Fusarium solani Upregulated Sesquiterpene Synthase Expression, Sesquiterpene Production and Allelopathic Activity in Piper betle
Kamrai Woranoot, Rattikarn Buaruang, Kodchakorn Aranyakanon, Kumrop Ratanasut, Anupan Kongbangkerd, Panatda Jannoey, Pranee Nangngam, Chonnanit Choopayak
UpregulatedExpression, Sesquiterpene Production and Allelopathic Activity in
Kamrai Woranoot1, Rattikarn Buaruang2, Kodchakorn Aranyakanon2, Kumrop Ratanasut3, Anupan Kongbangkerd1, Panatda Jannoey2, Pranee Nangngam1, Chonnanit Choopayak2
(Department of Biology, Faculty of Science, Naresuan University,Phitsanulok 65000, Thailand; Department of Biochemistry, Faculty of Medical Science,Naresuan University, Phitsanulok 65000, Thailand; Department of Agricultural Science, Faculty of Agriculture, Natural Resources and,)
Activators of() gene expression and sesquiterpene production inL.were examined using quantitative real time PCR and gas chromatography mass spectrometry methods,and the allelopathic activity of untreated and-treated betel extracts was tested on seed germination and on the shoot and root growth of Thai rice variety PSL2 (cv. Phitsanulok2) and three dominant paddy weeds (,and). The results demonstrated thatdramatically upregulatedexpression and productions ofb-cubebene, β-caryophyllene and germacrene D sesquiterpene when compared with the untreated control, and that betel extracts had a greater inhibitory effect on weeds than on rice. The effects were more clearly detected on seed germination and root growth than on shoot growth, and they were found to be dose-dependent. It is also noted that-treated extract had stronger effects than the untreated extract. The species most sensitive to the allelopathic effects was, germination of which was completely inhibited even at a dose of 0.1 mg/mL untreated extract. With regards to rice, although betel extract at 1.0 mg/mL showed no inhibitionon germination, it affected the elongation of rice roots, in addition to those of the tested weeds. The obtained data suggested thathas potential as an activator of sesquiterpene allelochemical production viaexpression, the latter leading to the treated betel extract having a stronger phytotoxic effect. These results were beneficial in the promotion of natural herbicide production using biotechnology.
L.; paddy weed;; sesquiterpene synthase; allelopathic activity
Weeds are a major problem encountered in paddy fields during rice production, as they reduce the productivity and quality of the crop (Xu et al, 2018), but as a solution, farmers routinely resort to the use of synthetic chemical herbicides that are toxic to users, consumers and the environment (Myers et al, 2016; de Brito Rodrigues et al, 2017). By avoiding the side-effects of this toxicity, natural products provide an alternative method for the control of paddy weeds (Reigosa et al, 2013), and these products include allelochemicals and secondary metabolites from plants and microorganisms that affect the growth and development of other biological systems (Poulson-Ellestad et al, 2014). Among these, sesquiterpenes (C15H24) obtained from a large number of plant sources have been reported as having allelochemical properties (Amri et al, 2013; de Miranda et al, 2014; Abd El-Gawad et al, 2018), sesquiterpenes themselves being volatile organic compounds. Their biosynthesis occurs primarily via the MVA pathway in the cytosol, using farnesyl diphosphate (FDP) as a substrate and sesquiterpene synthase (STS) as a key enzyme (Hattan et al, 2018). In plants, they play a role as direct or indirect defenses against herbivores and plant pathogens (Mithöfer and Boland, 2016), and as reported earlier, plants synthesized and emitted increased levels of sesquiterpenes following virulent fungi infection viagene induction (Park et al, 2014).
To investigate whether there were specific interactions between fungi and the species of plant tested, different fungi, including,,and, were investigated for their potential as activators ofexpression and sesquiterpene production. The virulent necrotrophiccauses root rot in soybean (Costa et al, 2016) and black pepper (da Luz et al, 2017).causes leaf spots in(Akram et al, 2014) and rice (Majeed et al, 2016).
is a devastating hemibiotrophic fungus and is a pathogen of over 72 plant species, including economically-important crops such as pepper.is a non-pathogenic fungus and it was included in the study as a biological control against other plant pathogenic fungi (Gajera et al, 2015) and paddy disease brown spot (Gupta et al, 2018).
plants exhibit an allelopathic effect by inhibiting germination and growth, which is observed in several weed species. The extract from(Kunth) Steudel leaves inhibits the germination and early growth of radish (L.) (Borella et al, 2012). The methanolic extract ofRoxb. inhibits seedling growth in narrow leaf weeds, includingLam.,Gaud.,L.,(L.) Nees.,(L.) Link,(L.) P. Beauv. andL. (Pukclai and Kato-Noguch, 2011). The ethyl acetate and n-butanol extracts ofL. show inhibitory activity on(Aubl.) Sw.,P. Beauv. andScop(Yan et al, 2006). Our preliminary study reported that ethyl acetate extract ofcontains high quantities of phenylpropanoids (eugenol acetate and 4-allyl-1,2-diacetoxybenzene) and a substantial amount of sesquiterpenes (β-caryophyllene, δ-cadinene,a-copaene anda-humulene). They have phytotoxic effects on paddy weed germination but not on crop germination (Woranoot et al, 2015b), and thuswas chosen as the plant to be tested for strong allelopathic activity enhancement.
To obtain and examine an extract which demonstrated high levels of allelopathic activity, the activators ofexpression and sesquiterpene production that promotes the allelopathic activity of betel extract were investigated in this study.
MATERIALS AND METHODS
Plant materials and tissue culture
L. were grown in Phisanulok Province, Thailand. They were transplanted in 12 cm diameter pots and watered daily in the nursery. Three-month-old plants were used for explants. The stem ofwas cut into pieces (about 5 cm in length) and washed in detergent-water and tap water, respectively. The stem pieces were soaked in 0.2% carbendazim for 10 min followed by 15% cloroxÒfor 5 min and 0.1%HgCl2for 5 min. After that, they were washed in sterile water for three times and placed on sterile petri dish. The node and shoot were cut into small pieces (about 1.5–2.0 cm) and cultured in Murashige and Skoog (MS) basal medium (Murashige and Skoog, 1962) without plant growth regulators. The tissues were grown at 25 ºC±2ºC under 12 h light/12 hdark photoperiod at 20 µmol/(m2·s). After 10 weeks, 15plantlets (about 5 cm in length) were used for further study.
For allelopathic activity assay, the seeds of narrow leaf weeds including swallen fingergrass (Sw.), barnyard grass ((L.) T. Beauv.) and broad leaf weed false daisy (L.) were collected from paddy in Sukhothai and Phitsanulok provinces, Thailand, in February 2015. Voucher specimens were deposited at plant specimen collection room at Department of Biology, Faculty of Science, Naresuan University. Thai rice varietyPhitsanulok2 (PSL2) was provided by Phitsanulok Rice Seed Center, Thailand. The seed of lettuce (L.) was purchased from Chiatai®, Thailand.
Microorganism
Four fungi were obtained from BIOTEC Culture Collection, National Center for Genetics Engineering and Biotechnology, Thailand, including(BCC No.31824),(BCC No.4805),(BCC No.15560) and(BCC No.17755). The fungi were cultured on potato dextrose agar (PDA) and incubated at (30±2) ºC for 7 d.
Elicitation for STS expression and sesquiterpene production study
Fungal disc agar assay was performed for fungal treatment. The 0.5 diameter agar disc with fungal mycelium was put on the upper of the 1st and 2nd betel leaf tissue culture. The control samples were treated with PDA without fungus. Leaf samples were collected at 2 d after inoculation. Untreated and treated leaves were examined forexpression and sesquiterpene production.
RNA isolation and cDNA synthesis
The easyREDTM kit (iNtRON Biotechnology, Korea) was used to isolate total RNA from betel leaves. The leaf (0.1 g) was grounded to fine powder by chilled mortar and pestle. Total RNA was obtained following the manufacturer’s instruction. Contaminated DNA was removed by RNase-free DNase I (Sigma-Aldrich, USA) for 30 min at 37 ºC. Afterwards, RNA quantity and quality were determined using NanoDrop 2000 UV-Vis Spectrophotometer (Thermo Scientific, USA) and 1% agarose gel electrophoresis.
The first strand cDNA was synthesized from total RNA by mixing 250 ng total RNA with 100 μmol/L Oligo(dT)18and incubated at 65 ºC for 10 min. Then, the reaction systemincluding 1× buffer, 20 U Ribulock (Thermo Scientific, USA), 1 mmol/L each dNTP, 200 U RevertAid Reverse Transcriptase (Thermo Scientific, USA) and deionized distilled water, was incubated at 42 ºC for 90 min and then terminated at 70 ºC for 10 min. The first stand cDNA reaction mixture was used as a template for quantitative real-time polymerase chain reaction (qRT-PCR) analysis.
qRT-PCR
qRT-PCR was performed for measurements of transcript abundance ofgene. The transcript forgene was amplified with forward primer 5′-CGATATAGAAGGCATGTTGAGC-3′ and reverse primer 5′-CA TGAGATTGACCTCCTTGC-3′. Transcipts ofwas traced by amplification with the forward primer 5′-CATGAGACTACATACAACTCCATC-3′ and reverse primer 5′-TCGTACTCAGCCTTGGCAATC CAC-3′. The reaction mixture contained cDNA, 0.25 µmol/L of each primer and RBC ThermOne™ Real-Time PCR Premix (SYBR Green) Kit (RBC Bioscience, Taiwan). PCR amplification was performed with each cycle: 95 ºC for 10 min, 95 ºC for 30 s, annealing for 30 s and 72 ºC for 30 s for 35 cycles using LightCycler® 96 Real-Time PCR (Roche, US). Then, the relative expression was analyzed using 2-ΔΔCtmethod of the LightCycler® 96 SW1.1 program. In addition to the non-template (water) control, the non-enzyme control was also performed. Triplication of all samples were done.
Preparation of volatile extracts
The volatile extraction was done following modified method of Jin et al (2015). Untreated and-treated fresh leaves were ground to small pieces with a blender. Leave pieces (50 g) were submerged in 250 mL ethyl acetate (GC grade) containing 10 mg/mL of camphor (GC grade, Sigma-Aldrich, USA) as an internal standard. The mixture was incubated at 4 ºC for 48 h. Afterwards, the mixture was filtrated with filter paper (Whatman No.1) and dehydrated with anhydrous Na2SO4(Sigma-Aldrich, USA). Then, the filtrate was collected and evaporated using a RotavaporÒR-124 rotary evaporator (BUCHI, Switzerland) at 40 ºC. The crude extracts were stored at 4 ºC in dark for further experiment.
Gas chromatography-mass spectrometry (GC-MS) analysis
The betel ethyl acetate extracts were analyzed for chemical composition using GC-MS. The extracts were dissolved in absolute ethanol at a ratio of 1:100, filtered through 0.22 µmol/L and then subjected to GC-MS (GC Agilent 6890N and MS Agilent 2577A 5973N Agilent Technologies, USA). The analysis was carried out using HP-5MS, 19091S-433 column (30 mm × 0.25 mm; film thickness 0.25 μm). The operating condition was as follows: injector at 150 ºC, detector at 280 ºC, helium carrier gas, oven temperature program at 40 ºC for 5 min then at 2 ºC/min up to 250 ºC and finally held isothermally for 20 min. The identification of components was established from their GC retention times, by comparison of their mass spectra with those reported in literature (Eleven Peak Index of Mass Spectra) and by computer matching with the Wiley 5 mass spectra library, as well as, by co-injection with the authentic commercial eugenol andb-caryophyllene GC standards. The amount of compounds was reported as relative area (RA, %) which was peak area relative to the total peak area.
Allelopathic bioassay
The betel ethyl acetate extracts were dissolved to different concentrations in 0.5% Tween 80. Extract (4 mL) was added onto Whatman paper No.1 which was placed in glass petri dish (9 cm diameter), whereas 0.5% Tween 80 was used as a negative control. Consequently, 30 seeds were placed in solution on paper and incubated in the growth chamber at 25 ºC±2 ºC for 12 h photoperiod under cool white fluorescent light [20 µmol/(m2·s)]. After 7 d, the number of germinated seeds, which was identified as 1 mm protruding of the radical, the root length and the shoot length of seedlings were recorded.
Statistical analysis
All experiments were done for three replications. Means were analyzed with one-way ANOVA. Significant differences between means were evaluated by Duncan’s new multiple range test by SPSS version 17.0 at the 0.05 level.
RESULTS
Affecting STS expression through fungi treatment
The application of four different fungi on true leaves oftissue cultures could stimulatetranscript level. The results demonstrated thatdramatically upregulatedexpression, whereas the transcripts of,andshowed no difference from the untreated (agar-treated) control (Fig. 1).
Identification of volatile constituents
The volatile compounds in ethyl acetate extract ofleaftissue were identified using GC-MS. The main compounds were grouped into two classes, consisting of approximately 40% phenylpropanoids and 20% sesquiterpenoids. Even though there were significantly more phenylproponoids than sesquiterpenes in the extract, followingtreatment, the sesquiterpenes showed stronger fold change relative to the untreatment than the phenylpropanoids (Fig. 2 and Table 1). The fold change increments for the sesquiterpenes were β-cubebene (3.8), β-caryophyllene (3.1), germacrene D (2.9), α-copaene (2.8), α-humulene (2.8), α-amorphene (2.8) and bicyclogermacrene (2.7) (Table 1). Whereas all sesquiterpenes were increased upon treatment, some phenylpropanoids were increased but some were reduced.There were increases in fold change (1.8) for isoeugenol and eugenol acetate, whereas for chavicol and allylpyrocatechol diacetate, fold changes were reduced by 1.5 and 1.2, respectively.
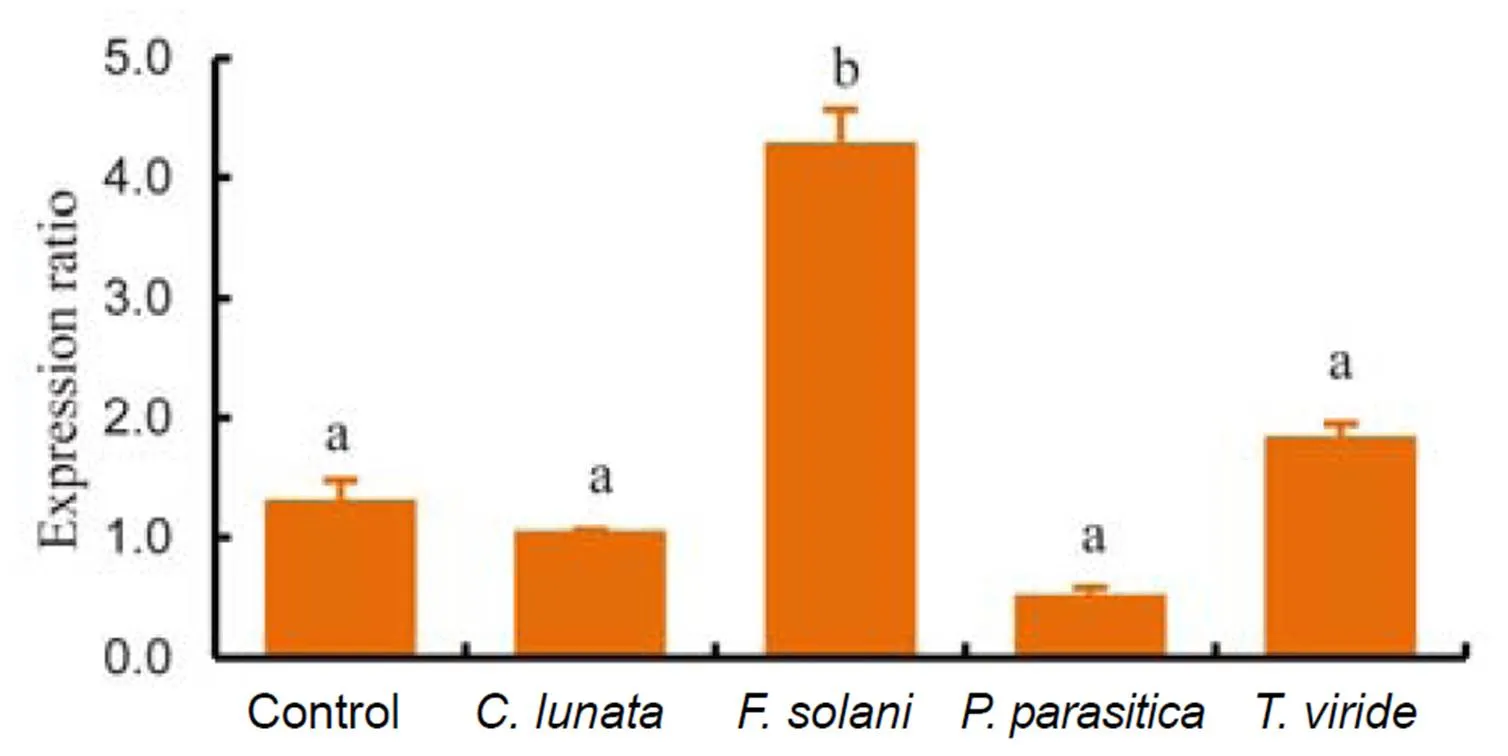
Fig. 1.Expression analysis by quantitative real-time PCR ofintissue culture following Fungi infection.
Bars are SE (= 3), and the lowercase letters indicate significant difference at the 0.05 level.
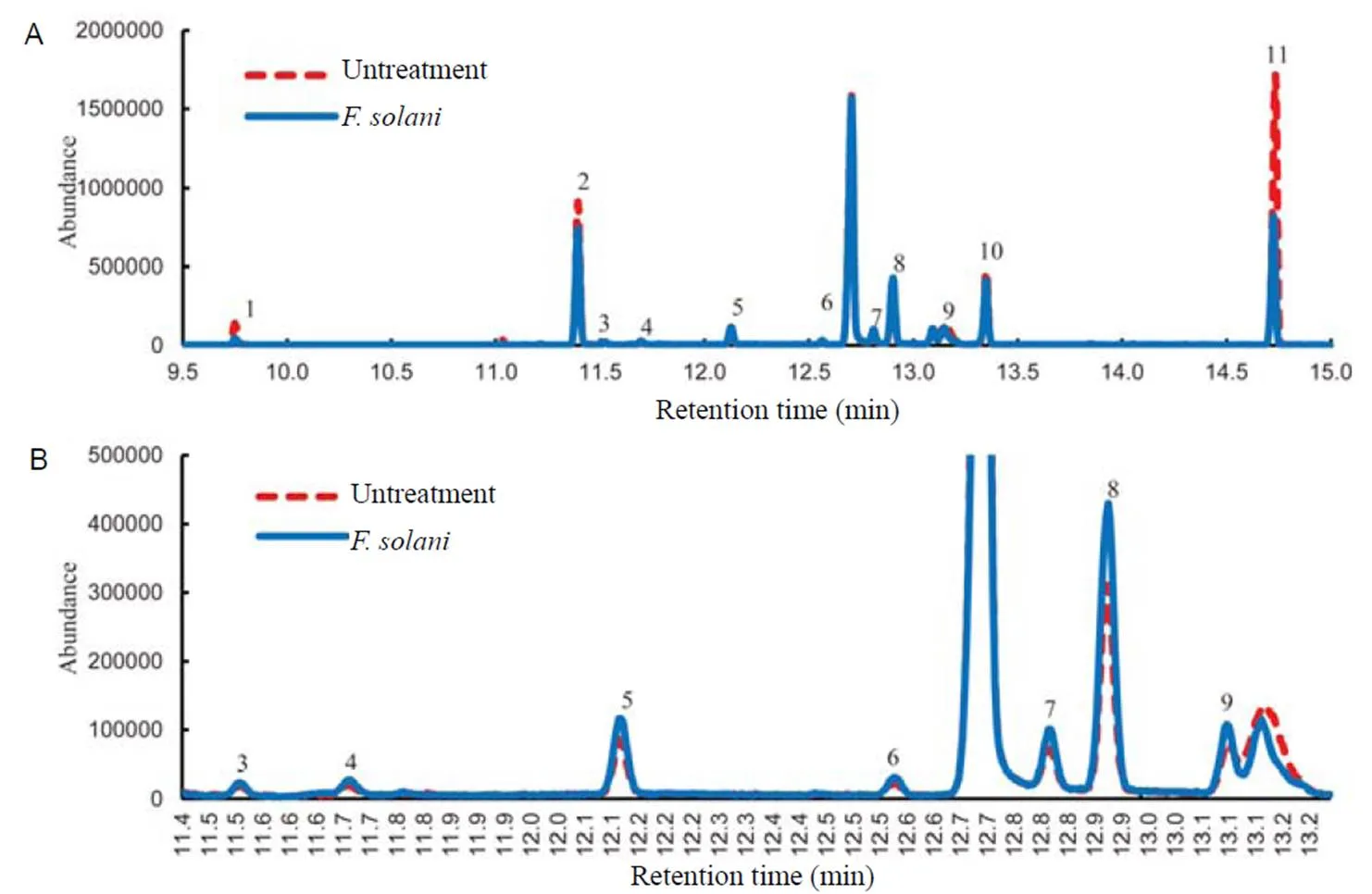
Fig. 2.Volatile organic compounds (VOCs) obtained fromtissue culture after 3 d oftreatment by gas chromatography-mass spectrometry.
A shows VOCs, including phenylpropanoids and sesquiterpenes, while B shows only sesquiterpene peaks for retention time of 11.4 to13.2 min.
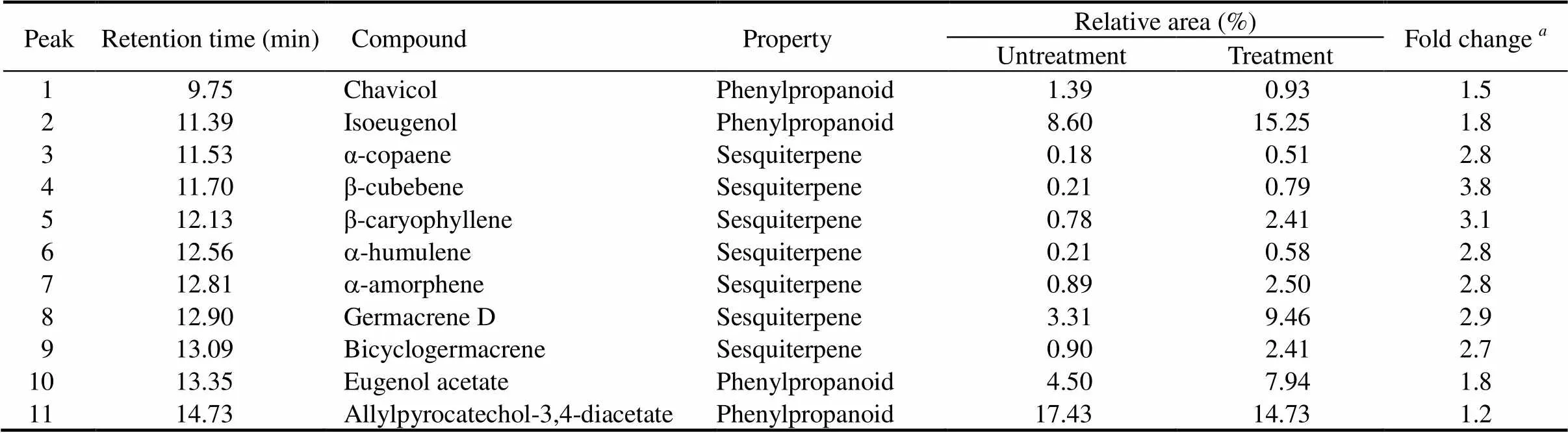
Table 1. Volatile organic compounds in untreated and F. solani-treated leaf tissue culture extracts.
Fold change compares compounds in untreated and treated samples.
Allelopathic effects of the extracts on germination and growth of crops and weeds
Extracts were obtained from the untreated and-treated leaves using ethyl acetate and used to study the allelopathic effects on seed germination and on the root and shoot lengths of,,and(Figs. 3–5). They were investigated over a period of 7 d. The results indicated that the extracts inhibited germination and growth of weeds and that treated leaf extract had a greater inhibitory effect than untreated leaf extract. The germination ofwas not inhibited by either extract (Fig. 3) but in the case of the narrow leaf weed, germination was dramatically decreased (it fell to less than 40%) at 1.0 mg/mL extract.
The treated leaf extract had a greater inhibiting effect on germination than untreated leaf extract (Fig. 3). The germination ofwas completely inhibited by the extract, even at doses as low as 0.1 mg/mL, and when applied at 0.5 mg/mL, the germination of the broad leaf weed,, was more completely inhibited by the treated than by the untreated extract, although germination was completely inhibited by both extracts at 1.0 mg/mL. The extracts inhibited root growth in all the treated plants (Fig. 4), although the weeds were more sensitive than the rice. In addition, the highest inhibitory effect was observed at 1.0 mg/mL.In the rice sample, the root length ofwas decreased at 0.1 mg/mL with no difference observed at increasing concentrations and although the shortest root ofwas recorded after treatment at 1.0 mg/mL, there was no difference between the effects of treated and untreated extracts. At a concentration of 0.1 mg/mL, the extracts had little effect on the root elongation of. In the case of shoot length, the extracts had no effect for(Fig. 5), while at 0.5 mg/mL, the shoot length ofdiffered significantly from the control. The highest inhibitory effect was recoded at 1.0 mg/mL. At a concentration of 0.1 mg/mL, the extracts had an effect on the shoot elongation ofand the inhibitory effect was greatly enhanced at 0.5 mg/mL. The shortestshoots were observed with 1.0 mg/mL treated extract, which was significantly different from the effect recorded for the untreated extract.
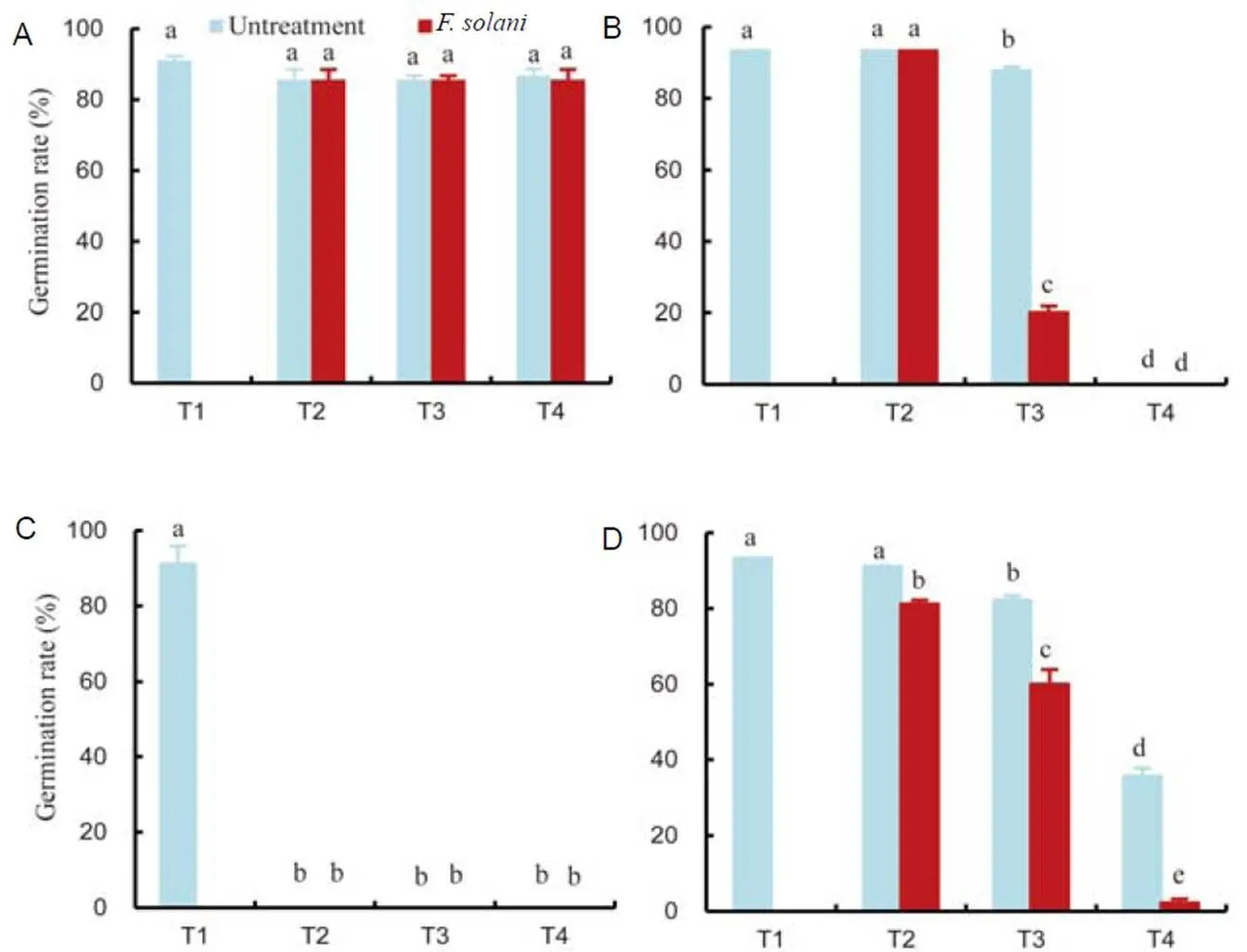
Fig. 3.Effects oftreated extracts ofon the germination of(A),(B),(C) and(D).
T1, 0.5% Tween 80 (control); T2, 0.1 mg/mL ethyl acetate extracts; T3, 0.5 mg/mL ethyl acetate extracts; T4, 1.0 mg/mL ethyl acetate extracts.
Bars are SE (= 3), and lowercase letters indicate significant difference at the 0.05 level.
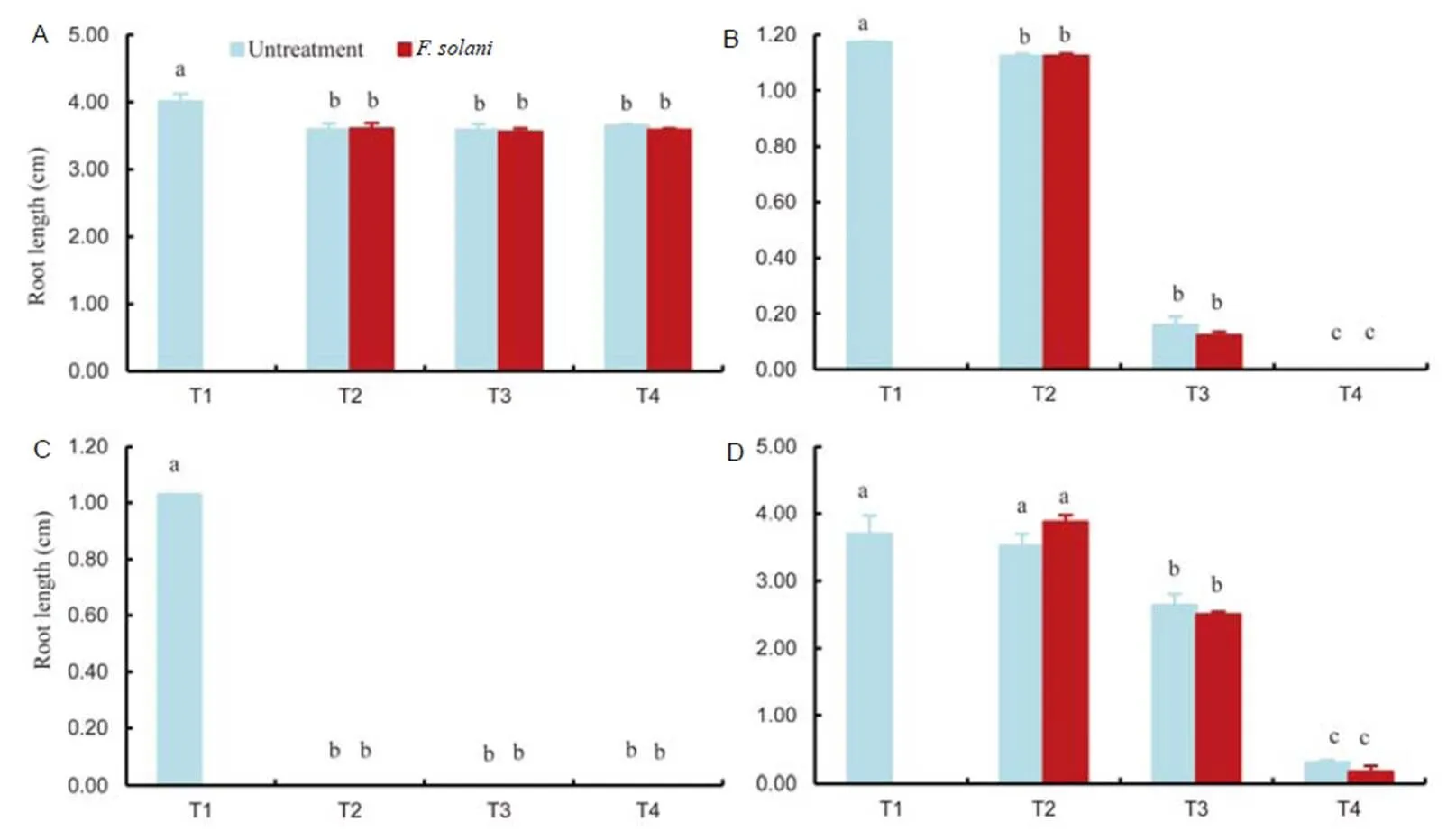
Fig. 4.Effects of untreated andtreated extracts ofon the root length of(A),(B),(C) and(D).
T1, 0.5% Tween 80 (control); T2, 0.1 mg/mL ethyl acetate extracts; T3, 0.5 mg/mL ethyl acetate extracts; T4, 1.0 mg/mL ethyl acetate extracts.
Bars are SE (= 3) and the lowercase letters indicate significant difference at the 0.05 level.
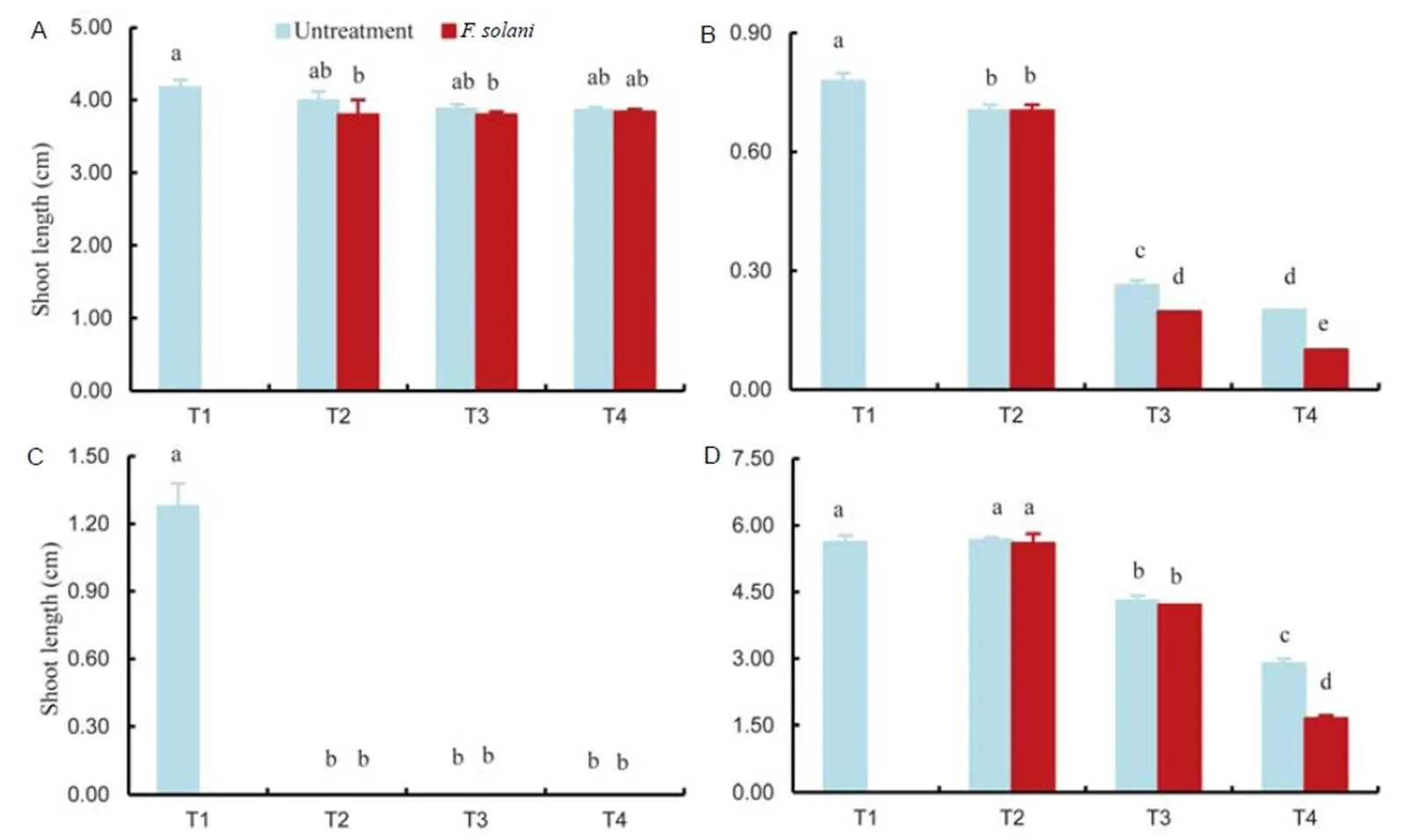
Fig. 5.Effects of untreated andtreated extracts ofon the shoot length of(A),(B),(C) and(D).
T1, 0.5% Tween 80 (control); T2, 0.1 mg/mL ethyl acetate extracts; T3, 0.5 mg/mL ethyl acetate extracts; T4, 1.0 mg/mL ethyl acetate extracts.
Bars are SE (= 3) and the lowercase letters indicate significant difference at the 0.05 level.
DISCUSSION
The goal of this research was to investigate a potent natural herbicide that could be used to control weeds in paddy fields.was chosen as the plant of choice for this study since it produces a substantial amount of phenylpropanoids and sesquiterpenoids that are already known to have allelochemical properties that protect the plant from pathogens and herbivores. Production of these chemicals is biosynthetically induced by biotic and abiotic stresses, including infection with necrotrophic fungi (Piesik et al, 2011), and this inducible production of sesquiterepenes, which occurs via sesquiterpene synthase expression up-regulation, plays a role in indirectly defending against fungal pathogens (da Luz et al, 2017). In line with previous reports, we applied a number of fungi with different characteristics tobut the results demonstrated that only the virulent necrotrophicup-regulatedexpression and promoted sesquiterpene production. Hence,was chosen as the activator ofexpression and sesquiterepene production in.spp. is a common plant pathogen that causes dark black discoloration to the root and stem of the betel (Alam et al, 2004) along with disease in betel vines, visible as yellowing of the leaves. Infected plants will wilt suddenly, accompanied by the drying up of the entire plant. A sesquiterpene cyclase (STC) family, 5-epi-aristolochene synthase (EAS), is significantly induced in the ripe pepper fruits uponinfection (Park et al, 2014). However, the transcription level ofb-pinene synthase is suppressed when juvenile leaves ofare treated withfungus (Lu et al, 2002). Silvar et al (2008) also reported that sesquiterpenes cyclase is up-regulated in pepper plants that are infected with. In, a high concentration of sesquiterpenes is observed afterf. sp. piperis infection (da Luz et al, 2017), while necrotrophic fungal infections (,and) induce sesquiterpene production, especiallyb-caryophyllene, in cereal crops (Piesik et al, 2011). Monoterpenes ((E)-b-ocimene and linalool) and sesquiterpenes (caryophyllene,b-elemene, α-farnesene) are also released from tobacco plants inoculated with avirulent and mutant strains of(Huang et al, 2003).
Production of sesquiterpene induced by infection with necrotrophic pathogens is principally regulated by phytohormone jasmonic acid (JA). We have previously demonstrated that methyl jasmonate inducesexpression in betel tissue culture (Woranoot et al, 2015a), but in response to hemibiotrophic pathogen infection, plants have evolved an alternate defense utilizing JA (Park et al, 2014). Taniguchi et al (2014) showed that JA spray oninduces monoterpene synthase gene expression and monoterpene production. JA-induced accumulation of monoterpene is regulated by the jasmonate ZIM domain protein (OsJAZ8) in the jasmonate signaling pathway. Piel et al (1997) reported that plants responded to the presence of cellulysin fromby increasing the biosynthesis of volatiles and endogenous jasmonic acid through the activation of the octadecanoid signaling pathway.
As regards sesquiterpene production due to the upregulation ofexpression afterinfection, the results showed a high level of fold change compared to the untreatment. Before induction, there were larger amounts of phenylproponoids, euganol and derivatives than those of sesquiterpenes.However, after induction, fold change increased for some of these compounds and decreased for others, while all sesquiterpenes increased in volume with greater fold changes than phenylproponoids. This underlines the significance of sesquiterpene action on response to pathogens.
The allelopathic results showed that ethyl acetate extract ofhad a phytotoxic effect, with a greater effect on narrow leaf than on broad leaf weeds, but with no effect on the germination of. It has also been shown that the roots of seedlings are more sensitive than the shoots (Woranoot et al, 2015b). Additionally, Nishida et al (2005) studied the allelopathic effects of monoterpenoids inand the results demonstrated that monoterpenes inhibit cell-nuclear and organelle DNA synthesis in the root meristem ofseedlings to a greater extent than in the shoot meristem. Other reports also indicated that root tissues are more permeable to allelochemicals, which causes modifications to DNA synthesis in the root cell meristem, changes in cell mitotic indices (Romagni et al, 2000), alterations to mitochondrial metabolism (Abrahim et al, 2000), reductions in the size of root xylem cells and marked changes in the primary root (Gatti et al, 2010). Trans-caryophyllene also inhibits weed germination, and induces plant water status alteration and oxidative damage in(Araniti et al, 2017). In addition, it is clear that treated leaf extract had a greater inhibitory effect than untreated leaf extract, and the higher allelopathic activity of this treated leaf extract is probably due to the relative abundance of sesquiterpenes and phenylpropanoids. Sánchez-Muñoz et al (2012) showed that theb-caryophyllene isolated fromextract has a major effect on the leaves ofplants, acting by inhibiting the photosystem II (PS II) and transforming active reaction centers into ‘heat sinks’ with silent reaction centers unable to reduce the plastoquinone electron acceptor pool. Moreover,b-caryophyllene also induces chlorosis in treated leaves. Other sesquiterpenes found inextract have also been reported as demonstrating allelopathic activity, which includes germacren D (Ayeb-Zakhama et al, 2016; Amri et al, 2017),a-humulene (Tellez et al, 2000) and phenylpropanoids (Namkeleja et al, 2014) such as eugenol (Vaid et al, 2010). Vaid et al (2011) have reported that eugenol decreases germination, seedling dry weight and seedling length inand, and also causes a reduction in photosynthetic efficiency, chlorophyll content and cellular respiration, as well as disruption to mitotic activity by disorganizing microtubules and altering cell wall biosynthesis.
CONCLUSIONS
was shown to be a potent activator ofexpression and thus sesquiterpene production in. Following this, ethyl acetate extract ofwas demonstrated to have an allelopathic effect on weeds without interfering with the development of rice seedlings. The betel extract had allelopathic effects on paddy weeds in terms of germination but had a greater effect on root growth. After being subject to, the phototoxicity on weeds of the application was stronger than the untreated application, and the effects of the applications were shown to be dose-dependent.-treated betel extract may therefore have potential as an alternative natural herbicide for the control of weeds in rice paddies. However, further studies are required to determine the relevant modes of action and the environmental safety of application in rice paddies.
ACKNOWLEDGEMENTS
We acknowledge research grant for graduate study (2016) from The National Research Council of Thailand (NRCT) for financial support.
Abd El-Gawad A M, El-Amier Y A, Bonanomib G. 2018. Allelopathic activity and chemical composition of(L.) DC. essential oil from Egypt., 15(1): e1700438.
Abrahim D, Braguini W L, Kelmer-Bracht A M, Ishii-Iwamoto E L. 2000. Effects of four monoterpenes on germination, primary root growth, and mitochondrial respiration of maize., 26(3): 611–624.
Akram W, Anjum T, Ahmad A, Moeen R. 2014. First report ofcausing leaf spots on sorghum bicolor from Pakistan., 98(7): 1007–1008.
Alam S, Islam M R, Sarkar M A, Chowdhury A N, Alam M S, Lee M W. 2004.effect of fungicides, plant extracts and smoke on conidial germination ofroot rot pathogen of., 32(1): 42–46.
Amri I, Hamrouni L, Hanana M, Jamoussi B. 2013. Reviews on phytotoxic effects of essential oils and their individual components: News approach for weeds management., 4(1): 96–114.
Amri I, Hanana M, Jamoussi B, Hamrouni L. 2017. Essential oils ofJ.F. Arnold subsp. laricio Maire: Chemical composition and study of their herbicidal potential Arabian., 10(2): 3877–3882.
Araniti F, Sánchez-Moreiras A M, Graña E, Reigosa M J, Abenavoli M R. 2017. Terpenoid trans-caryophyllene inhibits weed germination and induces plant water status alteration and oxidative damage in adult., 19(1): 79–89.
Ayeb-Zakhama A E, Sakka-Rouis L, Bergaoui A, Flamini G, Jannet H B, Harzallah-Skhiri F. 2016. Chemical composition and allelopathic potential of essential oils from(Benth.) Kuntze cultivated in Tunisia., 13(3): 309–318.
Borella J, Martinazzo E G, Aumonde T Z, do Amarante L, de Moraes D M, Villela F A. 2012. Responses in germination and early growth of radish under the influence of an aqueous extract of(Kunth) Steudel., 26(2): 415–420. (in Portuguese with an English abstract)
de Brito Rodrigues L, de Oliveira R, Abe F R , Brito L B, Moura D S, Valadares M C, Grisolia C K, de Oliveira D P, de Oliveira G A R. 2017. Ecotoxicological assessment of glyphosate-based herbicides: Effects on different organisms., 36(7): 1755–1763.
Costa S S, Matos K S, Tessmann D J, Seixas C D S, Pfenning L H. 2016.sp. nov., a member of thespecies complex causes root rot on soybean in Brazil., 120(1): 51–60.
da Luz S F M, Yamaguchi L F, Kato M J, de Lemos O F, Xavier L P, Maia J G S, de R Ramos A, Setzer W N, da Silva J K D. 2017. Secondary metabolic profiles of two cultivars of(Black Pepper) resulting from infection byf. sp.., 18(12): 2434.
de Miranda C A S F, Cardoso M D G, de Carvalho M L M, Figueiredo A C S, Nelson D L, de Oliveira C M, Gomes M S, de Andrade J, de Souza J A, de Albuquerque L R M. 2014. Chemical composition and allelopathic activity ofandweeds essential oils., 5: 1248–1257.
Gajera H P, Savaliya D D, Patel S V, Goalkiya B A. 2015.induces pathogenesis related defense response against rot pathogen infection in groundnut (L.)., 34: 314–325.
Gatti A B, Ferreira A G, Arduin M, Perez S C G D A. 2010. Allelopathic effects of aqueous extracts ofO.Kuntze on development ofL. seedlings., 24(2): 454–461.
Gupta V, Shamas N, Razdan V K, Mahajan S, Fatima K, Sharm S, Rai P K. 2018. Management of brown spot of rice (L.) caused byby bio-control agents., 7(4): 3472–3477.
Hattan J I, Shindo K, Sasaki T, Ohno F, Tokuda H, Ishikawa K, Misawa N. 2018. Identification of novel sesquiterpene synthase genes that mediate the biosynthesis of valerianol, which was an unknown ingredient of tea., 8(1): 12474.
Huang J, Cardoza Y J, Schmelz E A, Raina R, Engelberth J, Tumlinson J H. 2003. Differential volatile emissions and salicylic acid levels from tobacco plants in response to different strains of.,217(5): 767–775.
Isman M B. 2000. Plant essential oils for pest diseases management., 19: 603–608.
Jin J J, Kim M J, Dhandapan S, Tjhang J G, Yin J L, Wong L, Sarojam R, Chua N H, Jang I C. 2015. The floral transcriptome of ylang ylang (var.) uncovers biosynthetic pathways for volatile organic compounds and a multifunctional and novel sesquiterpene synthase., 66(13): 3959–3975.
Lu S, Xu R, Jia J W, Pang J, Matsuda S P T, Chen X Y. 2002. Cloning and functional characterization of ab-pinine synthase fromthat shows a circadian pattern of expression., 130(1): 477–486.
Majeed R A, Shahid A A, Ashfaq M, Saleem M Z, Haider M S. 2016. First report ofcausing brown leaf spots of rice in Punjab, Pakistan., 100(1): 219.
Mithöfer A, Boland W. 2016. Do you speak chemistry? Small chemical compounds represent the evolutionary oldest form of communication between organisms., 17(5): 626–629.
Murashige T, Skoog F. 1962. A revised medium for rapid growth and bio assays with tobacco tissue cultures., 15(3): 473–497.
Myers J P, Antoniou M N, Blumberg B, Carroll L, Colborn T, Everett L G, Hansen M, Landrigan P J, Lanphear B P, Mesnage R, Vandenberg L N, vom Saal F S, Welshons W V, Benbrook C M. 2016. Concerns over use of glyphosate-based herbicides and risks associated with exposures: A consensus statement., 15(1): 19.
Namkeleja H S, Tarimo M T C, Ndakidemi P A. 2014. Allelopathic effects of Argemone mexicana to growth of native plant species., 5(9): 1336–1344.
Nishida N, Tamotsu S, Nagata N, Saito C, Sakai A. 2005. Allelopathic effects of volatile monoterpenoids produced by: Inhibition of cell proliferation and DNA synthesis in the root apical meristem ofseedlings.,31(5): 1187–1203.
Park S, Park A R, Im S, Han Y J, Lee S, Back K, Kim J I, Kim Y S. 2014. Developmentally regulated sesquiterpene production confers resistance toinripe pepper fruits., 9(10): e109453.
Piel J, Atzorn R, Gäbler R, Kühnemann F, Boland W. 1997. Cellulysin from the plant parasitic funguselicits volatile biosynthesis in higher plants via the octadecanoid signaling cascade., 416(2): 143–148.
Piesik D, Pańka D, Delaneyc K J, Skoczekd A, Lamparskia R, Weaver D K. 2011. Cereal crop volatile organic compound induction after mechanical injury, beetle herbivory (spp.), or fungal infection (spp.).,168(9): 878–886.
Poulson-Ellestad K L, Jones C M, Roy J, Viant M R, Fernández F M, Kubanek J, Nunn B L. 2014. Metabolomics and proteomics reveal impacts of chemically mediated competition on marine plankton., 111: 9009–9014.
Pukclai P, Kati-Noguchi H. 2011. Allelopathic activity ofRoxb.,10(2): 147–152.
Reigosa M, Gomes A S, Ferreira A G, Borghetti F. 2013. Allelopathic research in Brazil., 27(4): 629–646.
Romagni J G, Allen S N, Dayan F E. 2000. Allelopathic effects of volatile cineoles on two weedy plant species., 26(1): 303–313.
Sánchez-Muñoz B A, Aguilar M I, King-Díaz B, Rivero J F, Lotina-Hennsen B. 2012. The sesquiterpene β-caryophyllene and caryophyllene oxide isolated fromact as phytogrowth and phtosynthesis inhibitors.,17(2): 1437–1447.
Silvar C, Merino F, Díaz J. 2008. Differential activation of defense-related genes in susceptible and resistant pepper cultivars infected with., 165(10): 1120–1124.
Taniguchi S, Miyoshi S, Tamaoki D, Yamada S, Tanaka K, Uji Y, Tanaka S, Akimitsu K, Gomi K. 2014. Isolation of jasmonate-induced sesquiterpenes synthase of rice: Product of which has an antifungal activity against,171(8): 625–632.
Tellez M R, Dayan F E, Schrader K K, Wedge D E, Duke S O. 2000. Composition and some biological activities of the essential oil of(L.)., 48(7): 3008–3012.
Vaid S, Batish D R, Singh H P, Kohli R K. 2010. Phytotoxic effect of eugenol towards two weedy species.,5(3): 339–341.
Vaid S, Batish D R, Singh H P, Kohli R K. 2011. Phytotoxicity of limonene againstL.,6(1): 163–165.
Woranoot K, Buaruaeng R, Kongbangkerd A, Ratanasut K, Choopayak C. 2015a. Methyl jasmonate and wounding inducedexpression intissue culture., 8(3): 182–190.
Woranoot K, Naree P, Kongbangkerd A, Wongkrajang K, Buaruaeng R, Choopayak C. 2015b. Phytotoxic effects ofL. extracts on germination ofL. andSw. weeds., 12(1): 11–24.
Xu G F, Shen S C, Zhang F D, Zhang Y, Hisashi K N, David R C. 2018. Relationship between allelopathic effects and functional traits of different allelopathic potential rice accessions at different growth stages., 25(1): 32–41.
Yan G J, Zhu C H, Luo Y P, Yang Y, Wei J J. 2006. Potential allelopathic effects of,and.,17(9): 1633–1636. (in Chinese with English abstract)
18 June 2018;
8 October 2018
Chonnanit Choopayak (chonnanitc@nu.ac.th)
Copyright © 2019, China National Rice Research Institute. Hosting by Elsevier B V
This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/)
Peer review under responsibility of China National Rice Research Institute
http://dx.doi.org/10.1016/j.rsci.2019.08.003
(Managing Editor: Fang Hongmin)
- Rice Science的其它文章
- Quantitative Trait Loci Mapping for Rice Yield-RelatedTraits Using Chromosomal Segment Substitution Lines
- Differential RNA Editing of Mitochondrial Genes in WA-Cytoplasmic Based Male Sterile Line Pusa 6A, and Its Maintainer and Restorer Lines
- Quinclorac Resistance in Echinochloa crus-galli from China
- Effects of Integrated Organic and Inorganic Fertilizers on Yield and Growth Parameters of Rice Varieties
- Strategies for Fermentable Sugar Production by Using Pressurized Acid Hydrolysis for Rice Husks
- CRISPR/Cas9: Development and Application in Rice Breeding