of CO and CO2were higher than the calculated values over the whole blend ratio ranges.It can be inferred that in the co-pyrolysis,both hydrogen and methyl radicals participated in the interaction between coal and biomass,increasing the yield of liquid products.The higher content of CO and CO2than the calculated values may be related to the reduction in the yield of gas products.
With the increase of temperature,the contents of H2,CO and CH4were increased continuously,and the CO2content expressed a little change.It can be seen that the experimental yields of CO and CO2were higher,while the yields of H2and CH4were lower than the calculated values.It showed that the interaction between the coal and biomass in the co-process,and individual pyrolysis behavior of two was not isolated independently.Moreover,the interaction mechanism of pyrolysis intermediate products with different temperatures might not be similar,it is necessary to conduct further research by analyzing the properties of tar and char products.

Fig.9.Influence of pressure on pyrolysis oil quality.

Table 4 The composition analysis of three kinds of oil (wt%) (BR 30%,600°C,0.3 MPa,N2)
3.4.Char analysis
Proximate and ultimate analyses of co-pyrolysis chars from different pressure were listed in Table 3.It can be seen that an increase of the carbon content and a decrease of the hydrogen content of co-pyrolysis chars can be observed when the pressure was increased.This was probably due to the extended residence time of the vapor phase within the solid particles,promoting secondary cracking and char polymerization,resulting in volatile content reduction and fixed carbon content promotion respectively,which were consistent with the results of the carbon and hydrogen content in the coal pressured pyrolysis by Ju [26].
3.5.Pyrolysis oil components
Fig.9 showed the influence of pressure on co-pyrolysis oil under the condition of bituminous coal and biomass blending ratio of 70:30 (w/w),600°C and nitrogen atmosphere.The yield of oil should be increased in the hydrogen atmosphere,which is consistent with the results in our experiments.In addition,with the increase of hydrogen partial pressure,more light fraction(<350°C) was obtained,and the quality of oil was improved correspondingly.
As showed above,the synergistic effects under the pressure of 0.3 MPa on production distribution were obvious,thus an experimental study on tar composition analysis among tar,biomass and co-pyrolysis oil under the pressure of 0.3 MPa was performed.
From Table 4,the tar mainly consisted of aromatic(42.52%),aliphatic (29.03%),phenol (13.80%) and O,N,and S compounds(14.65%),respectively.For biomass oil,it had a higher relative content of O,N,and S compounds (44.09%).While for blends of the samples,the phenol and O,N,and S compounds of the copyrolysis oil were different from that of the other samples.These can be explained by the fact that some interactions between volatiles of coal and biomass existed in the co-pyrolysis process.The volatile would preferentially interact with the coke to form the phenolate groups over the char surface [27,28].
The top 20 main components of three kinds of oil were listed in Table 5.Tar mainly contained phenol,naphthalene,olefins and alkanes.Oxygenated compounds were reported as main components in biomass oil.For co-pyrolysis oil,the components were relatively consistent with the tar,having an abundance of naphthalene and phenol.There might be two possible explanations for this.One was that the proportion of coal was higher.The other was that the proportion of oxygenated compounds was transformed into water and carbon dioxide,which was also consistent with the fact that phenol content was decreased [29].Therefore,distribution of oxygenated compounds in co-pyrolysis oil was mainly concentrated in phenol,methyl,ethyl phenol and dimethyl phenol,the relatively content was showed in Table 6.
A synergistic effect was found in phenol compounds when comparing species in co-pyrolysis oil with those in tar.Copyrolysis oil mainly contained phenol (16.82%),2-methyl-phenol(9.67%),p-cresol (21.92%),2-4-dimethyl-phenol (13.21%) and catechol (16.56%),which were consistent with the reported results.Wei Ligang[30]verified that the content of phenol,methyl phenol,dimethyl phenol and derivatives had increased by 5% than thecalculated values.Zhang Li [13]showed that the phenolic component with relatively simple structure increased in co-pyrolysis oil and hydrogenated aromatic content,decreased in aromatic hydrocarbon content,and the quality of tar was improved.

Table 5 Top 20 main compositions in three kinds of oil①(BR 30%,600°C,0.3 MPa,N2)

Table 6 Relative content of phenol in phenolic compounds (BR 30%,600°C,0.3 MPa,N2)

Table 7 Ultimate and physical analysis of samples (BR 30%,600°C,0.3 MPa,N2)
As discussed above,the noticeable synergies always could be obtained from the ultimate analysis of tar composition,the aromatic and aliphatic hydrocarbon yields of co-pyrolysis oil have greatly improved compared with that of biomass,and polycyclic products were hardly detected,while anthracene,phenanthrene and fluorine in the tar were distinctly existed,it demonstrated that molecule substance in the biomass was cracked into small ones by hydrogenation.From Table 7,for co-pyrolysis oil,carbon content was varied from 82.00%in tar to 71.16%in co-pyrolysis oil,and sulfur content was also reduced to 0.31%,which was removed in the form of H2S.Thus it can be seen that co-pyrolysis oil has better quality by hydrogenation,which provided an appropriate route to extract fine chemicals to manufacture the fuel.
4.Conclusions
The co-pyrolysis of bituminous coal and biomass at various blend ratio,temperature,pressure and atmosphere were carried out in a pressured fluidized bed reactor to investigate the occurrence of the synergistic effects.Compared with the calculated values,the tar yield increased and consequently the char yield decreased,the composition of the gaseous product and tar was not linearly proportional to the percentage of their individual fuels,indicating that there exist synergetic effects between bituminous coal and biomass in the pressured fluidized bed reactor.
Especially,the obvious synergetic effect was observed under the condition of bituminous coal and biomass blending ratio of 70:30(w/w),600°C,0.3 MPa and nitrogen atmosphere.The yield of char decreased by about 4.5% and that of tar increased by about 7.2%compared with the calculated values.The gaseous components such as the experimental yields of H2and CH4were lower,while the yields of CO and CO2were higher than calculated values.The phenol compounds of the co-pyrolysis oil were increased and relatively concentrated than those of their individual fuel,the aromatic and aliphatic hydrocarbon yields have greatly increased,which confirms the synergies in the co-pyrolysis of bituminous coal and biomass.
 Chinese Journal of Chemical Engineering2019年7期
Chinese Journal of Chemical Engineering2019年7期
- Chinese Journal of Chemical Engineering的其它文章
- Microencapsulated ammonium polyphosphate by polyurethane with segment of dipentaerythritol and its application in flame retardant polypropylene☆
- Distributed control and optimization of process system networks:A review and perspective☆
- Heat exchanger network synthesis integrated with flexibility and controllability☆
- Synthesis of flexible heat exchanger networks:A review☆
- Simulation and heat exchanger network designs for a novel single-column cryogenic air separation process☆
- A review of extractive distillation from an azeotropic phenomenon for dynamic control☆
