两个金属有机框架化合物对硝基芳香化合物在气/液相的荧光传感
李 江 韩 森 陈团结 苟召曦 张 琦 聂晓双 曹海如
(1长安大学材料科学与工程学院,西安 710064)
(2中交第一公路勘察设计研究院有限公司,西安 710062)
0 Introduction
The chemical sensing and detection play a crucial role in the anti-terrorism operation[1],environmental monitoring[2],medical science[3]and industrial production[4],etc.Recently,more and more researches focus on the development of the highly selective chemical sensor for the purpose of detection of nitro aromatic compounds in biological and environmental samples[1].In general,the nitro aromatic compounds are widely used industrially as organic solvents,pesticides,and reagents for chemical weapons.A series of nitro aromaticcompound areknown asacutelytoxic,organic pollutants that are frequently discharged into the environment by industrial production processes,and thus pose a threatto human health and environmental safety[5-8].Therefore,how to detect nitro aromatic compounds fast and quantitatively deserves more attention by scientists across different disciplines.So far,luminescent sensing was considered as an effective approach due to the rapid response,high selectivity,convenience and real-time monitoring characterizations.However,with the disadvantages as the low saturated vapor pressure and weak interactions with probe[9-10],this methods listed above are still facing the limitations to detect nitro aromatic in vapor-phase.
A variety of sensing methods and materials has been developed for the detection of low levels of such toxic nitro aromatic compounds,focusing not only on the rapid detection,but also on the operational ease[11-12].Particularly,multifunctional metal-organic frameworks(MOFs)was proposed as a new class of potential chemical sensors with luminescence and permanent porosity[13-19].The luminescent sensing ability of MOFs mainly relies on the interaction between the host backbones and the guest molecules[20-27].The majority of effective MOFs-based sensors are constructed with π-conjugated ligand,because the rich π-electron backbone can not only provide powerful luminescence but also supply active functional groups as the binding sites[28-29].In particular,the permanent pores in luminescentMOFs lead to increase interaction between the frameworks and the analytes,which could enhance the sensing ability of MOFs[30].
We have previously reported on the synthesis,structure and properties of these MOFs to detection of CuⅡions:[Cd2(DDCPB)(DMF)2(H2O)]n(CHD-1)and{[Zn2(DDCPB)(DMA)2].DMA}n(CHD-2)(H4DDCPB=1,1′∶3′,1″-terphenyl-3,3″,5,5″-tetracarboxylic acid)[31].However,we make full use of the high porosity and strong luminescence of two MOFs(CHD-1 and CHD-2)to sense nitro aromatics compounds.H4DDCPB was selected as the flexible V-shaped ligand for its aromatic π rings which may help to enhance the rigidity of the framework and promote luminescent character.CHD-1 and CHD-2 display ligand-based luminescence and high stability in those kinds of organic solutions.Therefore,both of them could detect NACs quantitatively and sensitively through luminescence quenching,which make them promising luminescence sensors.It should be noted that guest sensing incorporate pores or channels in CHD-1,while CHD-2 features a dense stacking mode without solvent accessible volume.Therefore,the detection limits of two MOFs are different and determined by luminescent sensor structure.Furthermore,two MOFsbased films were prepared and exhibit high sensitivity against the vapor of nitrobenzene (NB)and 2-nitrotoluene (o-MNT).Additionally,the mechanism of photo-induced electron transfer was systematically investigated.
1 Experimental
1.1 Materials and methods
All the reagents and solvents were obtained from commercial sources and used directly without further purification.Elemental analyses for C,H and N were performed on a Carlo-Erba CHNO-S microanalyzer.Infrared spectra(IR)were acquired with KBr discs via a Bruker EQUINOX-55 FT-IR spectrometer in a range of 400 to 4 000 cm-1.Thermogravimetric analyses(TGA)were carried out with NETZSCH STA 449 CTG/DTA equipment under N2stream at a heating rate of 5℃.min-1.The luminescence properties were conducted in Hitachi F4500 fluorescence spectrophotometer.The powderX-ray diffraction (PXRD)patternswere collected on a Bruker D8 ADVANCE X-ray powder diffractometer with Cu Kα radiation(λ=0.154 18 nm)in a 2θ range of 5°~50°at a speed of 0.02°.s-1.The operating voltage and current are 40 kV and 40 mA,respectively.The SEM images were operated by S4800 scanning electron microscope (SEM)(Hitachi,Japan)at voltage of 2 kV.The quantum yields of two MOFs were performed with Horiba Fluoromax-4 at room temperature.
1.2 Synthesis of[Cd2(DDCPB)(DMF)2(H2O)]n
(CHD-1)
A mixture of Cd(NO3)2.6H2O(1 mmol),H4DDCPB(1 mmol),DMF(2 mL)and H2O (1 mL)was put into 15 mL Teflon-lined stainless steel vessel,and then heated at 120℃ for 72 h[31].The colorless rod-like crystals were obtained after the vessel progressively cooled to room temperature.The productivity is in ca.57.0%yield based on Cd.Anal.Calcd.for C28H24N2Cd2O10(%):C,43.49;H,3.13;N,3.62.Found(%):C,43.55;H,3.25;N,3.75.
1.3 Synthesis of{[Zn2(DDCPB)(DMA)2].DMA}n
(CHD-2)
The synthesis of CHD-2 was similar to the synthesis process of CHD-1 except that the solvent was DMA.The productivity obtained in ca.63.0%yield based on Zn.Anal.Calcd.for C34H37N3Zn2O11(%):C,51.39;H,4.69;N,5.28.Found(%):C,54.42;H,4.73;N,5.30.
1.4 Luminescent sensing methodology
The luminescent properties were investigated in the solid state,in organic solvent suspensions and in air at room temperature.The as-synthesized samples(5.0 mg)of CHD-1 and CHD-2 were immersed in differentorganic solvents to check the sensing selectivity,including methanol,ethanol,acetone,N-methyl pyrrolidone, tetrahydrofuran, 1,4-dioxane,acetonitrile,chlorobenzene,bromobenzene,cyclohexane,dimethylsulfoxide,dichloromethane,benzene and a series of nitro aromatics compounds(nitrobenzene(NB),1,4-dinitrobenzene(1,4-DNB),2,4-dinitrotoluene(2,4-DNT),2-nitrotoluene(o-MNT)and 4-nitrotoluene(p-MNT).The finely ground sample powders were dispersed in 5 mL organic solvent by using ultrasonication for 2 h.All titrations were carried out by gradually adding solution of NACs into the solvent suspensions of the samples.To verify the stability of two compounds in the quenching suspensions,the PXRD patternsofthe immersed powderswere measured(Fig.S1 and Fig.S2).
1.5 Vapor-phase detection
The sample used as an in situ time-dependent luminescent sensor for solvent vapors was prepared as follows:the ground sample powder(5 mg)was pressed into a firm sheet.The sheet was attached to a thin plastic slide and placed into a bottle.The film was kept about 3 cm away from the bottom of the airtight bottle;then one drop of NB and o-MNT was added into the bottle.
2 Results and discussion
CHD-1 and 2 were synthesised by a modified solvothermal method based on our previous work[31].By the way,the MOFs structure description was displayed in Supporting Information(Fig.S15~S17).
The crystallinity and purity of the prepared samples were confirmed by the powderX-ray diffraction (PXRD)patterns as shown in Fig.S1 and S2.The very strong and sharp peaks imply the high crystallinity.Both samples showed similar diffraction peaks located at nearly the same angles and matched perfectly with simulated patterns from single crystal X-ray diffraction.The morphologies of MOFs-based films,which were prepared for sensing experimentation,were determined by scanning electron microscopy (SEM).The SEM images of films on the other hand demonstrate uniformity amongst crystals.
2.1 Solid state luminescent properties of CHD-1 and CHD-2
As depicted in Fig.2,the solid-state luminescent properties offree ligand and compounds were determined at room temperature.The ligand exhibited an intense emission at 406 nm upon excitation at 300 nm,which can be attributed to π*-n or π*-π transitions.Both of them showed intense emission maximum at 357 and 350 nm upon excitation at 300 nm,respectively.The quantum yield of CHD-1 and CHD-2 were 2.56%and 1.91%,respectively.Compared with the emission peak of ligand,the spectra of compoundsindicate the blue-shiftsthatcan be ascribed to π* →π transition involving a ligandcentered excited state[32-33].
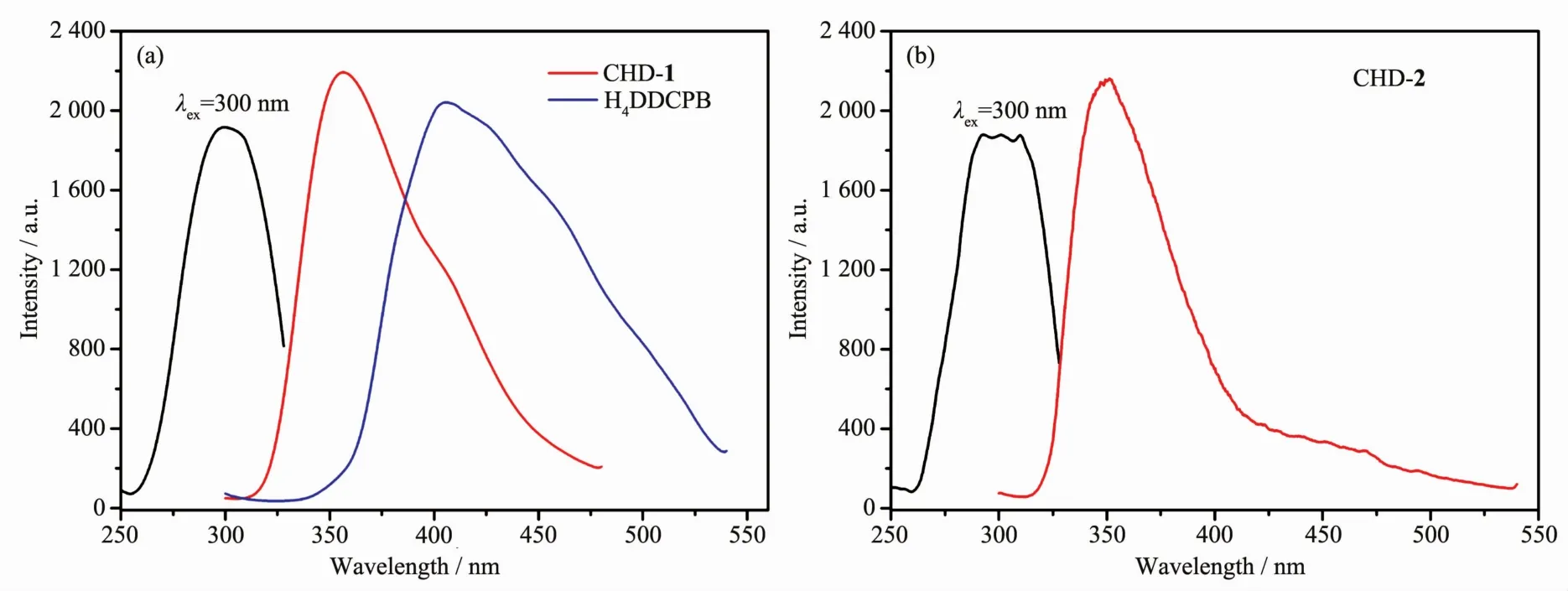
Fig.2 Luminescent spectra of CHD-1 and CHD-2 and free ligand in solid state at room temperature
2.2 Solution-based detection of nitro aromatic compounds
The potential of CHD-1 and CHD-2 for detecting of nitro aromatic compounds (NACs)have been investigated.The PXRD patterns for both CHD-1 and 2 after immersion in organic solvent were the same as those of CHD-1 and CHD-2,respectively,indicating the stability in the solvents(Fig.S1 and S2,supporting information).Asthe same treatmentabove,the excitations were conducted through the wavelength of 300 nm.
Firstly,the luminescent responses of CHD-1 and CHD-2 toward all organic solvent were performed.As shown in Fig.3,a series of nitro aromatic compounds,including nitrobenzene (NB),1,4-dinitrobenzene(1,4-DNB),2,4-dinitrotoluene(2,4-DNT),2-nitrotoluene(o-MNT)and 4-nitrotoluene (p-MNT),could completely quench emissive intensities of both compounds.In contrast,some organics,such as DMF,1,4-dioxane,toluene,could enhance the emissive intensities.However,the restofotherorganics,containing alcohols (methanol,ethanol),ketones (acetone,N-methylpyrrolidone),ethers (tetrahydrofuran,1,4-dioxane),nitriles(acetonitrile),arylhalide(chlorobenzene,bromobenzene),cyclane (cyclohexane),sulfoxides (dimethylsulfoxide),dichloromethane,and even aromatic compounds (benzene)have a slightly effect on the luminescence intensity.These results indicate that CHD-1 and CHD-2 can act as a luminescent probe for the recognition of nitro aromatic compounds.
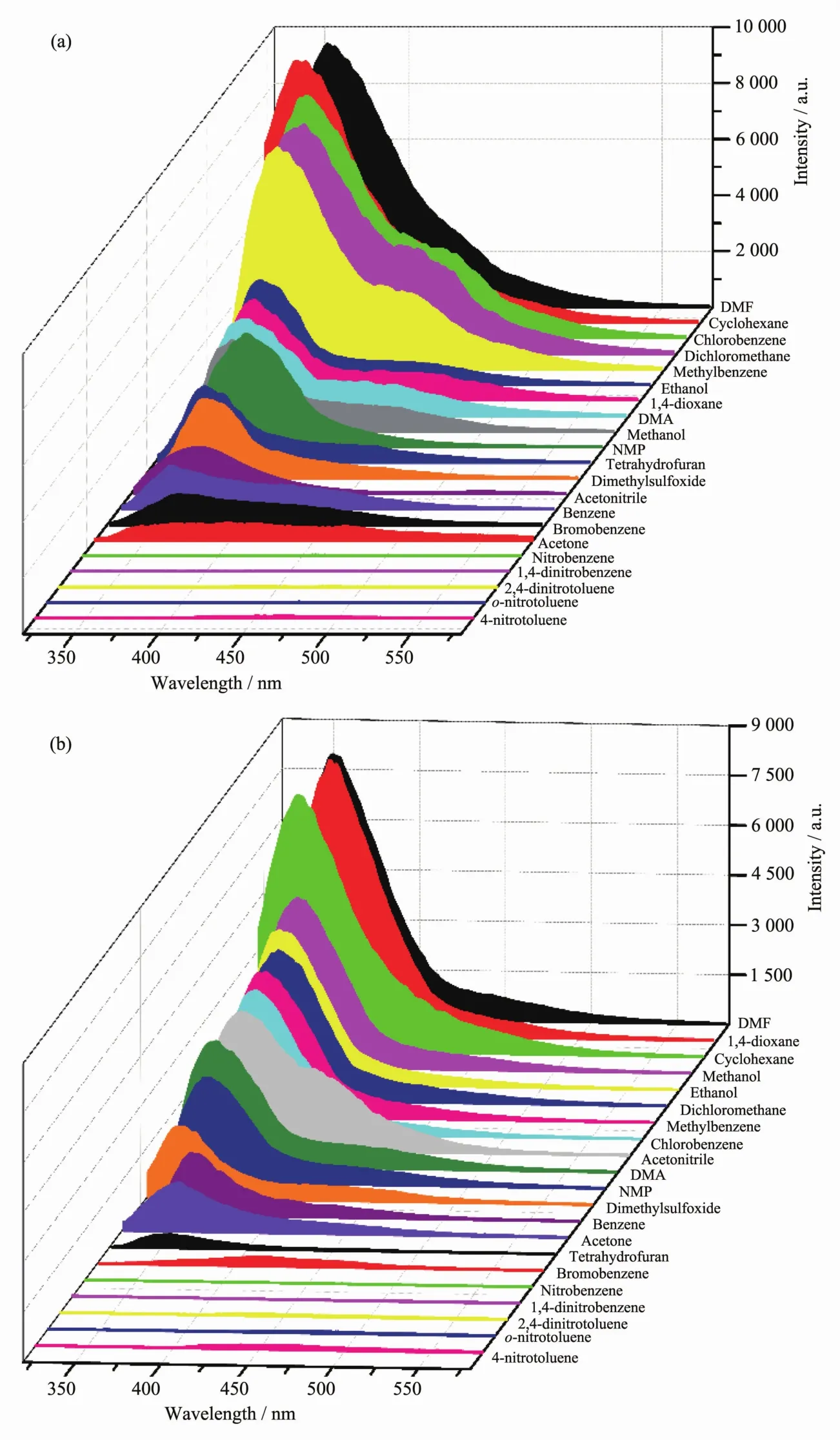
Fig.3 Emission spectra of CHD-1(a)and CHD-2(b)immersed into different solvents
Secondly,in order to further check the detection limits of both compounds,the titration experiments of NACs have been conducted.The sensitive quenching effect of CHD-1 and CHD-2 were examined with a batch of their suspensions(3 mL DMA)with a gradually increased concentration of all nitro aromatic compounds(CHD-1:Fig.S3~S7;CHD-2:Fig.S8~S12).Luminescence quenching efficiency must be quantized,which can be calculated by the following equation:

Where I0is the initial emission intensity of CHD-1 and CHD-2,and I is the intensity of analytesincorporated CHD-1 and CHD-2 and QE is quenching efficient.
Finally,as shown in Fig.4,in the all titration experiments,the quenching efficiency of CHD-1 and CHD-2 increased drastically and reached 50%with the analytes amount in the very low concentration(0.3 and 10 mg.L-1,respectively).In the case of CHD-1,the emission decreased to 64%of the initial intensity upon the addition of NB at 0.2 mg.L-1.However,for CHD-2,70%quenching of emission was observed at 10 mg.L-1.The concentrations of NB,1,4-DNB,2,4-DNT,o-MNT and p-MNT caused the complete emission quenching of CHD-1 at 0.3 mg.L-1or so.However,the concentration ofNACs fully quenched the luminescence of CHD-2 in a range of 30~45 mg.L-1.Totally,both compounds exhibit similar quenching for these NACs,but the response of CHD-1 to concentrations of nitro compounds is much lower than that of CHD-2.
In order to obtain the detection limits of two MOFs,the Stern-Volmer(SV)equation was established[34]:

Wherein KSVstands for the quenching constant(L.mol-1)and cMis the concentration of NACs.For the NACs,the plot of I0/I vs cMwas nearly linear in the range of low concentrations.The KSVvalues of NACs were calculated and listed in Table 1 and Table 2.
On the basis of the KSVvalues and standard deviations by measuring luminescence intensity of blank solutions 5 times(Table 1 and 2),the detection of limit(LOD)was also calculated[35-37]:

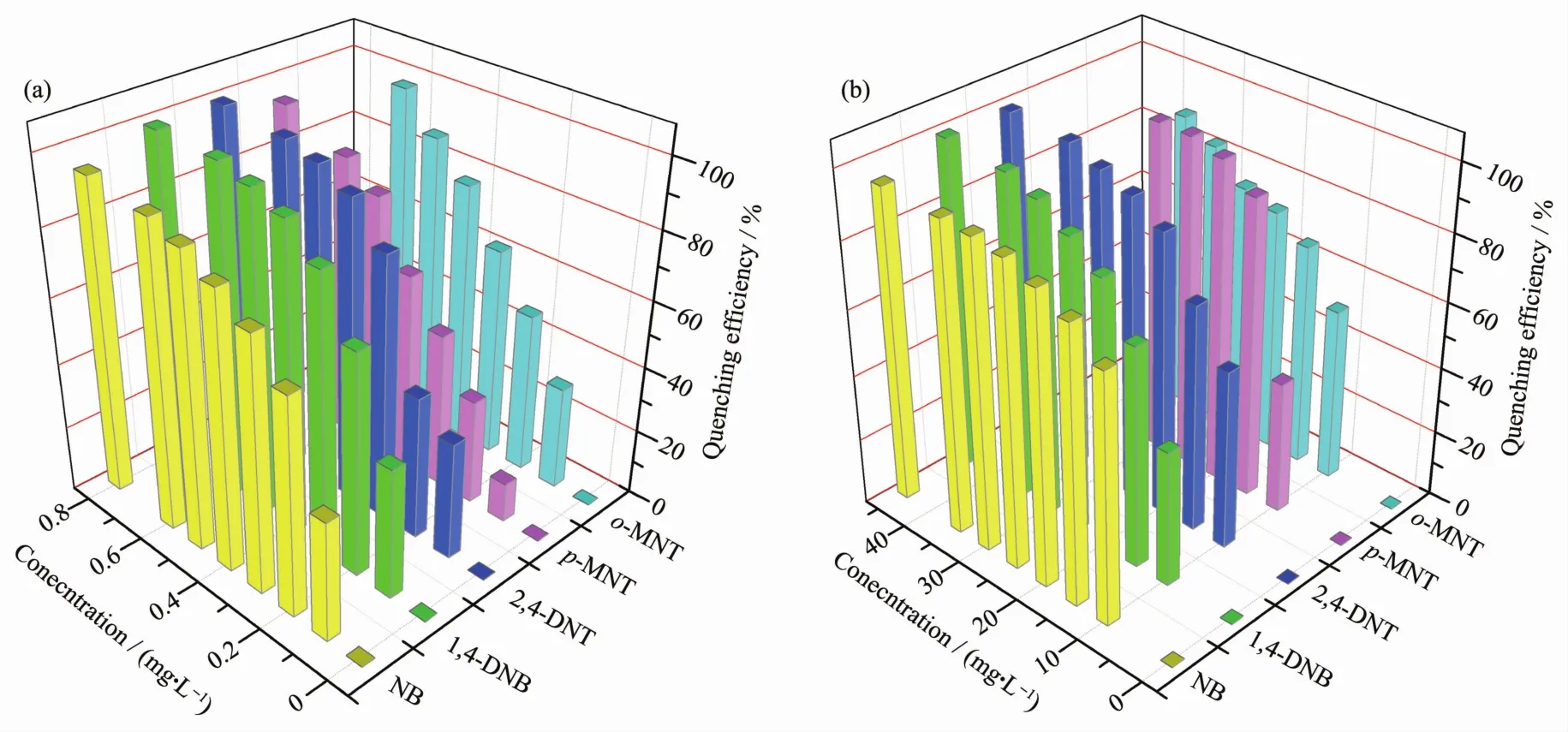
Fig.4 Plot of quenching efficiency of CHD-1(a)and CHD-2(b)immersed in DMA at different concentrations of several nitro aromatic compounds
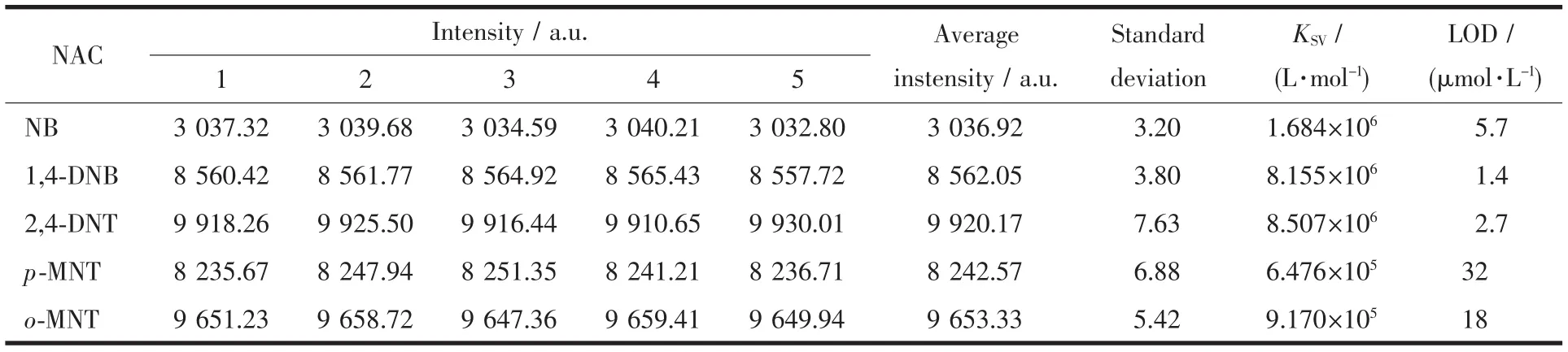
Table 1 Standard deviation(δ)and detection limits calculation for the detection of NACs by CHD-1

Table 2 Standard deviation(δ)and detection limits calculation for the detection of NACs for CHD-2
where δ is the standard deviation of blank measurements(N=5);the values I0and IRare the luminescence intensity of suspension in the absence and the average of I0,respectively.The high quenching constants KSVand the low detection limits(LOD)indicate extremely high sensitivity toward NACs.
The discrepancy in the response concentration between CHD-1 and CHD-2 can be explained as the pores inside CHD-1 that serve as recognition sites and enhance the host-guest interaction.So,comparison with CHD-1 and CHD-2,the detection response of CHD-1 is much more sensitive than that of CHD-2.It is noteworthy that the concentration of quenching the half luminescent intensity to nitro aromatics compounds locate in 0~0.3 and 0~15 mg.L-1,respectively,which is lower than that reported by Pang (0~1 000 mg.L-1)[38],Sun(0~100 mg.L-1)[39],Su(0~100 mg.L-1)[40]and Li(0~2 000 mg.L-1)[41].The results illustrate that the porous frameworks could not only have a highly sensitivity towards a wide range of NACs,but also detects the trace amount of them.
2.3 Vapor-phase detection of nitro aromatic compounds>
CHD-1 and CHD-2 were proved for their ability to act as solution-based sensory materials towards a series of nitro aromatic compounds.Compared to the common sol or gel sensing systems,the highly stable and porous CHD-1 and CHD-2 can provide a superior sensing platform to overcome the aggregation-caused quenching effect.Thus,the film based on two MOFs was prepared,and nitrobenzene (NB)and 2-nitrotoluene(o-MNT)was used as analytes to investigate the vapor-phase sensing capability of the film.Just as expected,the film displayed highly sensitive response towards the vapor of NB and o-MNT.The films of CHD-1 and CHD-2 were further exposed to analytes for 30,60,90,180 and 360 s,and the luminescent intensity was measured immediately after each time period (Fig.5).However,the luminescent intensity declined nearly 50%in as short as 60 s.The vapor pressure of NB and o-MNT were 0.02 and 0.03 kPa at 298 K,respectively,which were generated by depositing small amounts of the analytes into sealed bottle,creating a static headspace.The MOFs-based films were rapidly placed inside the sealed tubes for fixed amounts of time during the sensing procedure.
In a word,CHD-1 and CHD-2 not only act as luminescent probe for NACs,but also exhibit good vapor-phase detection ability towards them.
2.4 Mechanism of CHD-1 and 2 towards nitro aromatic compounds
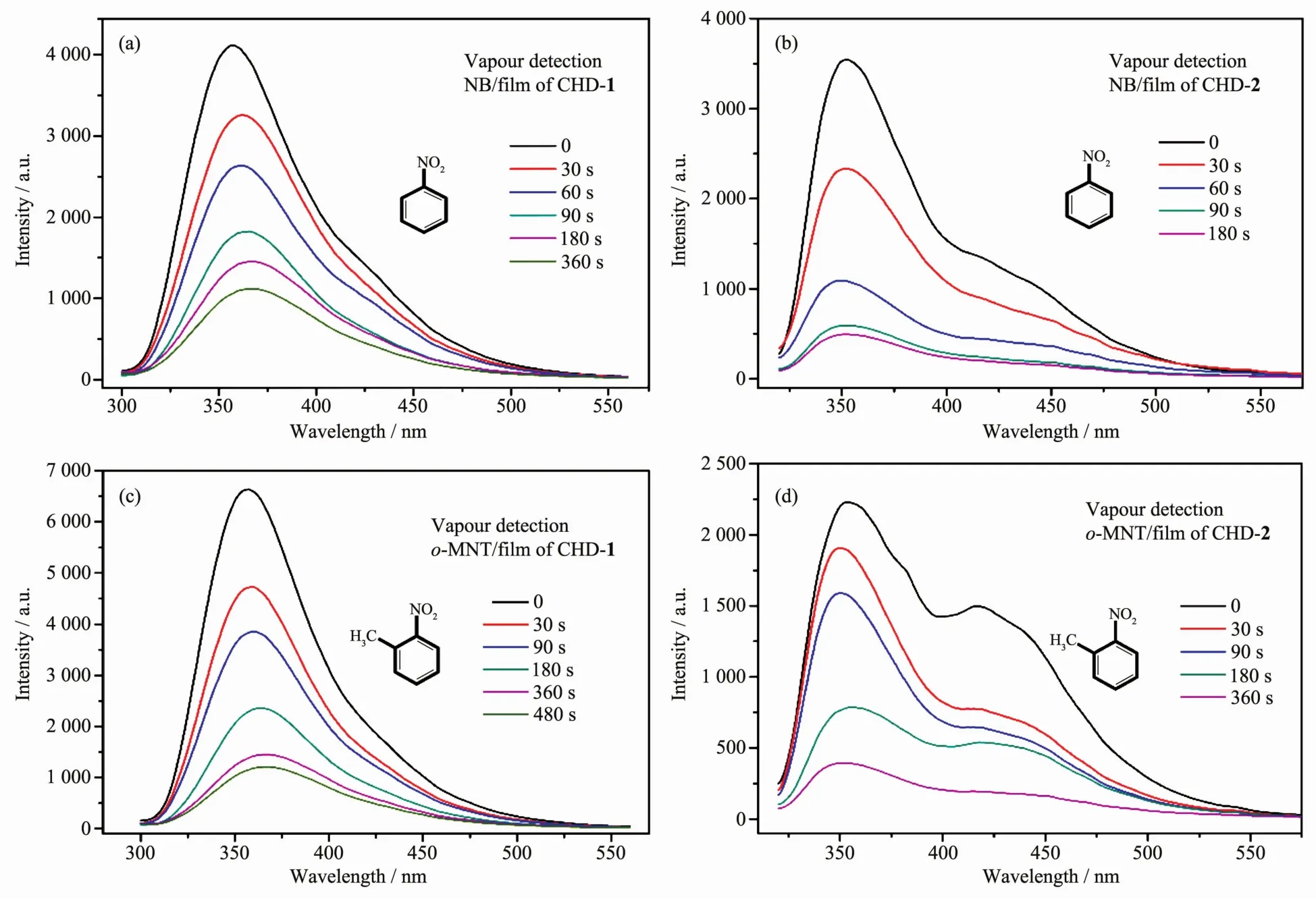
Fig.5 Luminescent emission intensity for film of CHD-1(a,c)and CHD-2(b,d)upon exposure to NB and o-MNT vapor for 0~480 s
Since the nitro-based compounds are typical electron-withdrawing substituents[41],the sensing mechanism can be proposed to be the photo-induced electron transfer(PET)[42-43].Generally,for electron-withdrawing nitro-analytes,the electrons from the electron-rich framework are transferred to the lower LUMO of the electron-deceit nitro-compounds,not to the ground state of the framework.The excited electron of MOF-based sensors would be so tightly bound to nitro aromatic that transitions from excited state to ground state are blocked.
To verify the hypothesis,the frontier molecular orbital of these nitro aromatics and H4DDCPB were calculated with density functionaltheory atthe B3LYP/6-31G*level[40].As shown in Fig.6,NB,1,4-DNB,2,4-DNT,o-MNT and p-MNT have the lower LUMO than H4DDCPB,which make them the strong electron acceptor in the excited state[39].Accordingly,the highest selectivity for detection of 1,4-DNB is consistent with its lowest LUMO energy level by comparison with all other NACs.However,the order of observed quenching efficiency(1,4-DNB>2,4-DNT>NB>p-MNT>o-MNT)is not fully in accordance with the LUMO energies.As discussed above,the luminescence of CHD-1 and 2 are both ligandcentered and electron rich analytes.The reducibility of H4DDCPB,which has a high LUMO energy(-0.5 eV for H4DDCPB,-2.3~3.9 eV for the tested nitro aromatics),and electron deficiency ofthe nitro aromatics with low LUMO energy together facilitates the excited electron to transfer from the frameworks to the electron deficient NACs,leading to the quenching of luminescence.

Fig.6 HOMO and LUMO energies for nitro aromatic analytes and the ligand
As shown in Fig.7,the absorption spectra of nitro aromatics have a large overlap with the emission spectra of both compounds.If the absorption band of the analyte has an effective overlap with the emission band of the fluorophore,the resonance energy transfer can occur from fluorophore to non-emissive analyte[44].Resonance energy transfer can dramatically enhance luminescence quenching efficiency and also improves sensitivity[45-47].
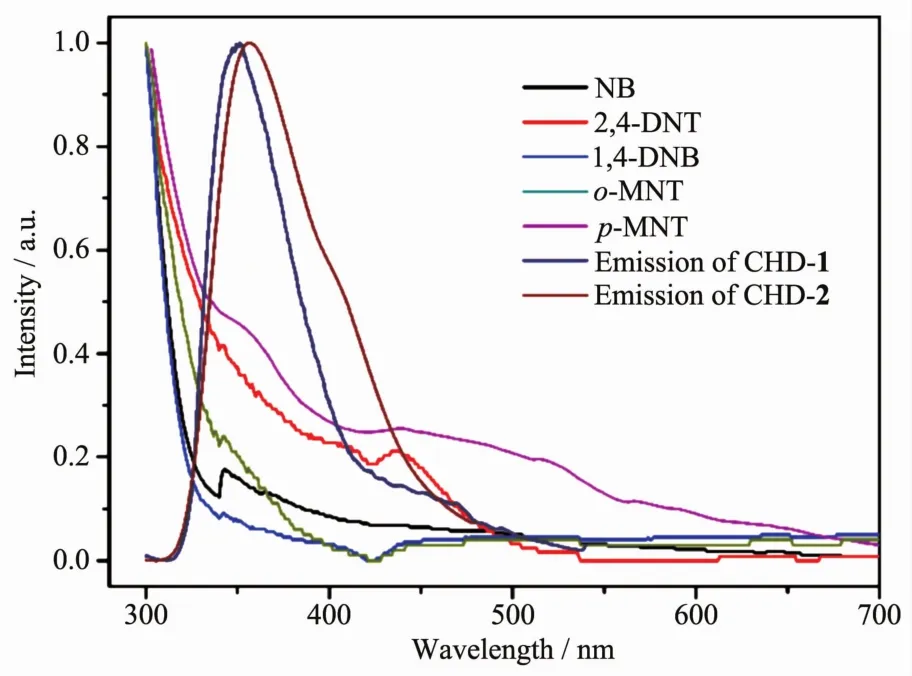
Fig.7 UV-Vis adsorption spectral overlap between normalized emission spectra of CHD-1,CHD-2 and normalized absorbance spectra of nitro aromatics
However,the discrepancy in structure factors between CHD-1 and CHD-2 in detection of NACs concentration also should be considered.The structure of CHD-1 and CHD-2 should play a significant role in the sensing process.The 2D microspores in the CHD-1 would enhance the interaction between nitro group and functional group (benzene of the ligand or coordinated DMF molecules)of frameworks,which result in quenching the luminescence with the lower concentration ofanalytes than thatofCHD-2.Therefore,in this case,it is resemblance to the previous studies that the pore structures of MOFs are beneficial to their interaction with analytes[42].The diameter of the tested nitro aromatics with van der Waals radius is within the range of 0.6~0.8 nm[48].Thus,three types of open channels(1.11 nmX1.12 nm,1.21 nmX1.61 nm and 1.15 nmX0.77 nm along with a,b-axis excluding the van der Waals radii)within 1 are large enough to take in nitro aromatics.It is supposed that,during the immersion,the nitro aromatics would act as guest molecules to enter the channel and interact internally with the host framework causing a luminescence quenching.
3 Conclusions
In summary,two highly stable and porous MOFs based on CdⅡ and ZnⅡ were successfully synthesized under solvothermal conditions.Both compounds display highly selective and sensitive luminescence quenching on nitro aromatic compounds in gas and solution phase.In particular,compared with that of CHD-2,CHD-1 with 2D microspores possesses the lowest detection limits for nitro aromatic compounds locating in 0~4 μmol.L-1,which are lower than the reported compounds.The presentworksgive a reference that to rationally design MOF-based senor with multidimensional microspores is beneficial to the detection of target;this may be useful under more realistic conditions in the future.
Supporting information is available at http://www.wjhxxb.cn
——中交第四航务工程勘察设计院有限公司

