二维材料膜在气体分离领域的最新研究进展
程龙,刘公平,金万勤
材料化学工程国家重点实验室,南京工业大学化工学院,南京 210009
1 Introduction
Graphene brings a new age of two-dimensional (2D)materials. Other 2D materials, including metal-organic frameworks (MOFs), layered double hydroxides (LDHs),transition metal carbides (TMCs) and graphdiyne are widely explored in electronics/optoelectronics, catalysis, energy storage and sensing platforms1. Owing to their unique atomic thickness and micrometer lateral dimensions, 2D nanosheets have become emerging nano-building blocks for separation membranes featuring distinct laminar structures and tunable mass-transport performance2. The ultimate thickness of nanosheets makes membrane as thin as possible, which will minimize transport resistance and maximize permeation flux. In addition, the aperture or channels can be precisely manipulated within nanoor even subnano-size for molecular separation, such as gas separation. This perspective highlights some latest progresses in 2D material membranes for gas separation and analyzes the challenges that must be overcome to further advance this new family of membranes for molecular separation.
2 Two-dimensional-material membranes for gas separation
2.1 Graphene-based membranes
The unique one-atom thickness of graphene impelled researchers to explore the gas transport through monolayer graphene. At first, the monolayer graphene membrane, however,was demonstrated to be impermeable to gases as small as helium3.This is due to the fact that graphene’s π-orbitals form a dense,delocalized cloud that blocks the gap within its aromatic rings.The impermeable property motivated the theoretical and experimental studies on creating nano-size pores on graphene membrane for gas separation. Jiang et al.4firstly investigated the gas permeability and selectivity of graphene sheets with designed subnanometer pores using first principles density functional theory calculations. For the designed N-functionalized (0.30 nm) and the all-H pores (0.25 nm) oneatom-thick graphene sheets, H2/CH4 selectivity was as high as the order of 108and 1023, respectively. Bai and co-workers5also explored the selective permeation of gas molecules through the graphene nanopore. The macroscopic and selective porous graphene membrane was firstly prepared by ultraviolet-induced oxidative etching technique6. The obtained membrane shows H2 permeance of 4.5 × 10-23mol·m-2·s-1·Pa-1and H2/CH4selectivity of 104, partially agreeing with simulation results. To realize large-scale physical perforation, Celebi et al.7developed an optimized CVD growth method followed by a focused ion beam approach to prepare bilayer graphene with narrowly distributed pore sizes ranging from < 10 nm to 1 μm and a large number of pores (~103to 106per membrane). As a result, they observed orders-of-magnitude enhancements for gas, water, and water vapor permeances.
Among the 2D material family, graphene oxide (GO), the derivative of graphene, is most widely studied for membrane separation, since its oxygen-containing groups besides make GO dispersible in water for easy processibility, also provide convenient sites for enhancing interactions with specific gas molecular. Few-layered GO membranes were firstly reported for gas separation8, and demonstrated the feasibility of ultrathin 2D laminar membranes. Molecular transport through the 2D materials-assembled laminate occurred in in-plane slit-like pores and then plane-to-plane intergalleries. However, the oxygencontaining groups distributing on GO nanosheets would cause electrostatic repulsive forces between nanosheets, resulting in disordered stacking structure in-plane and excessive interlayer spaces that cannot afford sufficient selectivity.
It is challenging to precisely control the GO channels within sub-nanometer size that is necessary for gas separation. In this case, we considered the intrinsic forces, that was repulsive forces between GO nanosheets, must be conquered by additional forces, so we rationally designed external forces applied both outside and inside the GO laminate to manipulate the structure of stacked nanosheets (Fig. 1)9. The “outer” external forces(EFs) referred to compressive, centrifugal, and shear forces,which were applied outside GO laminate; the “inner” EFs refer to molecular interactions that were applied inside the laminate,which was realized by building up molecular interactions via step-by-step depositing GO nanosheets and polymer molecules.As a result, they were synergistic to overcome the intrinsic repulsive electrostatic interactions between GO layers, leading to a highly ordered GO channels with an empty interlayer height of ≈0.4 nm for fast transport and selective sieving of gases. The subnanosized channels could offer a 2- to 3-orders of magnitude higher H2permeability and 3-fold enhancement in H2/CO2selectivity compared to commercial membranes. This work contributed a notable step to push 2D-material membranes toward implementation for precise molecular separation. Recently, we also reported the cationic control of the interlayer spacing of graphene oxide membranes with ångström precision10, which probably becomes another alternative for constructing the desired intergallery for gas separation.
Another efficient way for manipulating GO channels is crosslinking, which was first proposed for water treatment11and pervaporation12. Jiang and co-workers13fabricated ultrathin(< 10 nm) GO membranes by borate-crosslinking and realized the facilitated transport of CO2molecular. The interlayer spacing between two-dimensional GO nanosheets could be accurately tailored by covalently bonded borate groups and associated water of hydration. The borate groups could trigger reversible reactions with CO2molecules, facilitating their transport. In addition, humidified feed gases could control water content within the stacked GO nanosheets, forming desired sieving channels. Specifically, the GO membrane crosslinked by borate at 70 °C and tested in the wet state obtained a channel size of 0.35 nm, which rightly lies between the kinetic diameters of CO2(0.33 nm) and CH4(0.38 nm) molecules, enabling size sieving between the two gases. The as-prepared membrane exhibited a high CO2permeance of 650 GPU (gas permeation unit, 1 GPU =3.348 × 10-10mol·m-2·s-1·Pa-1), and a CO2/CH4 selectivity of 75,which is currently the best performance reported for GO-based composite membranes. Zhou et al.14employed a facile vacuum filtration coating process to prepare sub-20-nm GO hollow fiber membranes with CO2-philic agent (piperazine) alternating between GO layers for highly efficient CO2/N2 separation.Piperazine was introduced as a carrier-brush into the GO nanochannels with chemical bonding. The resulting CO2-facilitated-transport membrane exhibited excellent separation performance under simulated flue gas conditions with CO2permeance of 1020 GPU and CO2/N2selectivity as high as 680,demonstrating great potential for practical CO2 capture.
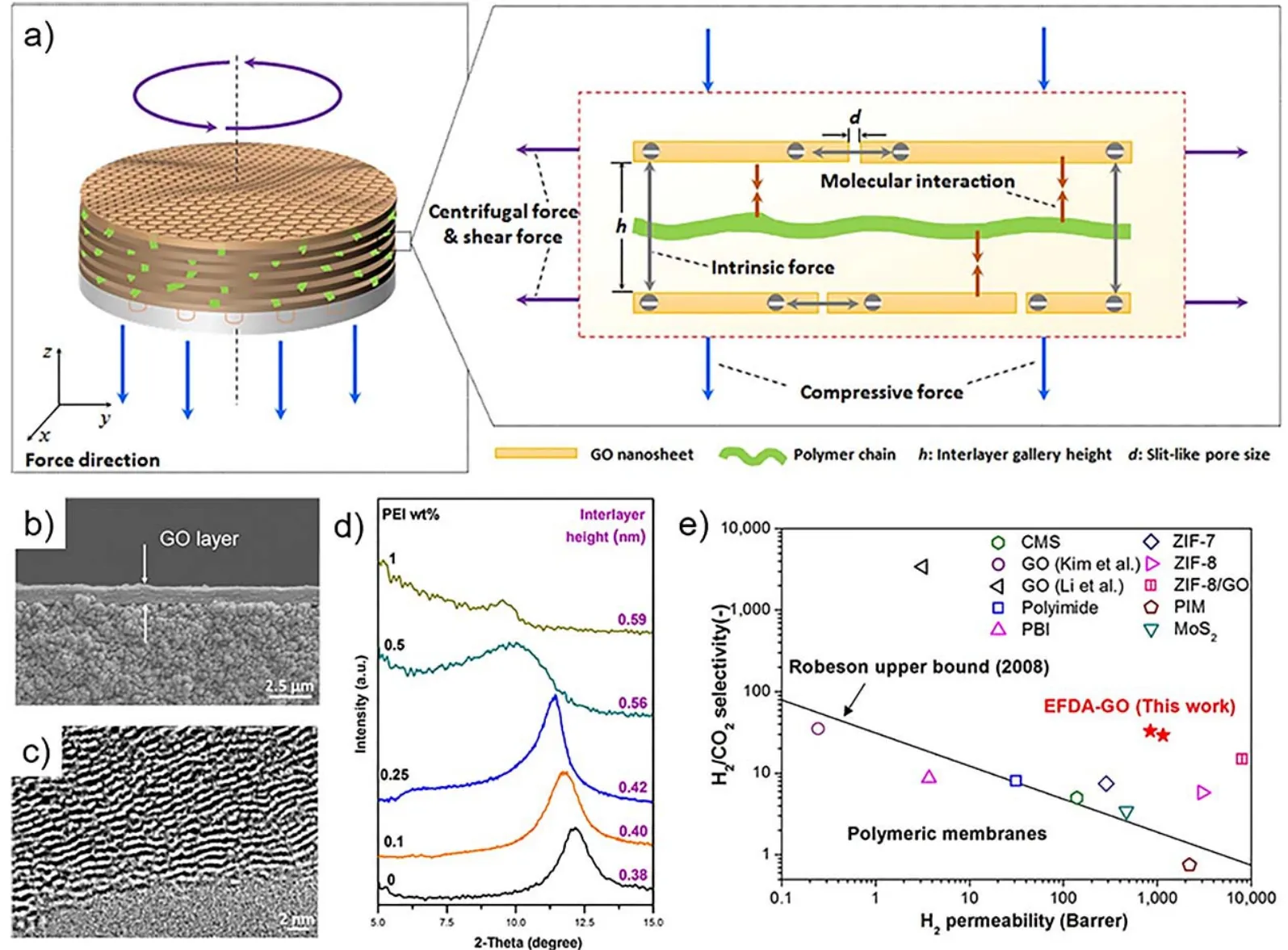
Fig. 1 Subnanometer 2D channels manipulated by external forces for ultra-fast gas sieving 9.a) External force-driven assembly (EFDA) approach for fabricating 2D channels, which involves 3D external forces in x, y, and z axes. Enlarged schematic shows force analysis for one 2D channel unit consisting of GO nanosheets and polymer chains. Three main forces are included: intrinsic force, outer external forces (compressive force,centrifugal force and shear force), which are applied outside the 2D channel unit, and inner external force (GO-polymer molecular interactions), which are applied inside the 2D channel unit. b) Cross-sectional SEM and c) TEM images of EFDA-GO membrane. d) Analysis for the average interlayer height of 2D channels in membranes. XRD spectra of EFDA-GO membranes with PEI solution concentration of 0-1% (weight percent). e) H2/CO2 separation performance of EFDA-GO membranes compared with state-of-the-art gas separation membranes. Reproduced with permission from American Chemical Society.
2.2 Metal-organic framework membranes
In recent years, metal-organic frameworks (MOFs)nanosheets have also been assembled for fabricating membranes. MOFs with layered structures, if successfully exfoliated, can serve as diverse sources for MOF nanosheets.The key challenge is how to maintain the structural and morphological integrity of nanosheets. Peng et al.15did pioneering work on pure 2D MOF nanosheet membranes. They exfoliated layered MOF precursors Zn2(bim)4(Fig. 2a) into 1-nm-thick nanosheets and assembled ultrathin membranes that own extremely high permeance while affording excellent molecular sieving properties for H2/CO2 separation. A gentle exfoliation method combining wet ball-milling and ultrasonication exfoliation in volatile solvent was proposed to maximally protect the nanosheets, and a mixture of methanol and propanol turned out to be the best solvent. To avoid the restacking of nanosheets back to ordered structures, they employed a hot-drop coating process leading to disordered stacking pattern in the membrane layer. The as-prepared ultrathin Zn2(bim)4membrane achieved a high permeance, up to several thousand gas permeation units (GPUs), and molecularsieving selectivity for H2/CO2(> 200) as well. They also found an unusual proportional relationship between H2 permeance and H2/CO2 selectivity for the membranes, and achieved a simultaneous increase in both permeance and selectivity by suppressing lamellar stacking of the nanosheets. As shown in Fig. 2c, H2permeance increased from 760 to 3760 GPU along with the variation of H2/CO2selectivity from 53 to 291. The membrane performance was demonstrated to be highly affected by the order of nanosheet stacking. For instance, the restacking of nanosheets would block the permeation pathway for H2, but had only a slight effect on CO2leakage, leading to a reduction in membrane performance.
Wang et al.16tried the freeze-thaw approach to exfoliate bulk MAMS-1 (Mesh Adjustable Molecular Sieve, Ni8(5-bbdc)6(μ-OH)4, 5-bbdc represents 5-tert-butyl-1,3-benzenedicarboxylate)crystals into discrete MAMS-1 nanosheets with high aspect ratios (Fig. 2e). Then they successfully assembled the 2D MOF nanosheets into molecular sieving membranes by the hot-drop casting approach. These membranes possessed pore openings parallel to gas concentration gradient allowing high gas permeation flux and high selectivity, and the 12-nm membranes exhibit H2/CO2separation factors of (34 ± 5) and H2permeance of (6516 ± 990) GPU. When the membrane thickness was increased to 40 nm, the membranes possessed H2/CO2separation factors of (235 ± 14) but with compromised H2permeance of(553 ± 228) GPU. Interestingly, the gas transport pathways of these membranes exhibited a reversed thermo-switchable feature. The H2/CO2separation factor exhibited a temperature dependent behavior, with the highest value of 245 at 20 °C and the lowest value of ca. 5 at 100 °C. They speculated that this abnormal behavior should be contributed by the molecular flexibility of the building metal-organic nanosheets, which was confirmed by in situ variable temperature PXRD (Powder X-ray diffraction) and molecular dynamics simulation. Therefore, the reversed thermo-switchable molecular sieving was firstly demonstrated in membranes composed of 2D MOF nanosheets,which may find novel applications in temperature-related gas separations.
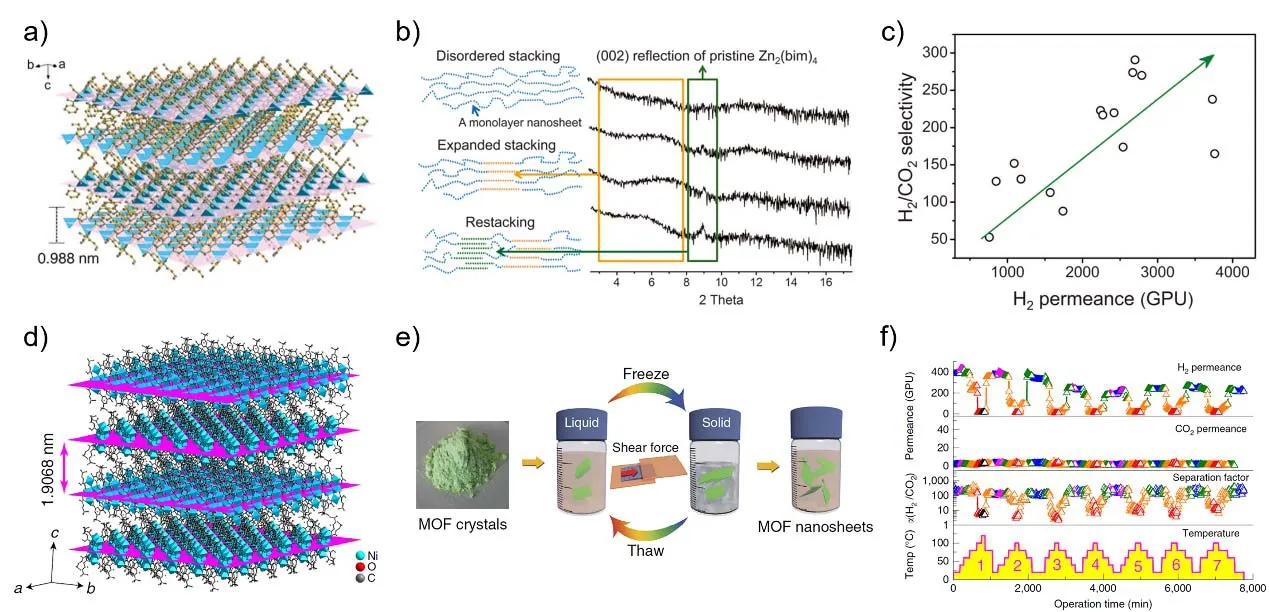
Fig. 2 Molecular sieving membranes composed of 2D metal-organic nanosheets for gas separation 15,16.a) Architecture of the layered Zn2(bim)4 precursor. The ab planes are highlighted in purple to better illustrate the layered structure 15. b) Powder XRD patterns of four membranes with different separation properties. The cartoons schematically illustrate the microstructural features of the nanosheet layers. The yellow and green portions correspond to the low-angle humps and the (002) peaks in the XRD patterns, respectively 15. c) Anomalous relationship between selectivity and permeance measured from 15 membranes. All the membranes were measured for equimolar mixtures at room temperature and 101.325 kPa 15. d) Crystal structure of MAMS-1. The ab planes are highlighted in magenta to illustrate the layered structure 16. e) The freeze-thaw exfoliation of MAMS-1 crystals into dispersed nanosheets 16. f) Gas permeance and H2/CO2 separation factors of the 40-nm membrane under seven heating/cooling cycles. Different colors represent various temperatures: blue, 20 °C; magenta, 40 °C; olive, 60 °C; orange, 80 °C; red, 100 °C and black, 120 °C 16. Reproduced with permission from the American Association for the Advancement of Science and Nature Publishing Group.
2.3 Layered double hydroxide membranes
Layered double hydroxides (LDHs), known as anionic clays,are a typical representative of layered compounds. They consist of positively charged brucite-like layers and interlayer galleries containing charge compensating anions17. The general formula is [M2+1-xM3+x(OH)2][An-]x/n·zH2O (M2+, M3+, An-and H2O represent di- and tri-valent metal ions, n-valent anions and the interlayer water, respectively). Different combination of metal ions and charge compensating anions can vary the gallery height from nano-meter to sub-nanometer scale, which provides application potential in gas separation. For the first time, Caro et al.18prepared a compact NiAl-CO3 layered double hydroxide membrane for H2purification by in situ growth method. The 2D intergallery height, calculated by basal spacing of 0.79 nm subtracting the thickness of a brucite-like layer (i.e., 0.48 nm),was estimated to be 0.31 nm, which was smaller than the kinetic diameter of most gas molecules such as CO2 (0.33 nm), N2 (0.36 nm) and CH4 (0.38 nm) except H2 (0.29 nm), indicating a molecular-sieving mechanism. As a result, the as-prepared membranes showed a remarkable selectivity of ~80 for the H2-CH4mixture. They also concluded that the deposition of α-Al2O3 intermediate layer on the surface of the substrate and the use of CO2-saturated water as the solvent were two critical conditions for preparing a high performance LDH membrane.
Furthermore, how to control the microstructures of the LDH layers, such as preferred orientation and thickness, has been explored in their another work19. They demonstrated that trace amounts of CO2 in the precursor solution led to the formation of ab-oriented 0.6 μm thick LDH membranes, while randomly oriented 5 μm thick LDH membranes formed from CO2-saturated precursor solutions (Fig. 3). By comparison, the randomly oriented LDH membranes exhibited a much higher H2selectivity than ab-oriented membranes, which possibly owed to the decreased density of mesoscopic defects. In addition, the gallery height of the prepared LDH membrane was tunable by intercalating different anions. The original reagent Ni(NO3)2was replaced by Zn(NO3)2, leading to a ZnAl-NO3LDH membrane with the gallery height of 0.41 nm. The adjustable sieving channel provides 2D LDH membranes with great potential in separation of various gas pairs.
2.4 MXene membranes
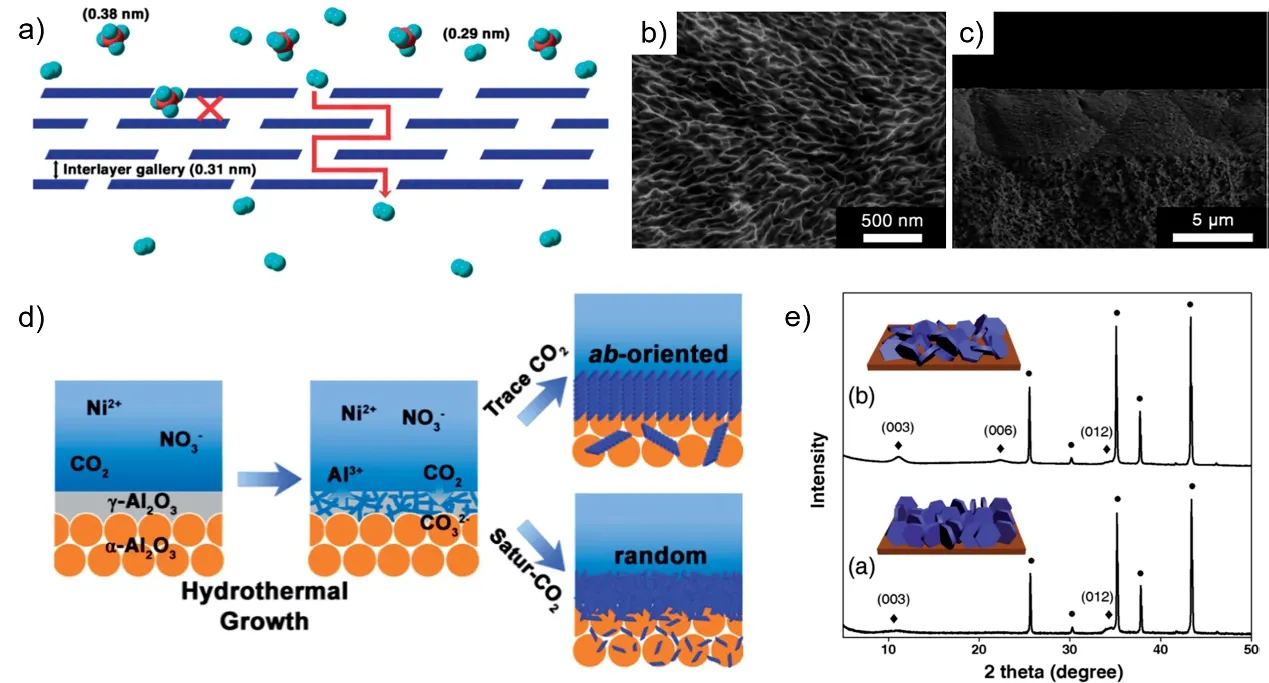
Fig. 3 Layered double hydroxide membranes with different microstructures 18,19.a) Schematic illustration of the concept of interlayer gallery-based separation. In the figure, layered compounds with a gallery height of 0.31 nm represent NiAl-CO3 LDH membranes.Gas molecules with kinetic diameters of 0.29 and 0.38 nm represent H2 and CH4, respectively 19. b) Top image and c) the cross-sectional image of the LDH membrane 18.d) Schematic illustration of the in situ hydrothermal growth of NiAl-CO3 LDH membranes with diverse microstructures. CO2 plays an important role in controlling the preferred orientation of the NiAl-CO3 LDH crystals in the membranes 19. e) XRD patterns of NiAl-CO3 LDH membranes prepared with (a) DI water and (b) CO2-saturated water as solvents,respectively. Inset: schematic illustration of the microstructure of each LDH membrane based on the XRD pattern and SEM image 19. Reproduced with permissions from RSC.
MXenes, as a young family of the 2D materials, was firstly reported by the Gogotsi group in 2011. With the formula of Mn+1XnTX, they are usually produced by selectively etching the A-group (mainly group IIIAor IVAelements) layers from Mn+1AXn phases (n = 1, 2, 3), where M is an early transition metal and X represents carbon and/or nitrogen20. Interestingly,during the etching and delaminating processes, abundant of surface-terminating groups (TX: = O, -OH, -F) are formed evenly on the entire surface of the nanosheets, whose size and content can control the structural and physicochemical properties of nanochannels between stacked MXene nanosheets.Therefore, MXenes can be employed to fabricate high performance membranes with tunable functionality. Wang et al.21reported lamellar stacked MXene membranes with aligned and regular subnanometer channels, which exhibited excellent gas separation performance with H2 permeability > 2200 Barrer (1 Barrer = 3.348 × 10-16mol·m-1·s-1·Pa-1) and H2/CO2selectivity > 160. They also utilized molecular dynamics simulations to support the experiments, confirming the subnanometer interlayer spacing between the neighboring MXene nanosheets as molecular sieving channels for gas separation.
Recently, we designed and engineered MXene flacks into 20-nm nanofilms with tunable gas transport property (Fig. 4c)22.Well-stacked pristine MXene nanofilms exhibited outstanding molecular sieving property for H2preferential transport with H2permeance as high as 1584 GPU and H2/CO2selectivity of 27.Interestingly, selectively permeating CO2 could also be realized by chemical tuning of the MXene nanochannels. Borate and PEI(polyethylenimine) molecules were well interlocked into MXene layers, which could delicately manipulate the stacking behaviors and interlayer spacing of MXene nanosheets. As a result, the functionalized MXene nanofilm showed a desirable CO2capture performance, CO2 permeance of 350 GPU with a CO2/CH4 selectivity of 15.3. The mechanisms within these nanoconfined MXene layers were discussed, revealing the transformation from“diffusion-controlled” to “solution-controlled” channels after chemical tuning. The pristine and functionalized MXene nanofilms were both mechanically stable, maintaining the high separation performance during long-term operation test over 100 h. Therefore, MXene nanosheets exhibit great potential in gas sieving and many other types of 2D nanosheets of MXene family are worthy of being explored.
3 Comparison of membrane structures and separation performance
In general, four kinds of 2D materials discussed above can be divided into two categories: porous materials (MOFs) and nonporous materials such as GO, LDHs and MXene. The porous materials can be fabricated into ultrathin membranes, only one or a few layers of nanosheets with intrinsic pores that can sieve small gases with different molecular sizes. Therefore,membranes made from porous 2D materials usually possess quite high flux and excellent molecular sieving performance at the same time. As shown in Table 1, the H2 permeance of Zn2(bim)4membrane represents the peak in all membranes for H2/CO2 separation, and the membrane exhibits quite high selectivity as well. For this kind of membrane, one key issue to be addressed is how to obtain high-quality nanosheets with integrated structure. What’s more, how to reduce the formation of defects in the membrane structure during the fabrication process is another challenge.
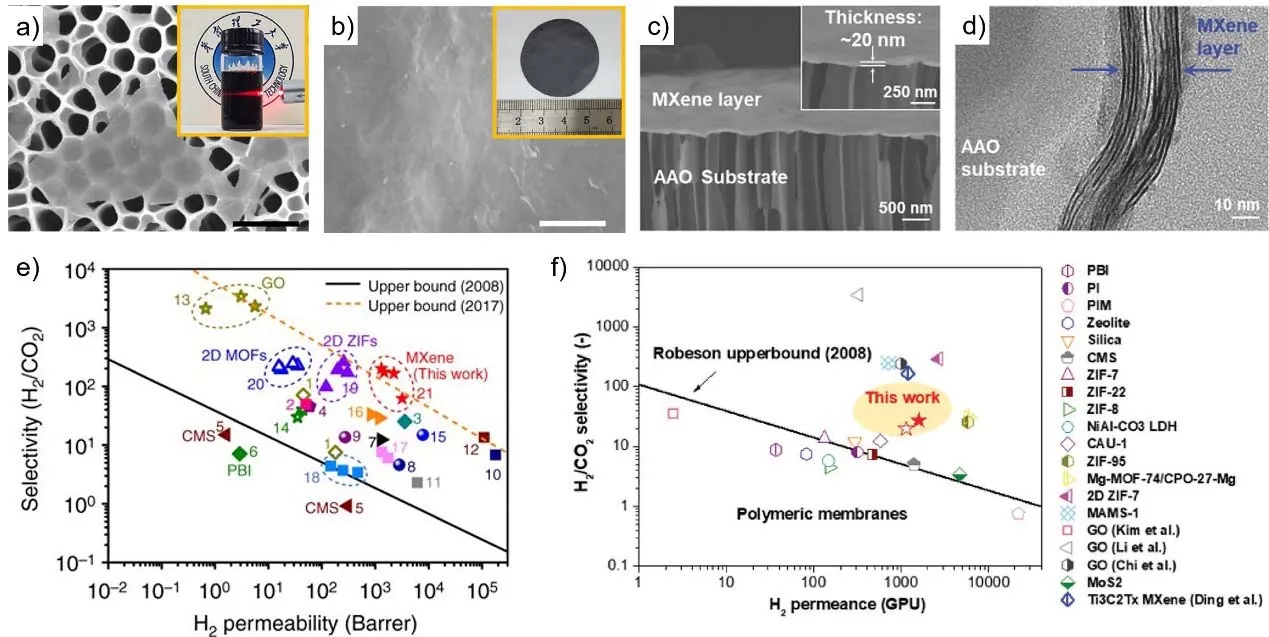
Fig. 4 MXene membranes for gas separation 21,22.a) SEM image of the delaminated MXene nanosheets on porous anodic aluminum oxide (AAO) (scale bar, 1 μm). Inset is the Tyndall scattering effect in MXene colloidal solution in water 21. b) SEM image of the MXene membrane surface (scale bar, 500 nm). Inset is a photograph of a MXene membrane 21. c) SEM image of 20 nm thick MXene nanofilms’ cross section. Inserted in panel c) is the enlarged cross-sectional SEM image of MXene nanofilms 22. d) HRTEM image of 20 nm thick MXene nanofilms 22. e) H2/CO2 separation performance of the MXene membrane compared with state-of-the-art gas separation membranes. The black line indicates the Robeson 2008 upper bound of polymeric membranes for H2/CO2 separation, and the orange dashed line represents the 2017 upper bound of the best current membranes for H2/CO2 separation 21. f) H2/CO2 separation compared with state-of-the-art gas separation membranes. The solid red star symbol represents the single gas permeation at 150 kPa and 25 °C, while the open red star symbol represents the mixed gas permeation (50 : 50 H2/CO2, volume percent) at 100 kPa and 25 °C 22. Reproduced with permission from Nature Publishing Group and John Wiley and Sons.
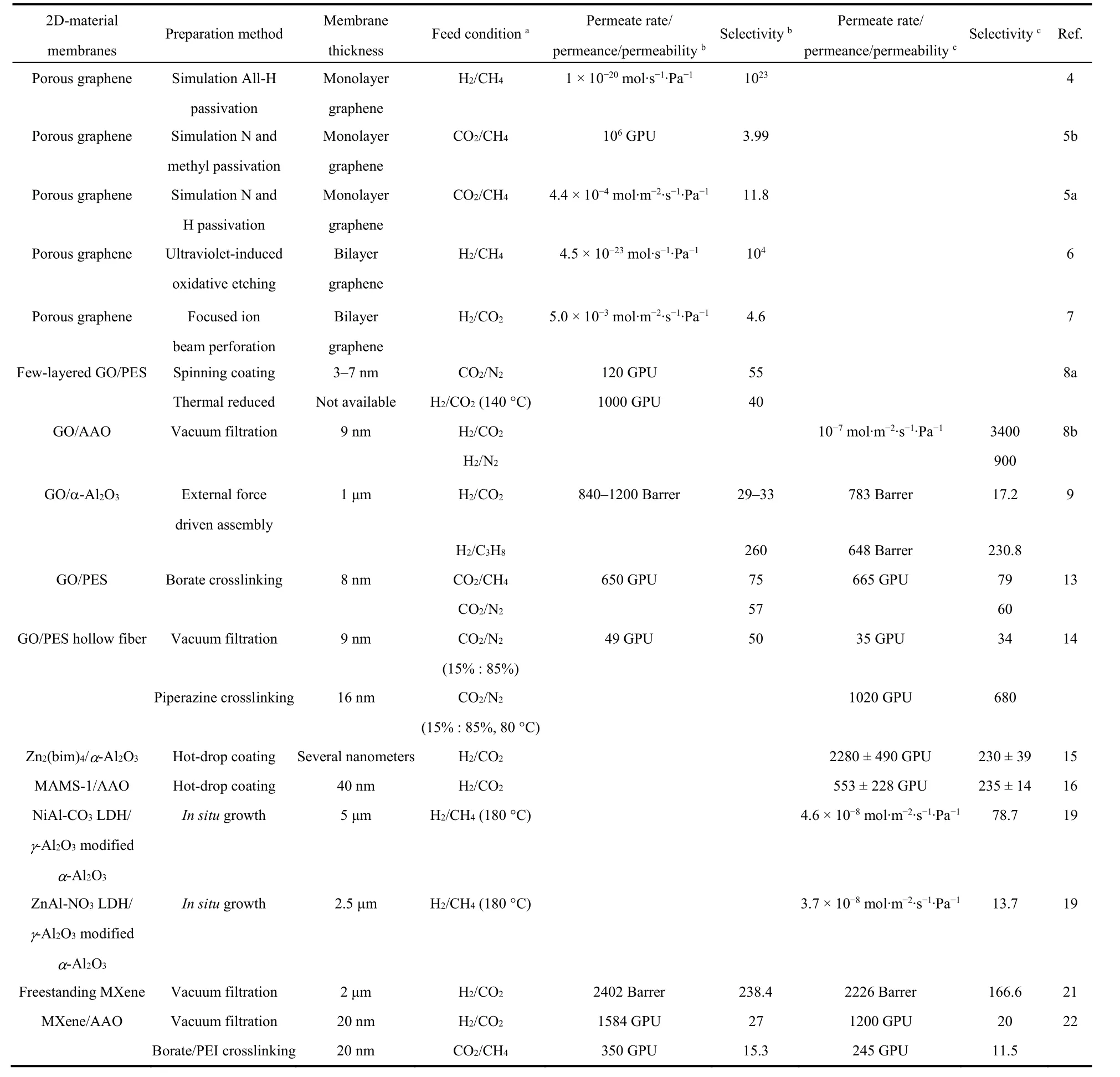
Table 1 Comparison of gas separation performance of 2D-material membranes.
The other category is nonporous materials featuring graphene oxide. The 2D nanosheets are usually assembled into laminar structure with interlayer passages for gas transport. As mentioned above, the gallery height can be precisely manipulated with sub-nanometer size for molecular sieving.However, the zigzag transport path will increase the transport distance, which means longer time to pass the membrane.Therefore, the gas permeance of membranes merely composed of GO nanosheets is usually relatively low unless creating more pores by thermal treatment. The facilitated transport carriers,such as borate and piperazine were employed to facilitate gas transport at the wet condition, achieving 20-fold enhancement in CO2 permeance. We consider that if nanosheets can be assembled into vertically aligned structure, the straight transport pass can greatly enhance the permeating rate. Qu and co-workers23firstly prepared the long-range vertically aligned graphene sheets membrane as the highly efficient solar thermal converter for generation of clean water. However, it is quite challenging to reduce the intergallery height to less than 1 nm for gas sieving.
4 Conclusions
In summary, great attention has been drawn to 2D-material membranes for gas separation. The key efforts have been made mainly involve the manipulation of 2D channels within subnanometer size, tunable 2D gas transport channels and how to obtain 2D nanosheets with structural integrity. Various membranes made from 2D materials exhibit excellent gas separation performance surpassing that of the state-of-art membranes. However, some critical issues still need to be addressed. First, the low yield of high-quality 2D nanosheets,such as GO or MOF nanosheets with integrated structure and narrow size distribution, will seriously block their mass production for industrial attempt. More efficient and universal exfoliation methods need to be explored for adequate nanosheets used in industrial membrane fabrication. Second, even though ultrathin membranes show exciting gas-sieving properties, it remains technical challenge to scale up to large enough membrane area with tolerant defects for practical application.Therefore, more advanced membrane fabrication process is required for controlling the pore size or interlayer height of 2D material membranes in large scale. For instance, we can combine the spray-coating with spinning process to achieve more uniformly coated surface. Third, the transport model for the confined transport through porous or laminar 2D channels is expected to be proposed with advanced characterization technology and molecular-level simulation. We should focus on the exciting breakthroughs that had been achieved and figure out connections between experimental and computational results.The relationship between gas transport and physicochemical properties of membranes such as pore size and functionalized groups should be thoroughly understood to further enhance membrane performance and guide the scale-up of membranes.Other issues such as membrane stability and complicated conditions where membranes operate must be taken into account as well. Finally, we anticipate that the new family of highperformance 2D membranes is promising material for efficient separation of gas pairs such as H2/CO2(H2recovery in petrochemical engineering), CO2/N2(CO2capture from flue gas), CO2/CH4(CO2removal from natural gas) or even meaningful yet challenging C2-C3 hydrocarbons.

