Comparison of Effectiveness of Gefitinib, Erlotinib,and Afatinib in Advanced Non-small Cell Lung Cancer Patients with EGFR Mutation Positive in Indonesian Population
Noorwati SUTANDYO, Arif HANAFI, Mulawarman JAYUSMANDivision of Hematology and Medical Oncology, Department of Internal Medicine;
2Department of Pulmonology, Dharmais National Cancer Centre Hospital, Jakarta, Indonesia
Abstract Background and objective EGFR-tyrosine kinase inhibitors (EGFR-TKIs) were used to treat non-small cell lung cancer(NSCLC) patients with EGFR mutation positive. This study aims to compare the effectiveness of first line TKIs; gefitinib,erlotinib, and afatinib in the treatment of advanced stage NSCLC patients with EGFR mutation positive in the Indonesian population.Methods A retrospective cohort study of 88 NSCLC patients with EGFR mutation positive treated with gefitinib (n=59),erlotinib (n=22), and afatinib (n=7) was performed in national cancer hospital in Indonesia.Inclusion criteria were stage IIIb or IV NSCLC with adenocarcinoma subtype. Subjects less than 18 years or with a history of other malignancy were excluded.Outcomes were treatment response, progression-free survival (PFS), and mortality rate.Results Complete response, partial response, and stable disease were shown in 1.1%, 35.2%, and 31.8% of subjects, respectively.There were 31.8% of subjects developed progressive disease during treatment. Regarding EGFR mutation positive profile, a total of 56.8% subjects had deletion in exon 19, 42% subjects had mutation in exon 21, and rare mutation in exon 18 was found in 3.4%of total subjects. Demography and clinical characteristics had no significant association with the risk of progressive disease. The median PFS of subjects was 11 months (95%CI: 6.8-15.2 months). There was no statistical difference of PFS between treatment groups.Conclusion Gefitinib, erlotinib, and afatinib have similar effectiveness in advanced stage NSCLC with EGFR mutation positive.Afatinib tends to be associated with longer PFS but further investigation is required.
Key words Lung neoplasms; EGFR mutation positive; Tyrosine kinase inhibitors
Introduction
Despite the global health effort on smoking cessation, lung cancer still retains its high mortality rate in the developed and developing countries until present[1]. It was estimated that lung cancer mortality in 2035 will be 86% higher than in 2012[2]. Throughout all types of lung cancer cases, the non-small cell lung cancer (NSCLC) accounts for 85% of them. Adenocarcinoma subtype found in more than 70%of NSCLC. In a majority of patients, NSCLC is usually diagnosed at an advanced stage where surgical therapy is no longer applicable[3].
In 10%-35% of lung adenocarcinoma, mutations in the epidermal growth factor receptor (EGFR) gene was found[4]. EGFR mutations were found in significantly higher proportion in female patients, Asian population, and nonsmokers. The most common mutations were the deletion in exon 19 and mutation in exon 21 L858R point[5]. Several experimental studies and meta-analysis reported that EGFR-tyrosine kinase inhibitors (EGFR-TKIs) treatment has better efficacy in advanced stage of NSCLC with these EGFR mutation positive compared with conventional chemotherapy treatment[6-9].
Currently, there are several EGFR-TKIs treatment such as gefitinib, erlotinib, afatinib worldwide approved for treating advance stage of NSCLC with EGFR mutation positive.Gefitinib and erlotinib are an oral reversible first-generation EGFR-TKIs. They bind to the ATP-binding sites to block the activation of the signal induced by EGFR. While afatinib is an oral irreversible second-generation EGFR-TKI. This drug was developed in response to the resistance of the first generations[10].
However, several studies comparing the efficacy of gefitinib, erlotinib, and afatinib in lung adenocarcinoma patients’ mortality and progression-free survival showed conflicting results[11-15]. In addition, there were only a limited number of similar studies in the South-East Asian population which possibly having different characteristics of EGFR mutations compared to East Asian, European,and American populations. So we conducted this study to compare the effectiveness of gefitinib, erlotinib, and afatinib in advance stage adenocarcinoma NSCLC patients with EGFR mutations in the Indonesian population.
Methods
Study design and population
This was a retrospective cohort study at Dharmais National Cancer Hospital, Indonesia. The study was approved by the Ethical Committee of Dharmais National Cancer Hospital.To optimize the power of the research, total sampling was performed in recruiting study subjects. Subjects were advanced non-small cell lung cancer (NSCLC) patients(adenocarcinoma subtype) with proven EGFR mutation positive, who were administered with gefitinib, erlotinib, or afatinib in the period of January 2013 to March 2015. EGFR mutations were analyzed in the Kalbe Genomic biomolecular laboratory, Indonesia. DNA was extracted from tumor tissue during the diagnostic procedure using the QIAamp blood kit (Qiagen, Hilden, Germany). Then, DNA amplification using high-resolution PCR protocol followed by direct DNA sequencing was performed to determine the EGFR mutation profile.
Inclusion criteria were stage IIIb or IV of lung adenocarcinoma according to American Joint Committee 2010.[16]Subjects less than 18 years or with a history of other malignancy were excluded. EGFR-TKIs treatment was administered orally with a daily dose of 250 mg for gefitinib,150 mg for erlotinib, or 40 mg for afatinib. Treatment would be discontinued if there was evidence of progressive disease or serious adverse event.
The demographic and clinical parameters were collected before the EGFR-TKIs treatment. These data included age, gender, body mass index (BMI), comorbidity, EGFR mutation status. We did a 60-month follow-up through the medical record to evaluate treatment response, progressionfree survival (PFS), and mortality rate. Treatment response was assessed on the basis of the Response Evaluation Criteria in Solid Tumors (RECIST) guideline.[17]Evaluation of the treatment response was performed every 3-6 cycles after starting EGFR-TKIs treatment. Clinical examination,laboratory tests, abdominal ultrasonography, and computed tomography (CT) scan were performed to determine treatment response.
Statistical analysis
Statistical analysis was performed using IBM SPSS software version 24. Study outcomes were treatment response and 24-months PFS. For the survival analysis, we performed right censoring for handling loss to follow-up subjects.
To assess the association between type of EGFR-TKIs treatment and study outcomes, the Chi-square test was performed. A P-value of less than 0.05 was considered statistically significant. Relative risks and their 95%confidence interval were calculated.
The Kaplan-Meier graph and log-rank test were performed to compare the survival probability of PFS regarding EGFRTKIs treatment.
Results
A total of 115 of NSCLC patients fulfilled inclusion criteria of study. However, 27 subjects had incomplete medical records, so a total of 88 subjects were included in analysis.
Subjects’ Characteristics
Characteristics of subjects treated with gefitinib, erlotinib,and afatinib were comparable with respect to the demographic, clinical, and molecular variables (Table 1).
The mean age of all subjects was 60 years. There was no significant difference in gender proportion. Most subjects were at stage IV. The most common sites of metastasis were pleura (51.4%), bone (31.1%), and brain (10.8%). More than 50% of total subjects were found to have exon 19 deletion in EGFR gene. Approximately 3% of total subjects had rare mutation in the EGFR gene (mutation in exon 18). There were two subjects having double mutations in exon 19 and exon 21.
Response rate
Complete response (CR), partial response (PR), and stable disease (SD) were shown in 1.1%, 35.2%, and 31.8% of subjects, respectively. However, there were 31.8% of subjects who developed progressive disease during treatment of TKIs.Demography and clinical characteristics had no significantassociation with the risk of progressive disease (Table 2).
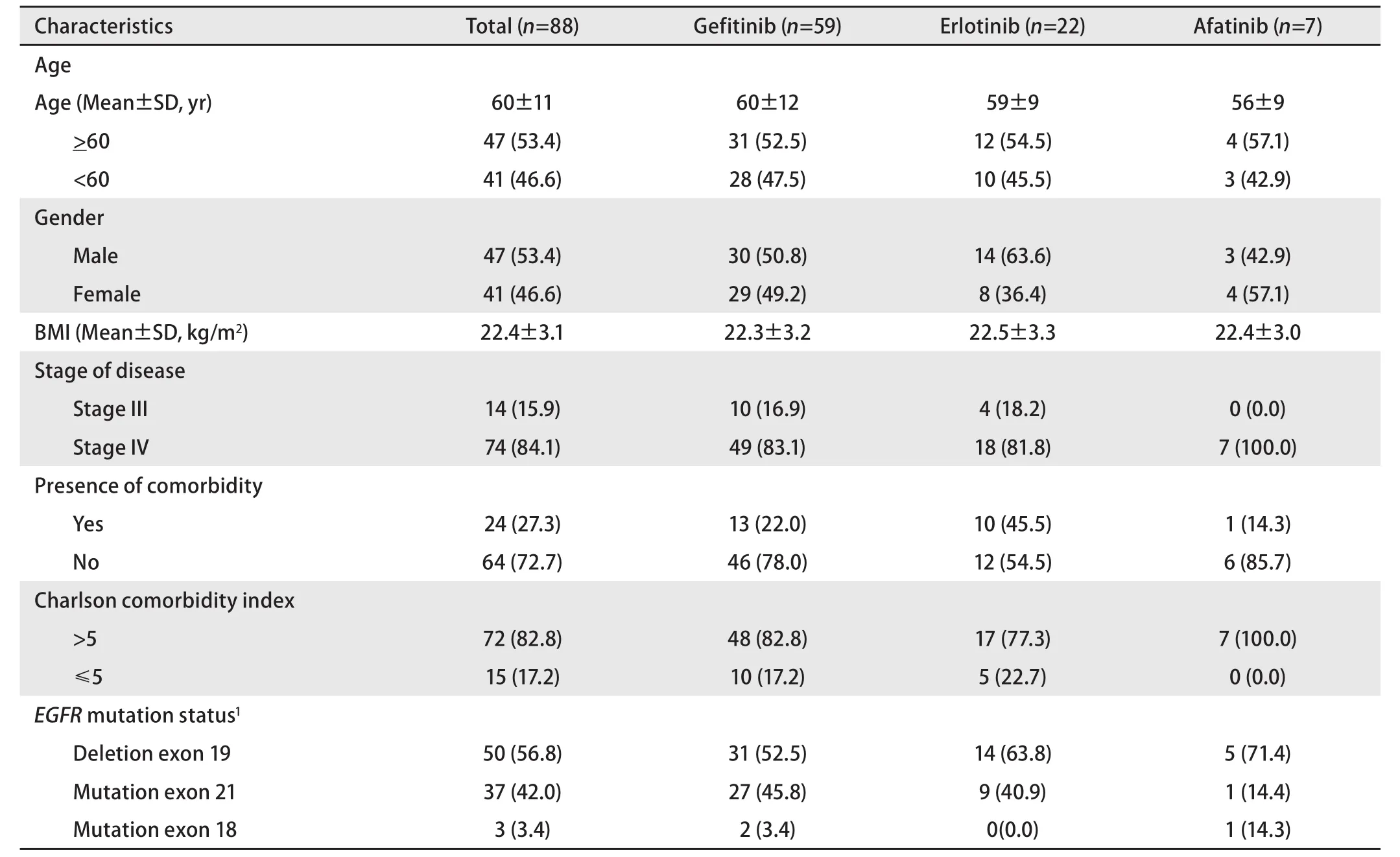
Table 1 Characteristics of subjects [n (%)]
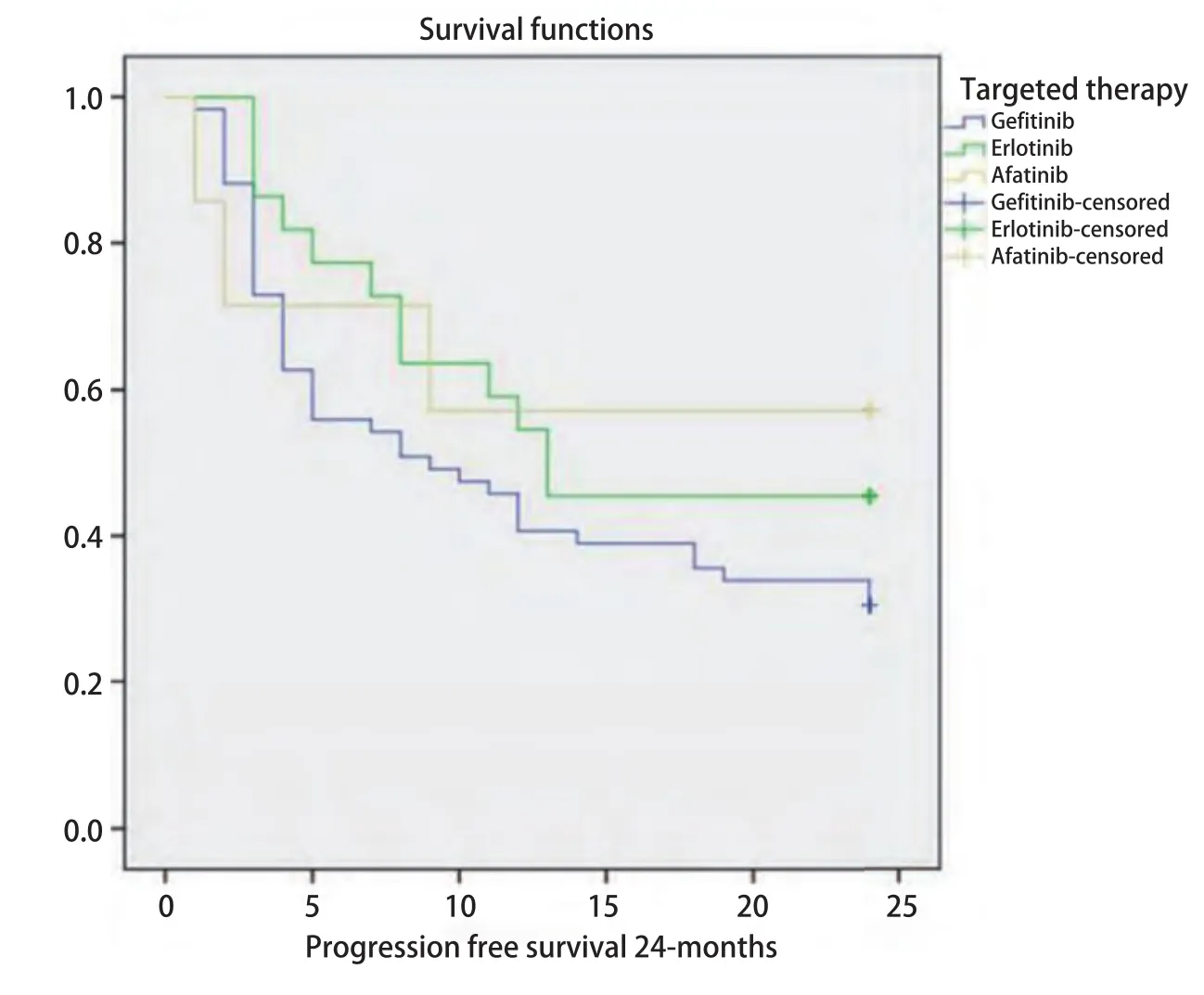
Fig 1 Kaplan-Meier graph of progression-free survival by EGFR-TKIs treatment group. TKI: tyrosine kinase inhibitors.
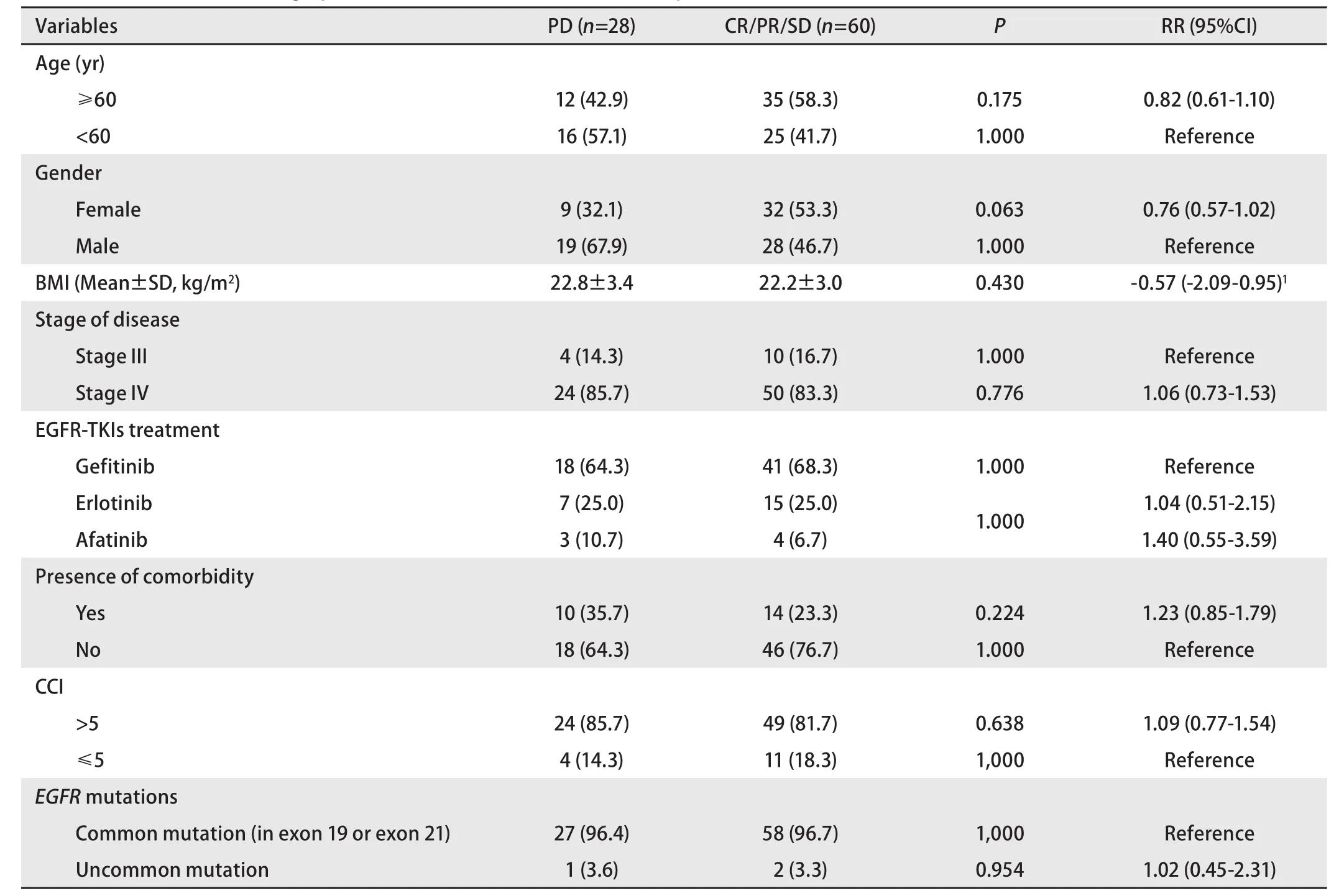
Table 2 Association of demographic and clinical characteristics with response to EGFR-TKIs treatment [n (%)]
Regarding the type of EGFR-TKIs treatment, the risk of progressive disease in subjects receiving gefitinib,erlotinib, and afatinib was similar. Subjects with uncommon EGFR mutations had no significantly difference of risk of progressive disease than subjects with a deletion in exon 19 or mutation in exon 21.
Progression-free survival
The median progression-free survival (PFS) of subjects was 11 months (95%CI: 6.8-15.2 months). Comparison of 24 months PFS by EGFR-TKIs treatment is shown in figure 1.The median PFS of subjects receiving gefitinib and erlotinib were 9 and 13 months, respectively. While subjects receiving afatinib did not reach median survival in a 24-months follow-up. Log-rank test showed that there was no significant difference in PFS between these three groups of treatment(P=0.28).
Discussion
To our knowledge, this was the first Indonesian cohort study which compared the effectiveness of EGFR-TKIs treatment in advanced stage NSCLC patients with respect of EGFR mutation profile. The results of our study showed that gefitinib, erlotinib, and afatinib had similar effectiveness in terms of treatment response and 24-months followup of PFS. Although not statistically significant, subjects receiving afatinib have marginally longer PFS compared to others receiving gefitinib or erlotinib. No progression found in patients with afatinib in 24 months follow up. Regarding EGFR mutation status, subjects with uncommon EGFR mutations had similar risk of progressive disease compared to subjects with mutation in exon 19 or exon 21 point.
A retrospective study by Krawczyk et al.[11]showed similar results. The study was done in Poland involving 180 NSCLC patients. They found that there was no significant in treatment response, PFS, and overall survival in NSCLC patients treated with gefitinib, erlotinib, and afatinib. There were 14.5% of subjects having progressive disease, compared to 31.8% in our study. The difference was probably due to several reasons. First, the study enrolled all subtypes of NSCLC in the Caucasian population compared to adenocarcinoma patients of the Asian population in our study. Second, there was a higher proportion of exon 19 mutation and a lower proportion of exon 21 in that study compared to ours. Patients with exon 19 deletion indicated to have a higher response rate after EGFR-TKI treatment compared patients having exon 21 mutation[18,19].
Similar to our study, Krawczyk et al.[20]also reported that subjects treated with afatinib have slightly longer PFS compared to subjects receiving gefitinib or erlotinib. A large phase 2B randomized controlled trial comparing the efficacy of gefitinib and afatinib performed by Park et al.(Lung-LUX 7) supports this finding. The study showed that median PFS in the afatinib group was 11 months (95%CI:10.6-12.9), while in gefitinib group was 10.9 months (95%CI:9.1-11.5). However, it is important to note that there was a slightly higher incidence of serious treatment-related adverse event and fatal adverse event in afatinib group compared to gefitinib group (11% in afatinib group vs 4% in gefitinib group, 9% in afatinib group vs 6% in gefitinib group,respectively).
On the contrary, a large retrospective cohort study involving 7,222 lung adenocarcinoma patients in Taiwan by Chang et al found a different result compares to our study[21].Gefinitib showed superior efficacy compared to erlotinib.Subjects treated with gefinitib have longer PFS and overall survival in 1-year follow up. These differences could be due to several explanations. First, the erlotinib group in the study has a higher proportion of cachexia which could affect treatment response and overall survival. Second, there was no data on the type of EGFR mutations. So, there might be a possibility that there was a difference in the profile of EGFR mutation compared to our study population.
In our study, the multivariate analysis to control all of the potential confounders was not performed due to two considerations. First, despite total sampling, the sample size was not adequate to perform a multivariate analysis. Second,the baseline characteristics between gefitinib, erlotinib, and afatinib group were comparable. Moreover, other similar retrospective study performed by Krawcyzk et al.[11]showed that patients’ characteristics such as age, gender, staging,performance status, and smoking history did not affect one-year and two-year overall survival after multivariate cox regression analysis. Other retrospective cohort study performed by Chang et al.[21]in Taiwan in previously treated NSCLC patients also reported that gender, comorbidities,and presence of cachexia had no association with PFS.
Our study had several limitations. Since afatinib were not covered in Indonesia National Health Insurance during the study period, we had only small number of subjects receiving afatinib. Therefore, although they had marginally longer PFS than other groups, the result must be interpreted cautiously.Further studies with larger sample size is needed to produce more precise result. Retrospective observational study designs was another limitation to our research. All potential confounders (e.g. smoking status, history of treatment) could not be analyzed due to incomplete data. In addition, time for assessing treatment response might be varied among study subjects.
Conclusion
The result of this study provides evidence that gefitinib,erlotinib, and afatinib have similar effectiveness in advance stage EGFR mutation lung adenocarcinoma patients. Afatinib tends to associate with longer PFS but further investigation is required.

