Quinclorac Resistance in Echinochloa crus-galli from China
Peng Qiong, Han Heping, Yang Xia, Bai Lianyang, Yu Qin, Stephen B. Powles
Quinclorac Resistance infrom China
Peng Qiong1, 2, 3, Han Heping3, Yang Xia1, 4, Bai Lianyang1, 2, Yu Qin3, Stephen B. Powles3
(Hunan Agricultural Biotechnology Research Institute, Hunan Academy of Agricultural Sciences, Changsha 410125, China; Collaborative Innovation Center for Field Weeds Control, Hunan University of Humanities, Science and Technology, Loudi 417000, China; )
is a major weed in rice fields in China, and quinclorac has been long used for its control. Over-reliance of quinclorac has resulted in quinclorac resistance in. Two resistant (R)populations from Hunan, China were confirmed to be at least 78-fold more resistant to quinclorac than the susceptible (S) population. No difference in foliar uptake of14C-labelled quinclorac was detected between the R and S plants. However, a higher level of14C translocation and a lower level of quinclorac metabolism were found in the R plants. Basal and induced expression levels of β-cyanoalanine synthase () gene and β-CAS activity were not significantly different between the R and S plants. However, the induction expression of 1-aminocyclopropane-1-carboxylic acid oxidase () gene by quinclorac treatment was evident in the S plants but not in the R plants. Quinclorac resistance in the two resistantpopulations was not likely to be related to foliar uptake, translocation or metabolism of quinclorac, nor to cyanide detoxification via. Thus, target-site based quinclorac signal reception and transduction and regulation of the ethylene synthesis pathway should be the focus for further research.
; quinclorac resistance; quinclorac metabolism; β-cyanoalanine synthase; 1-aminocyclopropane-1-carboxylic acid synthase; 1-aminocyclopropane-1-carboxylic acid oxidase; rice
Rice () is one of the most important global food crops. Rice-infesting weeds are a major annual challenge, with worldwide rice yield loss estimated at 35% due to weed competition (Oerke and Dehne, 2004).is one of the most widespread and severe weeds in rice fields. In recent decades, over-reliance on herbicides has resulted in the evolution of herbicide resistance in(Bajwa et al, 2015; Heap, 2018), and resistance in rice fields is on the increase in both frequency and complexity (Rouse et al, 2018). So far,has evolved resistance to herbicides including at least seven different chemical classes: synthetic auxins (i.e. quinclorac), acetolactate synthase (ALS) inhibitors, acetyl coenzyme A carboxylase (ACCase) inhibitors, photosystem II inhibitors, microtubule inhibitors, long-chain fatty-acid inhibitors and lipid synthesis inhibitors (Heap, 2018; Chen T et al, 2018).
The auxin herbicide quinclorac is a rice-selective herbicide, often used to controlspp. However, over-use has resulted in quinclorac resistance evolution in eightspecies including.(Lopez-Martinez et al, 1997),(Wright et al, 2018),(Yasuor et al, 2012) and(Sunohara and Matsumoto, 2004). The first case of quinclorac resistance inwas reported in southern Spain (Lopez- Martinez et al, 1996), and later in Arkansas, USA (Talbert and Burgos, 2007). Quinclorac has been used for more than 20 years in China forcontrol, and quinclorac resistance in China was firstly reported inin 2000 (Heap, 2018). Quinclorac resistance has become a serious problem in Chinese rice fields (Li et al, 2016; Yang et al, 2017; Zhu et al, 2018). Hunan Province is the major rice production area in China, andis a major yield constraint with many populations reported to be quinclorac resistant (Ma et al, 2013).
The mode-of-action of quinclorac has been suggested to particularly involve in the induction of cyanide as a coproduct of ethylene biosynthesis in plants (Grossmann and Kwiatkowski, 1993, 2000; Grossmann, 2010). Many attempts have been made to understand quinclorc resistance mechanisms in weedy grass species, with a focus on the ethylene biosynthesis pathway involving 1-aminocyclopropane-1-carboxylic acid synthase (ACS), 1-aminocyclopropane-1- carboxylic acid oxidase (ACO) and the cyanide detoxification pathway via β-cyanoalanine synthase (β-CAS). For example, lack of the induction ofandgene expression and/or ethylene synthesis, and increase ingene expression and/or enzyme activity are observed in resistantspp.(Grossmannand Kwiatkowski, 2000; Grossmann, 2010; Yasuor et al, 2012; Xu et al, 2013; Fipke and Vidal, 2016; Gao et al, 2017, 2018). In addition, the auxin uptake carrier AUX1(Hoyerova et al, 2018) and the auxin-conjugating Gretchen Hagen 3 (GH3) IAA-amido synthetases are also investigated (Li et al, 2016). Despite much effort, the molecular basis of quinclorac resistance inremains unclear.
In this study, we characterized quinclorac resistance and resistance to other herbicides in twopopulations collected from rice fields in Hunan Province, China, and investigated potential quinclorac resistance mechanisms.
MATERIALS AND METHODS
Plant materials
Quinclorac-resistantpopulations (R-DC and R-CH) were collected from rice fields in Changde (29º02′ N/111º68′ E)and Changsha (28º25′ N/113º07′ E) of Hunan Province, China, respectively. A known herbicide-susceptible (S)population collected from Longhui in Hunan Province (27º13′ N/111º04′ E) with no quinclorac use history was used as the control.
Whole-plant dose response experiments
Seeds of the R and S populations were germinated on 0.6% agar-solidified water in plastic containers. Uniformly-sized seedlings were transplanted to plastic pots (18 cm diameter, 20 seedlings per pot) containing potting mix (50% peat moss, 25% sand and 25% pine bark) and grown in a glasshouse at average 30 ºCdaytime / 20 ºC night temperature under natural sunlight. Seedlings were watered and fertilized as required.
Seedlings of R and S populations were treated with a commercial formulation of quinclorac (Quinclorac 75DF Select) plus 0.5% Hasten (surfactant) using a cabinet sprayer delivering 118 L/hm2water at a pressure of 200 kPa at the 3-leaf stage. The recommended field rate of quinclorac is 800 g/hm2. In the dose response experiments, quinclorac rates used were 0, 12.5, 25, 50, 75, 100, 200 and 400 g/hm2for S; 0, 50, 100, 200, 400, 800, 1 600 and 3 200 g/hm2for R-DC, and 0, 200, 400, 800, 1 600, 3 200 and 6 400 g/hm2for R-CH. Plants were returned to the glasshouse after herbicide application, and the mortality was recorded three weeks after treatment. Plants without new growth or active tiller formation were recorded as dead. The level of resistance was determined using the LD50(herbicide rate causing 50% plant mortality) which is estimated by the non-linear regression analysis. Each experimental treatment had three replicates, and the experiment was conducted at least twice with slightly adjusted rates.
Screening for multiple herbicide resistance
Seed germination and seedling transplanting were as described above. At the 3-leaf stage, five different herbicides commonly used in rice fields forcontrol were applied at the field rates or the rates fully controlling the S plants based on the preliminary experiments. These were penoxsulam (30 g/hm2), bispyribac-sodium (30 g/hm2), pyrazosulfuron- ethyl (120 g/hm2), imazamox (35 g/hm2) and cyhalofop-butyl (125 g/hm2). All herbicides except the last were applied with 0.1% BS1000 as a surfactant, and 0.5% uptake was used with cyhalofop-butyl. There were three replicate pots (each containing 20 seedlings) for each treatment. Mortality was assessed three weeks after treatment.
14C-quinclorac uptake and translocation
Individual plants at the one-leaf stage were transferred into small plastic pots (60 mm × 60 mm × 100 mm) and kept in a controlled environment room with 30 ºC daytime / 25 ºC night temperatures, 12 h light / 12 h dark photoperiod and a photon flux density of 650 μmol/(m2·s). At the 2- to 3-leaf stage, 1 µL14C-labeled quinclorac treatment solution (0.27 mmol/L), containing 0.45 kBq [14C]-quinclorac (BASF, Ludwigshafen, Germany) made up in distilled water plus 0.25% BS1000 and 1.0% Hasten, was applied to the midpoint of the adaxial surface of the second fully expanded leaf. This single droplet of quinclorac treatment solution applied to the plants was approximately equivalent to the rate of 6.7 g/hm2, and preliminary experiments established that early herbicidal symptoms appeared at 48 h after treatment (HAT). Therefore, seedlings were harvested at 6, 24 and 48 HAT. Treated plants were carefully removed from potting mix, and the treated leaf of each plant was rinsed with 20 mL washing buffer containing 20% methanol and 0.2% Triton X-100. The radioactivity present in the rinse solution was quantified by liquid scintillation spectrometry (LSS) to determine unabsorbed radioactivity. The root tissue of each plant was rinsed in 50 mL H2O, and the radioactivity in the root wash was found to be negligible and hence not shown. Quinclorac leaf uptake was calculated from the difference between the radioactivity applied and recovered in the wash solution. The plants were then pressed and oven dried at 65 ºC for 2 d. Quinclorac translocation was visualized using an Amersham Typhoon biomolecular imager 5.0 (GE Healthcare Life Sciences). After imaging, each plant was divided into three sections: root, stem plus new growth and treated leaf. The sections were combusted in a biological oxidizer (RJ Harvey Instrument Corporation, Hillsdale, NJ, USA). Released14CO2was trapped in the cocktail solution and measured by LSS. Herbicide translocation was expressed as the percentage of total absorbed radioactivity recovered from each plant section. The experiment had seven individual-plant replicates per population per time-point. The average radioactivity recovery rates (14C absorbed plus14C in the leaf wash) for the R-DC, R-CH and S populations were 96% ± 4%, 95% ± 2% and 110% ± 3%, respectively.
Metabolism of 14C-quinclorac
Experimental conditions, plant growth and herbicide treatment were the same as described for the leaf uptake and translocation experiment, except that the amount of14C in 1 µL droplet treatment solution was 1.09 kBq, with a final quinclorac concentration of 0.59 mmol/L. The treatment solution was spread along the adaxial surface (close to the leaf base) of the second fully expanded leaf. The aboveground material of treated plants was collected at 6, 24 and 48 HAT. The treated leaf of each plant was rinsed as described above, blotted dry, and the whole plant was snap frozen in liquid nitrogen and stored at -80 ºC. Six individual plants per treatment were bulked as the replicate, and three replicates per treatment were analyzed for each time point.
The samples were ground into powder with a pestle and mortar in liquid nitrogen, and then homogenised in 4 mL of 80% methanol prior to centrifugation at 8 000 ×for 15 min at 4 ºC. The supernatant was decanted and the residue was re-extracted with 1.5 mL of 80% cold methanol, followed by 1.5 mL of 50% cold methanol. The supernatants were pooled and recovered radioactivity was determined by LSS. The pooled supernatant (6.5–7.0 mL) was evaporated to dryness under vacuum with a SpeedVac (Savant, Farmingdale, NY, USA), and the residue was re-suspended in 300 µL of 50% methanol, and centrifuged at 14 000 ×for 5 min before HPLC injection. The average recovery of14C in extracts of the R-CH and S samples was 92% ± 5% and 82% ± 6%, respectively (the R-DC population was not analysed).
Plant extracts were separated on a 250 mm × 4.6 mm Waters Spherisorb ODS2 column (5 µm) by gradient reverse-phase HPLC equipped with a 600E dual-head pump with a 717 plus autosampler (Waters, Milford, MA, USA). Radioactivity was detected with an in-line β-RAM model 2B (IN/US Systems Inc., Pine Brook, NJ, USA) detector. The solvents used were acetonitrile (solvent A) and Milli-Q water with 0.1% formic acid (solvent B). The chromatographic conditions were according to Lamoureux and Rusness (1995), involving a 30 min linear gradient from 10% to 40% solvent B, followed by a 2 min linear gradient from 40% to 99% solvent B, then held at 99% solvent B for 10 min followed by re-equilibration with 10% solvent A for 10 min prior to the next injection. Injection volumes (33–50 µL) were adjusted to provide similar total loading of14C in extracts from both the R and S populations. The flow rate of both the HPLC and the Ultima Gold™ XR scintillant (Perkin Elmer, San Jose, CA) pump on the β-RAM detector was 1.5 mL/min. The relative proportions of14C-labelled parent quinclorac and its metabolites were expressed as a percentage of the total14C peak area in the sample injection. Rice seedlings at the 3-leaf stage, naturally tolerant to quinclorac, were also included in the metabolism study.
Gene expression of β-CAS, ACS and ACO
The 3-leaf stage seedlings ofpopulations were sprayed with 25 g/hm2quinclorac (lower than the LD50of the S population). Shoot materials were harvested at 6 HAT along with untreated control samples and snap-frozen in liquid nitrogen for RNA extraction. Total RNA was isolated using the ISOLATE II Plant RNA Kit (Bioline, London, UK). Genomic DNA was removed using the TURBO-DNA Free Kit (Ambion, USA). DNA contamination was checked using the exon-intron junction primers in Table 1, which can amplify a 750-bp tubulin fragment with genomic DNA but not RNA samples. About 2 μg total RNA was used for cDNA synthesis using SuperScript III Reverse Transcriptase Kit (Invitrogen, USA). Real-time PCR was performed on an ABI-7500 Fast RealTime PCR System (ABI, USA) with the SensiFAST SYBR Lo-ROX Kit (Bioline, London, England). Sequence information fromtranscriptome (Yang et al, 2013) and genome (Guo et al, 2017) was used to design primers for,,and, withas the reference gene (http: //sg.idtdna.com/scitools/Applications/RealTimePCR/) (Table 1). Becauseis a polyploid species, there are homeologous copies of these genes. Hence PCR primers in the relatively conserved regions were designed for amplification of all possible copies.The specificity of each PCR product was confirmed by the melting-curve analysis, and primer efficiency and slope were determined as 93% to 101% and -3.2 to -3.5, respectively. Relative changes in gene expression were determined using the comparative 2-ΔΔCtmethod.
β-CAS activity assay
plants were treated with 50 g/hm2quinclorac (close to the LD50of the S population) at the 3-leaf stage. The aboveground materials from treated and untreated control plants were harvested at 2, 6, 12 and 24 HAT foractivity analysis.extraction and activity assay were performed using the plant cyanoalanine synthase (CAS) ELISA Kit (Mlbio Corporation, China) according to the manufacturer’s instructions. About 2 g fresh aboveground materials were used for each measurement with three replicates.
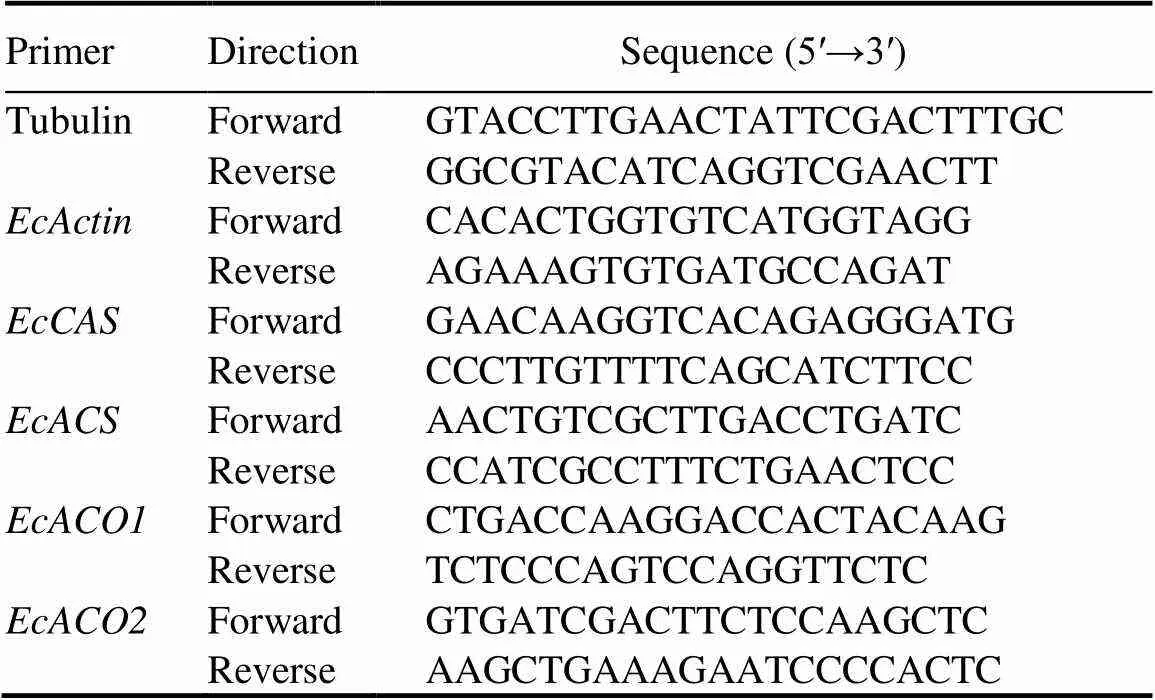
Table 1. Real-time PCR primer sequences.
Statistical analysis
For the dose-response study, the plant survival was assessed at three weeks after quinclorac treatment. The herbicide rate causing 50% plant mortality (LD50) was estimated using the four-parameter logistic equation (SigmaPlot 14.0, Systat Software, Inc., USA) according to Chen J Y et al (2018):
=+ (–) / [1 + (/0)]
Whereis the plant survival rate (%),is the quinclorac rate,0is the rate giving 50% plant mortality (LD50) andis the slope at0,is the lower limit representing plant survival at infinitely large herbicide rates,is the upper limit representing plant survival close to untreated controls. Significant differences of the LD50values among R and S populations were subject to-test at< 0.05. The R/S LD50ratio was calculated to indicate the level of resistance.
The data from14C-quinclorac and gene expression experiments were analyzed using the software SPSS, version 25.0 for Windows. Significant differences among and between means were analyzed using the Duncan’s test or the-test at the 5% level.
RESULTS
Dose-response to quinclorac
As expected, the S population was fully controlled by quinclorac, with no survivors at the rate of 200 g/hm2(Fig. 1) and the LD50of 41 g/hm2. However, the two R populations were not controlled even at the highest rates (3 200 and 6 400 g/hm2for R-DC and R-CH, respectively), meaning the LD50could not be calculated and giving R/S ratios of > 78 and > 156, respectively.
Multiple herbicide resistance screening
Five commonly used herbicide chemistries active against(four ALS inhibitors and one ACCase inhibitor) were tested. The two quinclorac-R populations remained susceptible to penoxsulam, bispyribac-sodium, imazamox and cyhalofop-butyl at the field rates, but there was a variable level of resistance to the ALS inhibitor pyrazosulfuron-ethyl (Table 2).
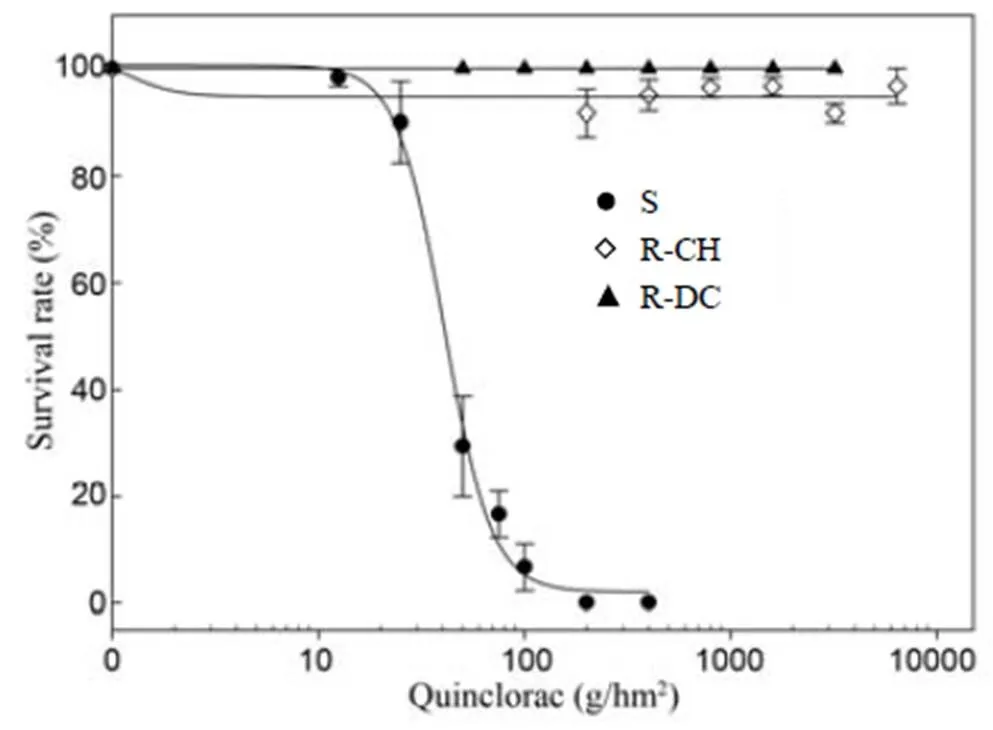
Fig. 1. Dose response to quinclorac ofpopulations at 3 weeks after treatment.
‘S’ indicates the susceptible population and ‘R-DC’ and ‘R-CH’ indicate two resistant populations.
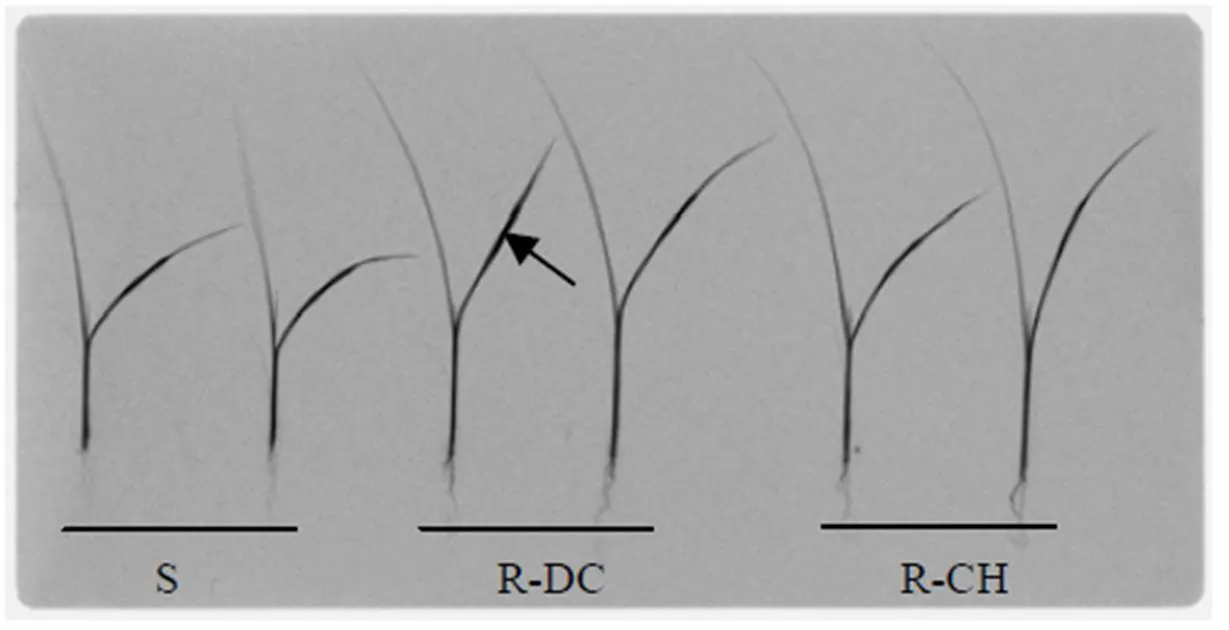
Fig. 2.Phosphor imaging comparing translocation pattern of14C-quinclorac inpopulations at 6 h after quinclorac treatment.
14C-quinclorac was applied to the midpoint of the adaxial surface of the second fully expanded leaf (arrowed) of each plant.
‘S’ indicates the susceptible population and ‘R-DC’ and ‘R-CH’ indicate two resistant populations.
Foliar uptake and translocation of 14C-quinclorac
Leaf uptake of quinclorac by R and S plants was rapid in the presence of 0.5% Hasten® (surfactant), with up to 97% quinclorac absorbed within 6 h of application. There was no significant difference in leaf uptake of quinclorac between the R and S plants at any time point (Table 3 and Fig. 2). Most14C was detected in the stem and new growth rather than the roots in both R and S plants, but significantly more14C was translocated to the stem and leaf in both the two R populations than in the S population, especially at 24 and 48 HAT (Table 3). The R-CH population also had more14C in the roots, as compared to the R-DC and S plants at 6 and 24 HAT (Table 3).
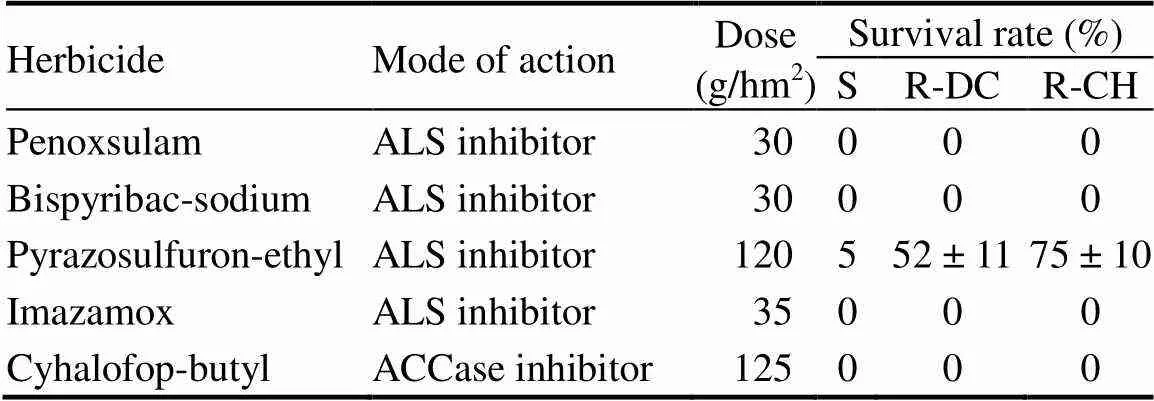
Table 2. Survival rate of E. crus-galli populations treated with herbicides.
‘S’ indicates the susceptible population and ‘R-DC’ and ‘R-CH’ indicate two resistant populations.
14C-quinclorac metabolism
Under our optimized HPLC condition, the14C-labelled quincloracstandard and the extractable parent quinclorac from treatedand rice seedlings eluted as a single peak at around 25 to 26 min (Supplemental Fig. 1). Two major quinclorac metabolites eluted at around 18 and 22 min, and were detected in both R and S plants, but the percentage of14C eluting as these metabolites was upto 2-fold lower in the R than in the S plants at 24 and 48 HAT (Table 4 and Supplemental Fig. 1). Quinclorac metabolism in rice was slightly different from that in, with only one major metabolite peak eluting at 22 min (Supplemental Fig. 1), accounting for only 6% of total14C recovered. So far, the nature of the metabolites in rice andremains to be determined.
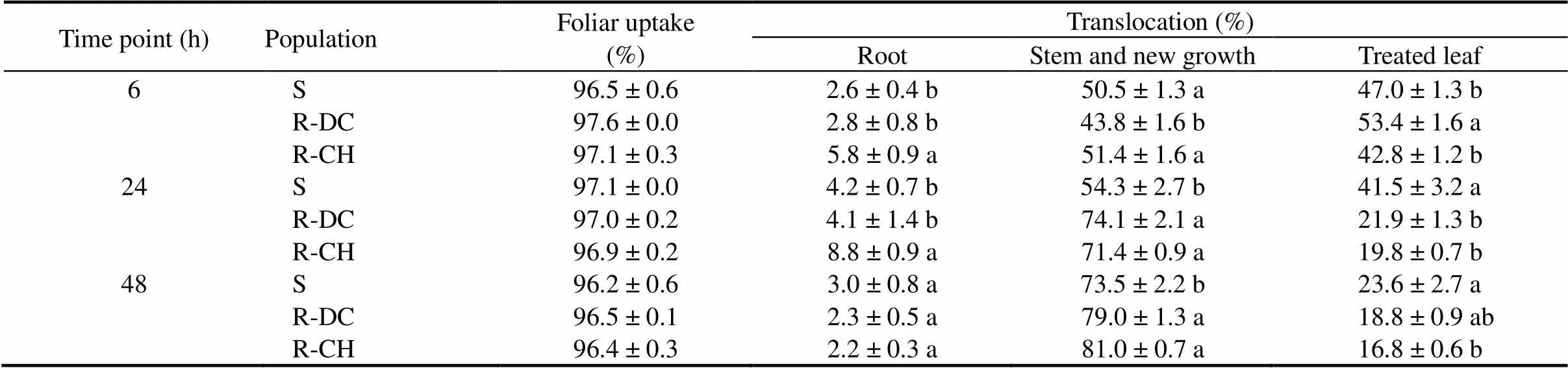
Table 3. Foliar uptake and translocation of absorbed 14C in E. crus-galli populations.
‘S’ indicates the susceptible population and ‘R-DC’ and ‘R-CH’ indicate two resistant populations.
Mean ± SE (= 5) labeled with the same letter in a column within a time point are not significantly different at the 0.05 level (-test).
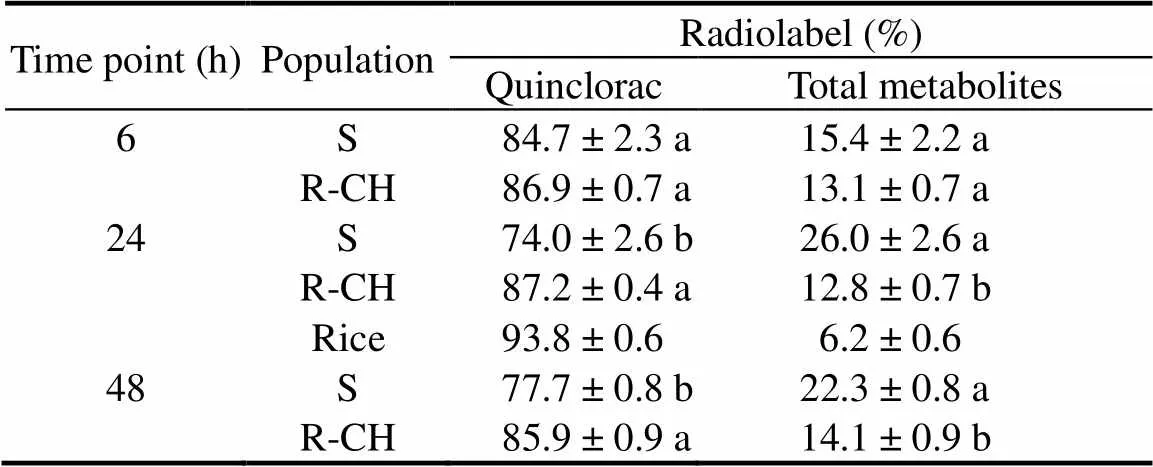
Table 4. Metabolism of 14C-quinclorac in E. crus-galli populations.
‘S’ indicates the susceptible population and ‘R-DC’ and ‘R-CH’ indicate two resistant populations.
Mean ± SE (= 7) labeled with the same letter in a column within a time point are not significantly different at the 0.05 level (-test).
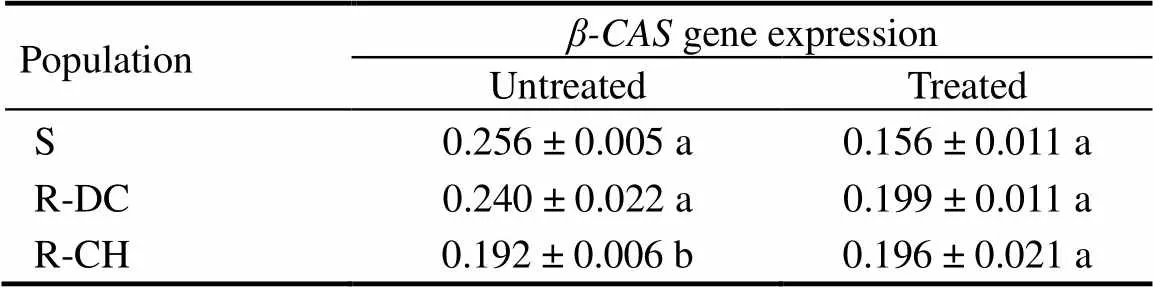
Table 5. Relative β-CAS expression in the shoot of E. crus-galli populations at 6 h after treatment.
‘S’ indicates the susceptible population and ‘R-DC’ and ‘R-CH’ indicate two resistant populations.
Data (Mean ± SE,= 3) are expressed as 2-ΔΔCt. Values labeled with the same letter in a column are not significantly different at the 0.05 level (Duncan’s test).
β-CAS gene expression
The basal (untreated) level ofexpression in the two R populations was either statistically to or lower than the S populations (Table 5). Similarly, there was no significant difference ingene expression in treated populations at 6 HAT (Table 5). In addition, relative to their respective untreated controls, the expression ofgene in the two treated R populations was only marginally changed, but it was decreased by nearly 40% in the treated S population.
β-CAS activity
In the absence of quinclorac, the basal level of β-CAS activity was similar between the R-CH and S samples, with a lower activity in the R-DC plants (Fig. 3). Following quinclorac treatment, consistent patterns in β-CAS activity in the R and S samples were observed up to 12 HAT. At 24 HAT, S plants started to show visual symptoms of quinclorac damage (data not shown), but β-CAS activity in these plants was the same as in R-DC population and lower than in R-CH.
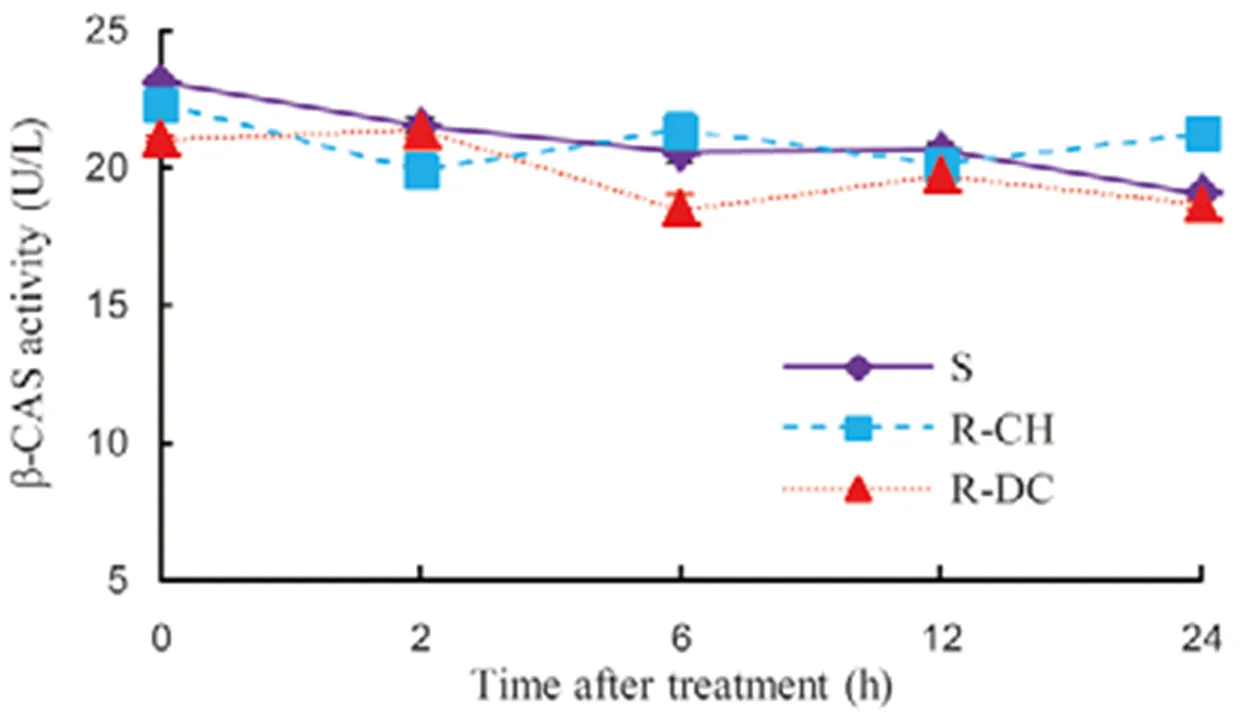
Fig. 3. Activity of β-CAS in the shoot ofpopulations treated with 50 g/hm2quinclorac.
‘S’ indicates the susceptible population and ‘R-DC’ and ‘R-CH’ indicate two resistant populations.
Gene expression of ACS, ACO1 and ACO2
In the absence of quinclorac, the expression levels ofandwere significantly higher in R-DC compared to the R-CH and S populations, but there were no significant differences among populations inexpression (Table 6). Six hours after quinclorac treatment, theexpression was significantly higher in the S plants compared to the two R populations. Expression level ofwas significantly higher in the S plants than in the R-CH (Table 6).
In the susceptible population, the relative expression levels of,andin treated plants were increased by 16%, 120% and 140% of untreated control, respectively. In contract, in R-DC population, the expression levels ofandin treated plants were decreased, and theexpression increased only by 13%. In the R-CH population, there was no significant change in the expression of, and a small increase in(4%) and moderate increase (44%) inat 6 h after quinclroac treatment (Table 6).
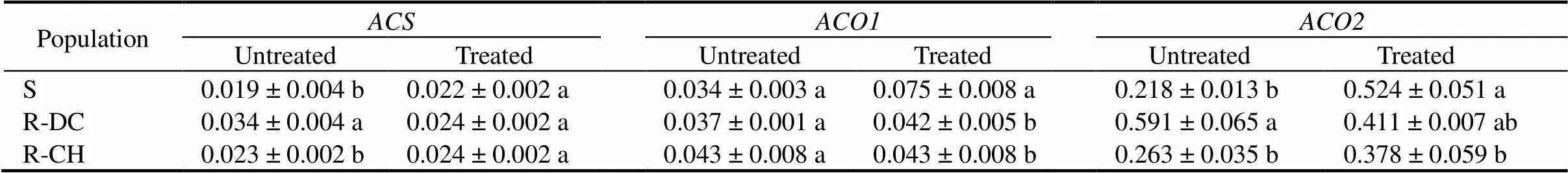
Table 6. Relative expression levels of ACS, ACO1 and ACO2 in the shoots of E. crus-galli populations at 6 h after quinclorac treatment.
‘S’ indicates the susceptible population and ‘R-DC’ and ‘R-CH’ indicate two resistant populations.
Data (Mean ± SE,= 3) are expressed as 2-ΔΔCt. Values labeled with the same letter in a column are not significantly different at the 0.05 level (Duncan’s test).
DISCUSSION
Quinclorac-resistant E. crus-galli populations can be controlled by alternative herbicides
In this work, we confirmed that the twopopulations (R-DC and R-CH) have evolved high- level resistance to quinclorac (Fig. 1). In addition, these two populations are also resistant to the ALS inhibitor herbicide pyrazosulfuon-ethyl,but are still controlled by other commonly used herbicides including bispyribac-sodium, penoxsulam, imazamox, pyrazosulfuron-ethyl and cyhalofop-butyl. Therefore, alternative herbicides in rotation, sequence or mixture remain effective in control of the R-DC and R-CH populations. As there are more quinclorac-resistantpopulations in Hunan Province (Ma et al, 2013), their resistance patterns to the above herbicides need to be determined. The above-mentioned ALS and ACCase inhibitor herbicides are commonly used in paddy fields forcontrol, and resistance evolution to these modes of action is inevitable if weed control relies solely on herbicides. In fact, resistance to the ALS inhibitor herbicide penoxsulam has been reported in quinclorac-resistantpopulations from other provinces in China (Chen et al, 2016). Thus, non-herbicide control tactics should be considered in order to reduce selection pressure for resistance evolution.
Reduced quinclorac uptake or translocation and enhanced metabolism are not resistance mechanisms in these two E. crus-galli populations
In this study, we showed that quinclorac uptake was rapid (6 HAT), with a high level (97% applied) of absorption by the leaf tissue in the R and S populations (Table 5). Hence, reduced foliar uptake of quinclorac did not contribute to resistance in these two R populations. The same conclusion is also drawn from studies on quinclorac-resistantpopulations from other countries (Lopez-Martinez and de Prado, 1996; Grossman and Kwiatkowski, 2000; Lovelace et al, 2007).
Interestingly, a greater level of14C translocation from the treated leaf to the stem and new growth was recorded in the R compared to the S population at 24 HAT (Table 3). This observation differs from the report by Grossmann and Kwiatkowski (2000), but is similar to that of Lovelace et al (2007).Most importantly, the results suggest that reduced quinclorac translocation is unlikely to be a resistance mechanism in the R-DC and R-CH populations. The reason for the higher14C-translocation observed in the R populations is currently unknown, but could be due to inhibition of translocation in the S plants through tissue damage caused by the toxicity of quinclorac itself. In order to avoid quinclorac phytotoxicity in the translocation study, the14C-quinclorac was applied at a very low rate, but there were still some visual symptoms in the S plants at 48 HAT.
The translocation pattern of14C-quinclorac in the S plants may also have been altered by the greater level of quinclorac metabolism observed in this population (26%) compared to the R populations and the quinclorac-resistant rice (6%–14%). The relatively low level of metabolism in all plants tested is consistent with previous studies showing limited quinclorac metabolism in weedy grass species and tolerant rice (Grossmann and Kwiatkowski, 2000; van Eerd et al, 2005). The lower level of quinclorac metabolism in the R and S plants would rule out a metabolism-based potential resistance mechanism unless the quinclorac metabolites are more phytotoxic than the parent molecule (data not shown). Quinclorac translocation and metabolism needs to be investigated in morepopulations to clarify if the unusual patterns observed here (i.e. higher metabolism in S and higher translocation R plants) are consistent across the species, and thus represent the consequence of the primary yet-to-be-discovered quinclorac resistance mechanism.
Quinclorac signal reception and transduction and ethylene biosynthesis regulation may be involved in resistance
Cyanide was found to be the primary phytotoxic compound of the quinclorac mode of action in(Grossmann and Kwiatkowski, 1995; Grossmann, 2003, 2010). Quinclorac selectivity in rice and several resistant populations of weedy grasses, including, was found to be due to cyanide detoxification via β-CAS activity (Grossmann and Kwiatkowski, 2000; Gao et al, 2018). However, in this study,gene expression and β-CAS activity in the R and S populations did not follow a clear pattern either before or after quinclorac treatment (Table 5 and Fig. 3), indicating a negligible role of cyanide detoxification in quinclorac resistance in the two R populations. Detailed genotyping and qPCR analysis in quinclorac resistant and susceptiblecrossing lines (F6progeny) also revealed no correlation between enhanced β-CAS activity and quinclorac resistance (Chayapakdee et al, 2017). The discrepancy between studies in the role of β-CAS in quinclorac resistance may be related to plant species and experimental conditions (such as hydroponic versus soil experiments, low versus field rates and treatment duration). In this study, to differentiate primary and secondary responses ofto quinclorac, soil-grownseedlings were foliar-treated at the quinclorac rate close to the LD50of the S plants, andgene expression and β-CAS activity were measured both in the response stimulation phase (6 HAT and earlier) and inhibition phase (12 HAT and later) (for details of three-phase response to auxinic herbicides (Grossmann, 2010).
As cyanide is a co-product of ethylene biosynthesis (Grossmann, 2010), it can be assumed that ethylene biosynthesis in the R plants may not be up-regulated. Indeed, the expression ofandgenes involved in ethylene biosynthesis was induced by 2.7- and 2.4-fold, respectively, in the S but not in the R plants following quinclorac treatment (Table 6). Therefore, it is likely that upstream events in quinclorac signal perception and transduction are responsible for resistance in the twopopulations.
The synthetic auxin herbicides contain several classes of different chemical structure, all of which mimic the endogenous plant hormone, indole-3-acetic acid (IAA) (Grossmann, 2010). Previous research showed that auxin herbicides bind to the plant auxin receptors TIR1/AFB1-5 and the Aux/IAA co-receptors to modulate auxin-responsive gene expression (Grossmann, 2010; Christoffoletti et al, 2015). Modification of the auxin receptor(s) affecting the binding of auxin herbicides may lead to resistance. For example, in, an AFB5 mutant line was found to be resistant to the auxinic herbicide picloram (Walsh et al, 2006). The mutantline is resistant to 2,4-D (Takahashi et al, 2017), and anmutant line is resistant to 2,4-D but not to dicamba (Gleason et al, 2011). Recently, a mutation in an/gene in the highly conserved degron region has been confirmed to endow resistance to dicamba, 2,4-D and fluroxypyr in(LeClere et al, 2018). Clearly, mutations of different auxin receptors/co-receptors can confer resistance to different auxin herbicides. Taking the advantage of the availability of thegenome sequence (Guo et al, 2017), work is under way on identification of possible mutations in auxin receptor and related proteins using RNA-sequencing.
ACKNOWLEDGEMENTS
This work was financially supported by the National Natural Science Foundation of China (Grant Nos. 31701803 and 31772182), the Natural Science Foundation of Hunan Province (Grant No. 2017JJ3164) in China andthe Australian Grains Research and Development Corporation (GRDC).
SUPPlemental DATA
The following material is available in the online version of this article at http://www.sciencedirect.com/science/ journal/16726308; http://www.ricescience.org.
Supplemental Fig. 1. HPLC chromatograms of14C-quinclorac and its metabolites in extracts ofand rice.
Bajwa A A, Jabran K, Shahid M, Ali H H, Chauhan B S, Ehsanullah. 2015. Eco-biology and management of., 75: 151–162.
Chayapakdee P, Iwakami S, Kamidate Y, Uchino A, Fan L, Sunohara S, Matsumoto H. 2017. Enhanced activity of β-cyanoalanine synthase does not confer quinclorac resistance in multiple-herbicide resistant. The 26th Asian-Pacific Weed Science Society Conference, Weed Science for People, Agriculture and Nature. Kyoto, Japan.
Chen G Q, Wang Q, Yao Z W, Zhu L F, Dong L Y. 2016. Penoxsulam-resistant barnyardgrass () in rice fields in China., 16(1): 16–23.
Chen J Y, Yu Q, Owen M, Han H P, Powles S. 2018. Dinitroaniline herbicide resistance in a multiple-resistantpopulation., 74: 925–932.
Chen T, Zhang S L, Zhao L, Zhang Y D, Zhu Z, Zhao Q Y, Zhou L H, Yao S, Zhao C F, Liang W H, Wang C L. 2018. Development and verification of a functional marker associated with resistance to ALS inhibitor herbicide., 32(2): 137–145. (in Chinese with English abstract)
Christoffoletti P J, de Figueiredo M R A, Peres L E P, Nissen S, Gaines T. 2015. Auxinic herbicides, mechanisms of action, and weed resistance: A look into recent plant science advances.,72(4): 356–362.
Fipke M V, Vidal R A. 2016. Integrative theory of the mode of action of quinclorac: Literature review., 34(2): 393–402.
Gao Y, Pan L, Sun Y, Zhang T, Dong L Y, Li J. 2017. Resistance to quinclorac caused by the enhanced ability to detoxify cyanide and its molecular mechanism invar.., 143: 231–238.
Gao Y, Li J, Pan X K, Liu D R, Napier R, Dong L Y. 2018. Quinclorac resistance induced by the suppression of the expression of 1-aminocyclopropane-1-carboxylic acid (ACC) synthase and ACC oxidase genes invar.., 146: 25–32.
Grossmann K, Kwiatkowski J. 1993. Selective induction of ethylene and cyanide biosynthesis appears to be involved in the selectivity of the herbicide quinclorac between rice and barnyard grass.,142(6): 457–466.
Grossmann K, Kwiatkowski J. 1995. Evidence for a causative role of cyanide, derived from ethylene biosynthesis, in the herbicide mode of action of quinclorac in barnyard grass., 51(2): 150–160.
Grossmann K, Kwiatkowski J. 2000. The mechanism of quinclorac selectivity in grasses.,66(2): 83–91.
Grossmann K. 2003. Mediation of herbicide effects by hormone interactions.,22(1): 109–122.
Grossmann K. 2010. Auxin herbicides: Current status of mechanism and mode of action.,66: 113–120.
Gleason C, Foley R C, Singh K B. 2011. Mutant analysis inprovides insight into the molecular mode of action of the auxinic herbicide dicamba., 6(3): e17245.
Guo L B, Qiu J, Ye C Y, Jin G L, Mao L F, Zhang H Q, Yang X F, Peng Q, Wang Y Y, Jia L, Lin Z X, Li G M, Fu F, Liu C, Chen L, Shen E H, Wang W D, Chu Q J, Wu S Y, Wu S L, Xia C Y, Zhang Y F, Zhou X M, Wang L F, Wu L M, Song W J, Wang Y F, Shu Q Y, Aoki D, Yumoto E, Lou Y G, Qian Q, Yamguchi H, Yamane H, Kong C H, Timko M P, Bai L Y, Fan L J. 2017.genome analysis provides insight into its adaptation and invasiveness as a weed., 8: 1031.
Heap I. 2018. International survey of herbicide resistant weeds. http://www.weedscience.com. 27 July 2018.
Hoyerova K, Hosek P, Quareshy M, Li J, Klima P, Kubes M, Yemm A A, Neve P, Tripathi A, Bennett M J, Napier R M. 2018. Auxin molecular field maps define AUX1 selectivity: Many auxin herbicides are not substrates., 217(4): 1625–1639.
Lamoureux G L, Rusness D G. 1995. Quinclorac absorption, translocation, metabolism, and toxicity in leafy spurge ()., 53(3): 210–226.
LeClere S, Wu C X, Westra P, Sammons R D. 2018. Cross- resistance to dicamba, 2,4-D, and fluroxypyr inis endowed by a mutation in angene., 115(13): 2911–2920.
Li G, Xu M F, Chen L P, Cai L M, Bai L Y, Wu C X. 2016. A novelgene with a different expression pattern in quinclorac- resistant and susceptible barnyardgrass ()., 5: 65–70.
Lopez-Martinez N, de Prado R. 1996. Fate of quinclorac in resistant.: Second International Weed Control Congress. Copenhagen, Denmark: Eurekamag: 535–540.
Lopez-Martinez N, Marshall G, de Prado R. 1997. Resistance of barnyardgrass () to atrazine and quinclorac., 51(2): 171–175.
Lovelace M L, Talbert R E, Hoagland R E, Scherder E F. 2007. Quinclorac absorption and translocation characteristics in quinclorac- and propanil-resistant and -susceptible barnyardgrass () biotypes., 21(3): 683–687.
Ma G L, Liu D C, Liu X Y, Tang T, Peng Y J. 2013. Resistance of barnyard grass () to quinclorac in double-harvest rice area in Hunan Province of China., 4: 23–27.
Oerke E C, Dehne H W. 2004. Safeguarding production-losses in major crops and the role of crop protection., 23(4): 275–285.
Rouse C E, Roma-Burgos N, Norsworthy J K, Tseng T M, Starkey C E, Scott R C. 2018.resistance to herbicides continues to increase in Arkansas rice fields., 32(1): 34–44.
Sunohara Y, Matsumoto H. 2004. Oxidative injury induced by the herbicide quinclorac onvasing. and the involvement of antioxidative ability in its highly selective action in grass species., 167(3): 597–606.
Takahashi M, Umetsu K, Oono Y, Higaki T, Blancaflor E B, Rahman A. 2017. Small acidic protein 1 and SCFTIR1ubiquitin proteasome pathway act in concert to induce 2,4-dichlorophenoxyacetic acid-mediated alteration of actin inroots., 89(5): 940–956.
Talbert R E, Burgos N R. 2007. History and management of herbicide-resistant barnyardgrass () in Arkansas rice., 21(2): 324–331.
van Eerd L L, Stephenson G R, Kwiatkowski J, Grossmann K, Hall J C. 2005. Physiological and biochemical characterization of quinclorac resistance in a false cleavers (L.) biotype.,53(4): 1144–1151.
Walsh T A, Neal R, Merlo A O, Honma M, Hicks G R, Wolff K, Matsumura W, Davies J P. 2006. Mutations in an auxin receptor homolog AFB5 and in SGT1b confer resistance to synthetic picolinate auxins and not to 2,4-dichlorophenoxyacetic acid or indole-3-acetic acid in., 142(2): 542–552.
Wright A A, Rodriguez-Carres M, Sasidharan R, Koski L, Peterson D G, Nandula V K, Ray J D, Bond J A, Shaw D R. 2018. Multiple herbicide-resistant junglerice (): Identification of genes potentially involved in resistance through differential gene expression analysis., 66(3): 1–8.
Xu J Y, Lv B, Wang Q, Li J, Dong L Y. 2013. A resistance mechanism dependent upon the inhibition of ethylene biosynthesis., 69(12): 1407–1414.
Yang X, Yu X Y, Li Y F. 2013.assembly and characterization of the barnyardgrass () transcriptome using next-generation pyrosequencing., 8(7): e69168.
Yang X, Zhang Z C, Gu T, Dong M C, Peng Q, Bai L Y, Li Y F. 2017. Quantitative proteomics reveals ecological fitness cost of multi-herbicide resistant barnyardgrass (L.).,150: 160–169.
Yasuor H, Milan M, Eckert J W, Fischer A J. 2012. Quinclorac resistance: A concerted hormonal and enzymatic effort in., 68(1): 108–115.
Zhu J W, Wang J, DiTommaso A, Zhang C X, Zheng G P,Liang W, Islam F, Yang C, Chen X X, Zhou W J. 2018. Weed research status, challenges, and opportunities in China.,doi.org/10.1016/j.cropro.2018.02.001.
27 July 2018;
9 December 2018
Yu Qin (qin.yu@uwa.edu.au)
Copyright © 2019, China National Rice Research Institute. Hosting by Elsevier B V
This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/)
Peer review under responsibility of China National Rice Research Institute
http://dx.doi.org/10.1016/j.rsci.2019.08.004
(Managing Editor: Li Guan)
- Rice Science的其它文章
- Fusarium solani Upregulated Sesquiterpene Synthase Expression, Sesquiterpene Production and Allelopathic Activity in Piper betle
- Quantitative Trait Loci Mapping for Rice Yield-RelatedTraits Using Chromosomal Segment Substitution Lines
- Differential RNA Editing of Mitochondrial Genes in WA-Cytoplasmic Based Male Sterile Line Pusa 6A, and Its Maintainer and Restorer Lines
- Effects of Integrated Organic and Inorganic Fertilizers on Yield and Growth Parameters of Rice Varieties
- Strategies for Fermentable Sugar Production by Using Pressurized Acid Hydrolysis for Rice Husks
- CRISPR/Cas9: Development and Application in Rice Breeding