Combustion Characteristics of Coal Slime, Corn Stalk and Their Blends via Thermogravimetric Analysis*
MA Zhibin CAO Liqiong YANG Fengling CHENG Fangqin
ABSTRACT The combustion characteristics of coal slime, corn stalk, and their blends were studied via thermogravimetric analysis in the present study. The effects of volatile and inorganic matters in the fuelson the combustion characteristics were investigated.The results show that by adding corn stalk,the igniting during the co-combustion of blends can be accelerated and the combustion performance of coal slime is improved significantly.The inorganic matters in coal slime hinder the combustion of coal slime while the inorganic matters in corn stalk promote the combustion of corn stalk significantly. The remaining matters in biomass char still promote the ignition and burn out of coal char. The activation energy of coal slime is close to the activation energy of the low rank coals including peat and lignite. The adding of corn stalk decreases the active energy of coal slime and corn stalk blends combustion.
KEYWORDS coal slime, corn stalk, co-combustion behavior, thermogravimetric analysis
0 Introduction
A large amount of coal slime is produced from coal washing industries in China each year, which is difficult to be utilized due to its high content of moisture, hazardous elements, and inorganic matters.The storage of coal slime not only occupiesa large area of land, but also leads to dust during windy days. Corn stalk is also largely produced as solid waste in the agriculture sector of China. The co-combustion of coal slime with corn stalkin the circulating fluidized bed boiler for power generation is a promising method for the utilization of these waste materials.Biomass utilization for energy production is vital for reducing the consumption of the fossil fuel[1]. Furthermore, co-combustion of biomass could reduce the emission of CO2per unit of energy produced and also the emissions of SO2and NOxdue to the highn(H)/n(C) ratio of biomass[2-4]. The high content of volatile matter and alkali and alkali earth metals (AAEM)in biomass has significant effects on the combustion of coal when their blends are processed in boilers[5]. Understanding the combustion characteristics of coal slime, corn stalk and their blends is of vital importance to the design and operation of boilers.
Co-combustion of coal and biomass were widely reported in literatures.Thermogravimetric analysis (TGA) has been extensively used for investigating the combustion properties of coal and biomass. For instance, GIL et al[6-7]compared the thermal properties and kinetic behaviors of coal, pine sawdust and their blends by using TGA. No significant interactions were observed between coal and pine sawdust combustion. The release of volatiles and char combustion occurred at different temperatures during the combustion of the pine sawdust. In contrast, the coal was characterized by only one combustion stage. The combustion of the blends showed all the stages of both coal and pine sawdust. They indicated that the first-order chemical reaction model was effective for describing the release of volatile from pine sawdust and the combustion of coal, and the diffusion mechanisms could be used for interpreting the combustion of biomass char. IDRIS et al[8]observed no synergistic behaviors during the co-combustion of biomass and coal, but the presence of biomass was found to promote the coal combustion. They discovered that the combustion performance of biomass was better than coal although the activation energy of the former was higher.However, SAHU et al[9]found that more reactive biomass chars were not necessarily better than the less reactive ones in terms of the promotion of the combustion of coal.As reviewed in the aforementioned studies, the combustion characteristics of different materials depend on the specific features of the materials. Few studies investigated the co-combustion characteristics of coal slime with corn stalk, and the effects of volatile and inorganic matters on combustion.
This work aims to study the co-combustion behavior of coal slime and corn stalk from the above aspects. The effects of volatile matters on the combustion performance were studied by comparing the combustion characteristics of raw materials with their chars. The combustion test of demineralized samples was conducted to investigate the effect of inorganic matters. The kinetic parameters were calculated via Coats-Redfern integral methods.
1 Experimental
1.1 Coal and biomass samples
Coal slime and corn stalk from Shanxi Province in China were used in this work. The proximate and ultimate analysis of them were listed in Table 1. The chemical composition of ash from coal slime and corn stalk was listed in Table 2.
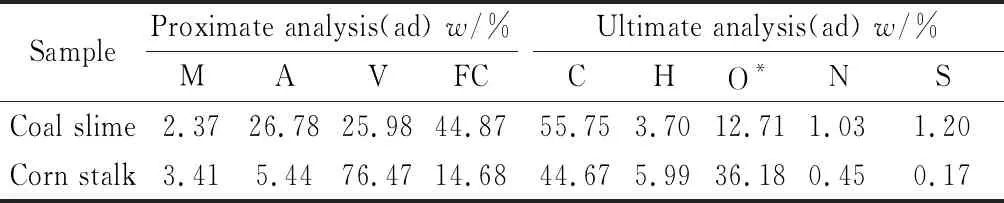
Table 1 Proximate and ultimate analysis of coal slime and corn stalk
* By difference.
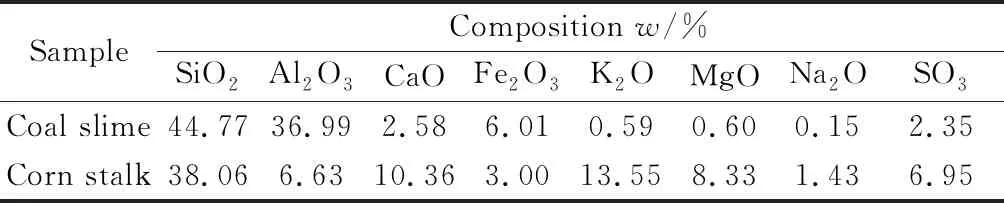
Table 2 Composition of ash prepared from coal slime and corn stalk
The samples were crushed and sieved to the particle size less than 77 μm. Several blends of coal slime and corn stalk were manually homogenized at several ratios. The mass ratio of coal to biomass in each blend was 100∶0, 80∶20, 50∶50, 20∶80, 0∶100, and denoted as 100C, 80C20B, 50C50B, 20C80B, 100B, respectively.
To investigate the effect of volatile matters on co-combustion, the blends were pyrolyzed in a muffle furnace at 400 ℃ for 2 h to remove most of the volatile matters. The char samples were denoted as 100C-P, 80C20B-P, 50C50B-P, 20C80B-P, 100B-P, respectively.
To investigate the effect of inorganic matters on the co-combustion characteristics, the blends were demineralized by HCl and HF solutions according to the national standard GB/T 7560-2001. The demineralized samples were denoted as 100C-D, 80C20B-D, 50C50B-D, 20C80B-D, 100B-D, respectively. The 100C-D was pyrolyzed in the muffle furnace at 400 ℃ for 2 h and the obtained char was named as 100C-D-P.
1.2 Thermogravimetric analysis
Combustion of the aforementioned samples were performed in a TGA instrument (Perkin Elmer Pyris1) via a non-isothermal method.10 mg samples were used in each experiment. Samples were heated in air at a flow rate of 60 mL/min from the room temperature (25 ℃) to 1 000 ℃ at the rate of 10 ℃/min.
Ignition temperature (Ti), burnout temperature (Tb) and the peak temperature at the maximum rate of combustion (Tp) were employed to evaluate the combustion performance of the samples, and these parameters were determined according to the method proposed by REN et al[10].
1.3 Kinetic analysis
The apparent activation energies (E)of coal slime, corn stalk and their blends were calculated according to the Coats-Redfern integral method[11], and the formula is as follows:
(1)
Wherenis the reaction order,αis the conversion rate,βis the heating rate,Ais the pre-exponential factor,Ris the gas constant 8.314 J/(K·mol),Eis the apparent activation energy, andTis temperature.
2 Results and discussion
2.1 Combustion characteristics of coal slime and corn stalk blends
There are significant differences in the combustion characteristics between coal slime and corn stalk. As can be seen from Table 1, the coal slime is characterized by its high content of ash, fixed carbon and relatively low content of volatiles. By contrast, the content of volatiles in corn stalk is up to 76.47% while its ash content is very low. Coal slime contains less oxygen than corn stalk, and then(H)/n(C) ratio of the former is also lower than that of the later.
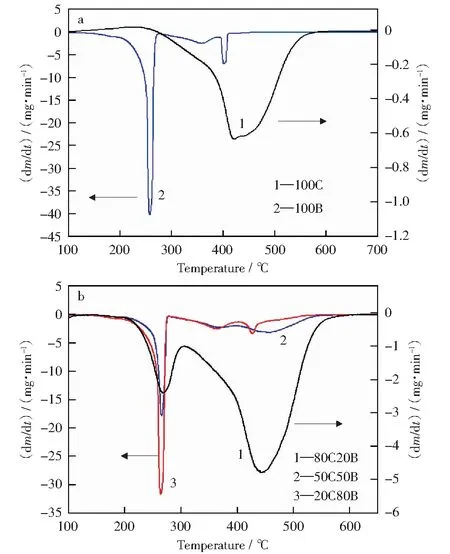
Fig.1 DTG curves during co-combustion of coal slime and corn stalk blends
The DTG curves during co-combustion of coal slime and corn stalk blends were shown in Fig.1. The combustion of corn stalk finishes at lower temperature than that of coal slime.The combustion of coal slime mainly occurs between 227 ℃ and 581 ℃. The release and burning of volatile matters and fixed carbon combustion cannot be distinguished in the DTG curve although the volatile content in coal slime is high (25.98%). In contrast, the corn stalk combustion has three peaks in its DTG curve during combustion. The corn stalk shows trimodal DTG curve in the lower temperature region, reflecting the existence of structures with different reactivities. Some researchers[1,6,12]claimed that the first stage was attributed to the release and burning of volatile matters and the second one was related to the char combustion. Some other scholars[13-15]explained this phenomenon as follows: the complete decomposition of hemi-cellulose and cellulose, and the partial decomposition of lignin takes place over a wider temperature range, leading to the first stage of the weight loss; the decomposition of the remaining lignin and the char combustion is responsible for the second one.The latter is regarded as more convincing and gains more recognition. In this work, there are three peaks in the DTG curve of corn stalk during combustion, though the second peak is relatively weak. The release and burning of volatile matters corresponding to the complete decomposition of hemi-celluloseand cellulose, and the partial decomposition of lignin are responsible for the first peak between 195 ℃ and 274 ℃.The weight loss at this stage is about 61.9%, which is lower than the content of volatile matters in corn stalk. The remaining volatile matters such as lignin decompose within a higher temperature range, leading to the second peak between 274 ℃ and 385 ℃. The third peak between 385 ℃ and 410 ℃ should be caused by the combustion of char from corn stalk.
in Fig.1b, the DTG curves of blends combustion before 300 ℃ are similar with that of corn stalk, and the DTG curves after 300 ℃ are similar with that of coal slime. There are two peaks within different temperature ranges for 80C20B in the DTG curves, and three peaks for 50C50B and 20C80B are observed in the DTG curves.
The typical combustion parameters of samples were listed in Table 3. TheTi,TpandTbof coal stalk are much lower than those of coal slime. The DTG curves (Fig.1a) show that the mass loss rate of corn stalk is much higher and the combustion of corn stalk is earlier than coal slime under some conditions. These results demonstrate that the combustion performance of corn stalk is much better than that of coal slime. The combined effet of the high content of volatile matters and the largern(H)/n(C) ratio of corn stalk reduces its ignition temperature,and the large amount of O is effective for enhancing its thermal decomposition and oxidation[16-17].
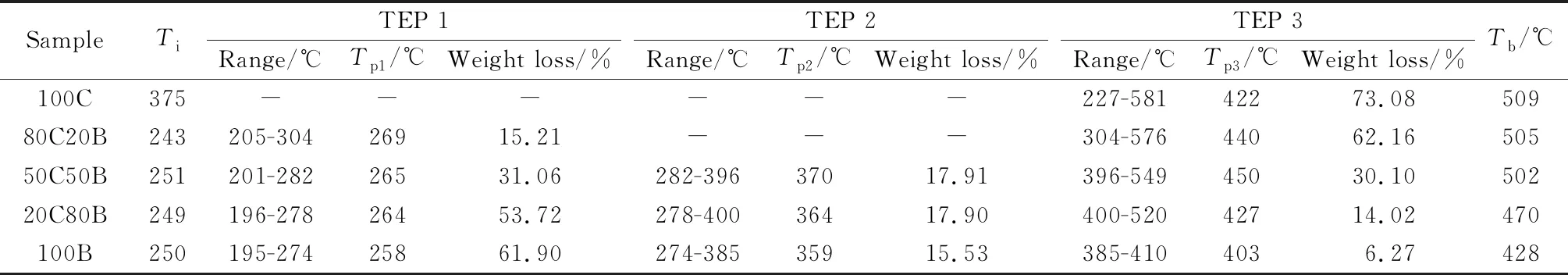
Table 3 Combustion parameters of coal slime, corn stalk and their blends
TheTiof 80C20B decreases dramatically compared to the single coal slime, but theTiof mixtures do not always decrease with the increasingproportion of corn stalk. The volatile matters in corn stalk firstly ignite and combust below 300 ℃, leading to the rise in the surface temperature of coal slime particles and thus promoting the ignition[17]. The peak temperatures at the maximum combustion rate (Tp) at different stages decrease with the rising ratio of corn stalk in the blends. In contrast, the burnout temperature of the blends does not decrease significantly when the proportion of corn stalk is lower than 50%, whileTbdecreases appreciably for 20C80B. The temperatures for the complete combustion of the blends at the final stage decrease with the increasing proportion of corn stalk. These results indicate that the addition of corn stalk to coal slime could accelerate the ignition process and improve combustion performance of coal slime, but no linear relationship exists between the improvement and the additionratios of corn stalk. For 80C20B, the weight loss peaks between 400 ℃ and 500 ℃, which is stronger than that of the 100C, indicating that the blending of corn stalk increases the maximum combustion rate of coal slime obviously. The DTG curves in Fig.1a show that the reactivity of corn stalk char is better than that of coal slime char. Therefore, the corn stalk char combusts at a lower temperature and releases heat that could increase the coal particle temperature and thereby enhance the reactivity on the surface of the coal slime.
2.2 Effect of volatile matters on the combustion characteristics
The DTG curves for raw materials and chars were shown in Fig.2. There are only minor differences in the DTG curves between 100C-P and 100C due to the low volatile matters content in coal slime. The combustion rate of coal char should be lower than that of the raw coal as reported[18-19], but Fig.2a shows the adverse variation. Less than 20% of weight loss occurs when the coal slime is pyrolyzed at 400 ℃, indicating that the effect of pyrolysis at 400 ℃ on the structure of coal char is insignificant[20]. Therefore, the combustion performance of 100C-P does not decrease appreciably compared to the raw coal. In contrast, the effect of pyrolysis at 400 ℃ on the combustion characteristics of corn stalk could not be neglected. The weight loss peak below 300 ℃ that is caused by the decomposition of volatile matters disappears in the DTG curve of 100B-P. This implies that the volatile matters such as hemi-cellulose and cellulose in corn stalk could decompose before 400 ℃ at the inert atmosphere. The oxidation of remaining matters in 100B-P and the char combustion are independently responsible for the two peaks in the DTG curve of 100B-P. The weight loss rate of 100B-P is much smaller than that of 100B because volatile matters in corn stalk are released during the pyrolysis. The DTG curves of blend chars including 80C20B-P, 50C50B-P and 20C80B-P exhibit the integrated weight loss peaks of both coal slime and corn stalk. The combustion of coal char and biomass char could not be distinguished in the DTG curves of 80C20B-P and 50C50B-P. For 80C20B-P, 50C50B-P and 20C80B-P, the weight loss rate of coal char in blend chars is greater than that of coal char because the peak at around 450 ℃ in the DTG curves of blend chars is stronger. This means that the combustion of coal char in blend chars is accelerated by the blending of biomass char. In addition, it is interesting to note that the peak temperature between 300 ℃ and 350 ℃ in the DTG curves of blend chars shifts to the low temperature zone with the increasing proportion of biomass in blends. This may be because coal slime char in the samples hinders the contact of oxygen and biomass char and thereby inhibits the oxidation of biomass chars. Therefore, there is some interaction between coal char and biomass char during co-combustion.
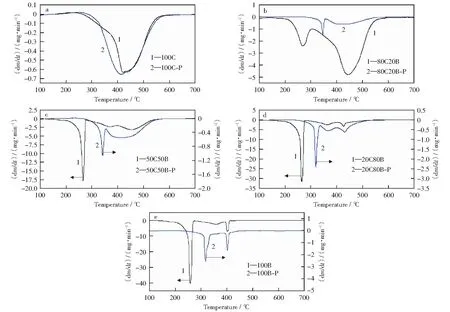
Fig.2 Co-combustion DTG curves for raw materials and char samples
2.3 Effect of inorganic matters on the combustion characteristics
The DTG curves for raw materials and demineralized samples are shown in Fig.3. There are significant differences inthe DTG curves between 100C-D and 100C. in Fig.3a, there are four peaks in the DTG curve of 100C-D combustion as compared to the single peak in the DTG curve of raw coal slime combustion. The release of volatile matters with different forms and the combustion of carbon are distinguished from the DTG curve of demineralized coal slime combustion. The decomposition and oxidation of the volatile matters should be responsible for the first and second peaks in the DTG curve of 100C-D because they disappear in the DTG curve of 100C-D-P combustion (Fig.4). The other peaks are caused by the combustion of carbon in the demineralized coal slime. The weight loss rate in the DTG curve of 100C-D combustion increases compared to that in DTG curve of raw coal slime combustion. These results imply that the inorganic matters in coal slime inhibit the decomposition and release of volatile matters as well as the combustion of fixed carbon.Table 2 shows that the coalslime contains abundant inert components such as SiO2and Al2O3. LI et al[21]found that the inherent mineral matters in coal inhibited the decomposition of macerals and its presence increased the initial and maximum decomposition temperature. The OH radical and oxygen atoms around the clay minerals easily form an interlayer with electric charge, which binds with the polar functional group in the organic layer to form a mineral-organic compound body. This phenomenon enhances the thermal stability of macerals in coal. Furthermore, the presence of a large amount of inert inorganic matters in the coal slime hinders the contact of oxygen and carbon and thereby decreases the pyrolysis and combustion rate of coal slime.
in Fig.3e, the peak position in the DTG curve of 100B-D combustion shifts to a higher temperature zone and the intensity of peak decreases remarkably as compared to 100B, indicating that the inorganic matters in corn stalk could promote the release of volatile matters and the biomass char combustion. The metal salt in the biomass could accelerate the decomposition of cellulose and the increase in the metal salt concentration results in a lower pyrolysis temperature[22-23]. According to the previous studies[2,24-25], the AAEM in biomass catalyzed its combustion and improved the combustion rate.Table 2 shows that the concentrations of CaO, K2O and MgO in ash prepared from corn stalk are relatively high, showing that the corn stalk contains abundant AAEM. Therefore, the inorganic matters including CaO, K2O and MgO in the corn stalk catalyze the decomposition of volatile matters and accelerate the combustion of biomass char.
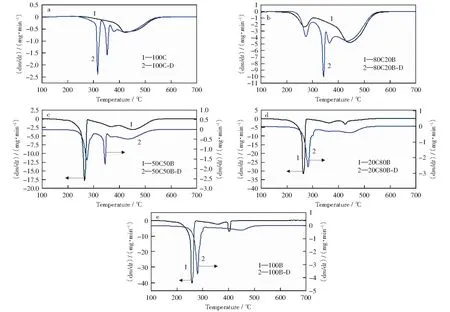
Fig.3 Co-combustion DTG curves for raw materials and demineralized samples
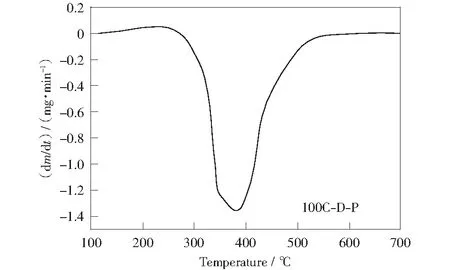
Fig.4 DTG curve of 100C-D-P combustion
2.4 Kinetics analysis
The reaction order (n) of sample combustion is determined by Eq.(1).The variations of regression coefficients (R2) with the value ofnfor blends combustion are shown in Fig.5. The highR2values indicate that the corresponding reaction order satisfactorily fits the experimental data. The value ofnthat leads to the maximum regression coefficient is determined as the reaction order of sample combustion in this work. The coal slime combustion is considered as a series of consecutive first order reactions according to Fig.5a, which is consistent with previous results[6,17].Fig.5e displays that the corn stalk combustion approaches the second order reaction. GIL et al[6]found that the first-order chemical reaction was the most effective solid-state mechanism for the release and oxidation of volatile matters in biomass and for coal combustion, but diffusion mechanisms were found to be responsible for the biomass char combustion. To date, there is no more explanation for this phenomenon. It is interesting to note that the reaction order of blends combustion increases with the increasing proportion of corn stalk. LIAO et al[26]investigated the co-combustion characteristics and kinetics of coal and paper mill sludge and found that the value of reaction order for coal/paper mill sludge co-combustion changed from 1 to 2.
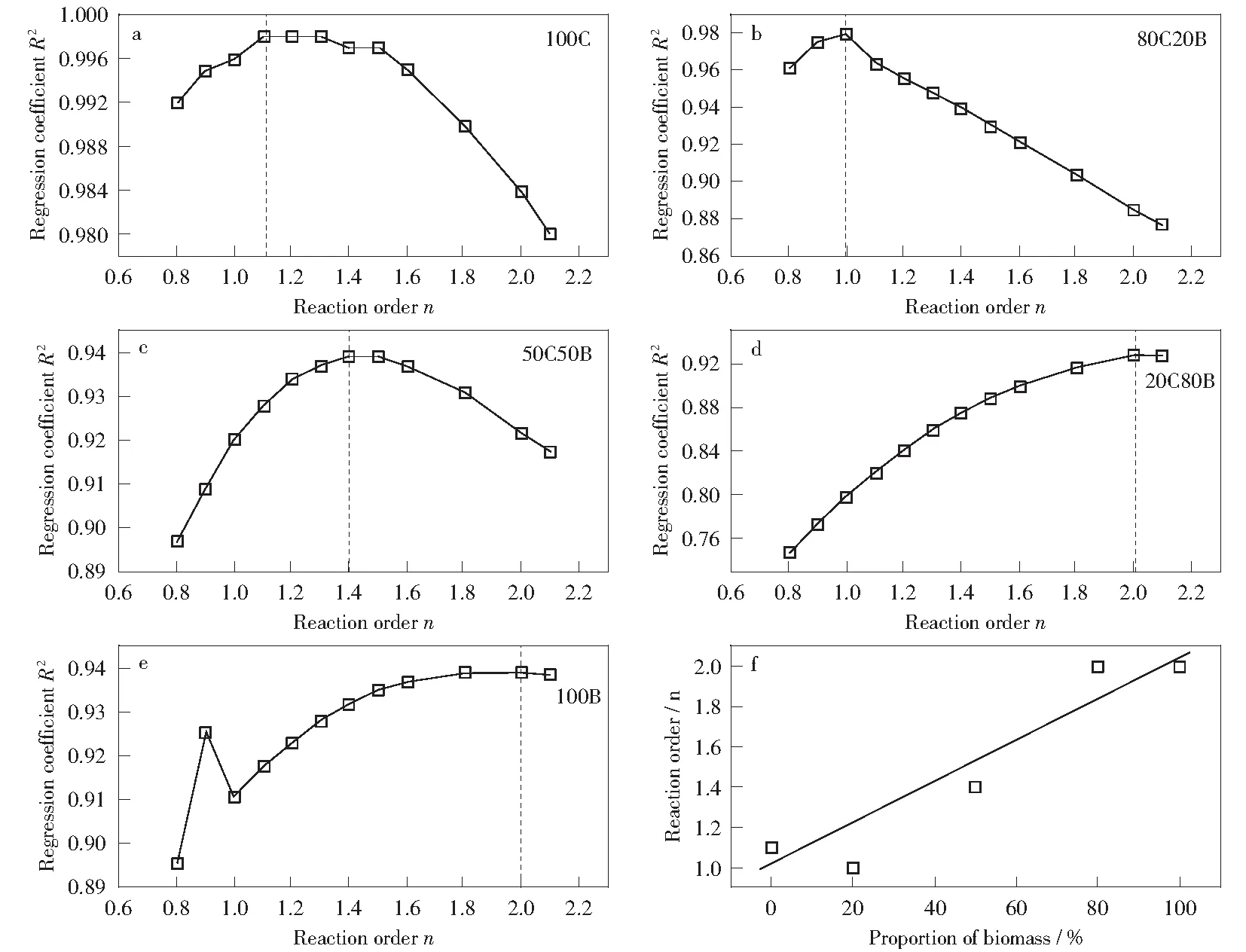
Fig.5 Variation of reaction order for raw fuel blends combustion
The activation energy of samples combustion is calculated by the Eq.(1) and the value ofnis determined according to Fig.5 during the calculation. These results are shown in Fig.6. The activation energy of coal slime combustion is about 71 kJ/mol and this value approaches the activation energy of the low rank coals including peat and lignite. This result indicates that the combustion performance of coal slime approaches that of the low rank coal and coal slime has relatively high combustion reactivity as compared to the high rank coals. The activation energy of corn stalk combustion is around 62 kJ/mol whenn=2. MUNIR et al[27]found that the activation energy of cotton stalk, sugar cane bagasse and shea meal during combustion were 1 138 kJ/mol, 1 168 kJ/mol and 108 kJ/mol, respectively. The activation energy of animal manure, sewage sludge and municipal solid waste combustion studied by SANCHEZ et al[28]were 1 408 kJ/mol, 1 438 kJ/mol, and 173 kJ/mol, respectively. OTERO et al[29]reported that the activation energy of sewage sludge combustion was around 99 kJ/mol. In contrast, the activation energy of corn stalk combustion in this work is much lower than that of other biomass resources in the literatures, suggesting that the corn stalk used in this work has good combustion performance. Some previous studies[8,30]reported that the combustion characteristics including combustion rate, ignition temperature, maximum weight loss temperature and burnout temperature of biomass were better than those of coal, but the Arrhenius activation energy of coal was lower than that of sewage sludge combustion. The other studies[26]showed that the activation energy of biomass was lower than that of coal combustion. The difference is resulted from the various raw materials (biomass) used. The results in this work agree with the latter cases.
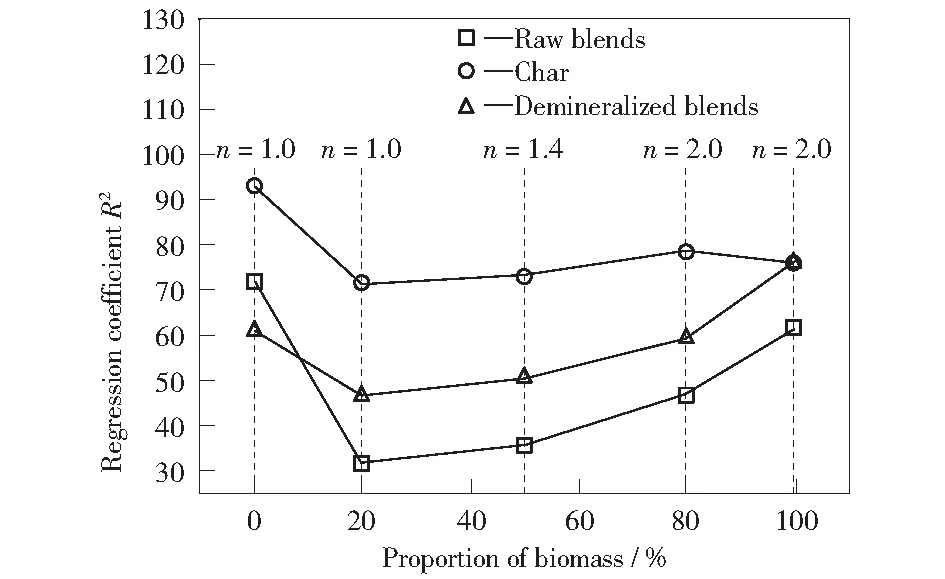
Fig.6 Variation of activation energy with the proportion of biomass at different reaction orders
The activation energy of blends co-combustion is lower than that of single coal slime combustion, implying that the blending of corn stalk could enhance combustion performance of coal slime. The activation energy of 100C-D combustion is lower than that of raw coal slime combustion, which shows that the inorganic matters in coal slime inhibit the combustion reaction of coal slime. The activation energy of the demineralized corn stalk combustion is greater than that of raw corn stalk combustion, which indicates that the inorganic matters in corn stalk could accelerate the corn stalk combustion. These results are consistent with the results in Section 2.3. The combustion performance of char is poorer than that of raw samples as the activation energy of the char combustion is much greater than that of the raw sample combustion. The volatile matters have a significant effect on the co-combustion of coal and biomass.
The activation energy of the coal slime/corn stalk blends co-combustion increases with the increasing proportion of corn stalk when the proportion of corn stalk in blends is greater than 50%. This may be resulted from the decomposition and release of volatile matters with endothermic in corn stalk. But the activation energy of blends co-combustion is lower than that of single coal slime combustion, implying that the blending of corn stalk could accelerate the coal slime combustion.
3 Conclusions
1) The coal slime is characterized by only one combustion step between 227 ℃ and 581 ℃. The corn stalk combustion takes place with three steps: (1) the release and burning of volatile matters, which contains the total decomposition of hemi-cellulose and cellulose as well as the partial decomposition of lignin, occurs between 195 ℃ and 274 ℃; (2) the remaining volatile matters such as lignin decompose between 274 ℃ and 385 ℃; (3) the biomass char combusts between 385 ℃ and 410 ℃.
2) The addition of corn stalk could accelerate ignition during the blends co-combustion and improve the combustion performance of coal slime remarkably due to a large amount of volatile matters in corn stalk.But there is no linear relationship between the improvement and the amount of biomass added in the blends.
3) Most of the volatile matters in corn stalk are released during pyrolysis, but the remaining matters in biomass char still promote the ignition and burn out of coal char. The inorganic matters in coal slime hinder the combustion of coal slime. On the other hand, the inorganic matters in corn stalk promote the combustion of corn stalk significantly.
4) The coal slime combustion is considered as a series of consecutive first order reactions, whilethe corn stalk combustion approaches the second order reaction. The activation energy of coal slime is around 71 kJ/mol and this value approaches the activation energy of the low rank coals including peat and lignite.

