复方透骨香乳膏中黄柏的质量控制研究
郭璐玫 姜莲 申璀


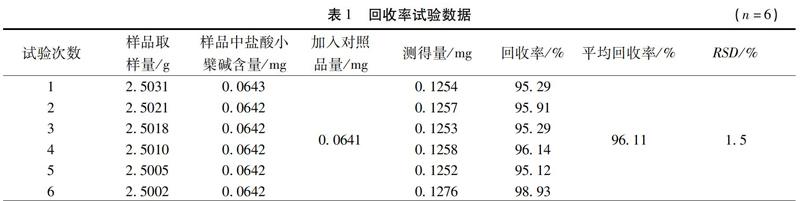
【摘 要】目的:研究并控制复方透骨香乳膏中黄柏的质量。方法:采用薄层色谱法对黄柏进行鉴别研究;采用高效液相色谱法测定黄柏中盐酸小檗碱的含量,色谱柱为Waters Xbridge C18柱(250mm×4.6mm,5μm),流动相为乙腈-0.05mol/L磷酸二氢钾溶液(30∶70),柱温为30℃,流速为1.0 mL/min,检测波长346nm。结果:薄层色谱斑点清晰,分离度较好;高效液相色谱测定盐酸小檗碱对照品在0.02688~0.5375μg范围内线性关系良好(r=0.9999),平均回收率为96.11%,RSD=1.5%(n=6)。结论:本研究方法准确可靠、重复性好,可用于复方透骨香乳膏中黄柏的质量控制。
【关键词】 复方透骨香乳膏;黄柏;薄层色谱法;盐酸小檗碱;高效液相色谱法
【中图分类号】R284.1 【文献标志码】 A【文章编号】1007-8517(2019)12-0031-03
Quality Control of Phellodendri Chinensis Cortex
in Compound Touguxiang Cream
GUO Lumei JIANG Lian SHEN Cui*
Guizhou Institute for Food and Drug Control,Guiyang 550004,China
Abstract:Objective To study and control the quality of phellodendri chinensis cortex in Compound Touguxiang Cream.Methods TLC was used for qualitative identification of phellodendri chinensis cortex.HPLC was used for content determination of berberine hydrochloride in phellodendri chinensis cortex on a Waters Xbridge C18(250 mm×4.6 mm,5μm),and the mobile phase was acetonitrile-0.05mol/L KH2PO4( 30∶70) at the column temperature of 30℃.The flow rate was 1.0 mL/min and the detection wavelength was 346nm.Results The TLC spots were clear and showed a good separation.HPLC showed the linear range of berberine hydrochloride was 0.02688~0.5375 μg (r=0.9999),the average recovery was 96.11%,and the RSD was 1.5 % (n=6).Conclusion The method is accurate with good reproducibility,which can be used for the quality control of phellodendri chinensis cortex in Compound Touguxiang Cream.
Key words:Compound Touguxiang Cream;phellodendri chinensis cortex;TLC; Berberine Hydrochloride;HPLC
复方透骨香乳膏由透骨香、黄柏、川芎、当归等11味药材组成,具有活血祛瘀、消肿止痛之功效,用于跌打损伤所致的局部软组织损伤、疼痛。方中黄柏为主要药味,具有明显的消肿作用。实验以黄柏對照药材和盐酸小檗碱对照品为指标,对复方透骨香乳膏中的黄柏进行薄层鉴别研究;含量测定则采用高效液相色谱法以盐酸小檗碱为指标性成分对黄柏质量进行控制。
1 仪器与材料
1.1 仪器 ThermoFisher Ultimate 3000(DAD)液相色谱仪;CAMAG薄层点样仪;AE 200型(十万分之一)电子天平(瑞士梅特勒托利多)、XP26型(百万分之一)电子天平(瑞士梅特勒托利多);millipore超纯水机制备超纯水。
1.2 材料 复方透骨香乳膏(贵州健瑞安药业有限公司),盐酸小檗碱对照品(批号:110713-201212,含量以86.7%计)、黄柏对照药材(批号:121510-200501)均来自中国食品药品检定研究院;乙腈为色谱纯,水为超纯水,其余试剂均为分析纯。
2 方法与结果
2.1 黄柏TLC鉴别 取本品内容物10g,加甲醇40mL,回流30min,放冷,离心,取上清液,蒸干,加水20mL溶解,加氨试液调pH值至11,加20mL饱和氯化钠溶液,以二氯甲烷萃取2次,合并二氯甲烷液,蒸干,加盐酸甲醇溶液(1∶100)2mL溶解,作为供试品溶液。另取黄柏对照药材0.1g,同法制成对照药材溶液。再取盐酸小檗碱对照品,加甲醇制成每lmL含0.5mg的溶液,作为对照品溶液。按处方比例称取除黄柏外其他药材适量,制成缺黄柏的阴性对照样品,同法制备缺黄柏的阴性对照溶液。照薄层色谱法(2015年版中国药典四部通则0502)试验,吸取供试品溶液5μL,对照药材溶液2μL,对照品溶液1μL,分别点于同一硅胶G 薄层板上,正丁醇-冰乙酸-水(7∶1∶2)为展开剂,展开,取出,晾干,置紫外光灯(365nm)下检视。供试品色谱中,在与对照药材、对照品色谱相应的位置上,显相同颜色的荧光斑点,且缺黄柏的阴性对照无干扰。如图1所示。

