Acute Toxicity of Tetrabromodiphenyl Ether on Three Model Aquatic Organisms
Shunlong MENG Xi CHEN Liping QIU Chao SONG Limin FAN Yao ZHENG Jiazhang CHEN Pao XU
Abstract The acute toxicity of 2,2′,4,4′tetrabromodiphenyl ether on Chlorella vulgaris, Dqohnia magna and Barchydanio rerio was analyzed using semistatic water contacting acute toxicity method. The results showed that the 96 hEC50 of 2,2′,4,4′tetrabromodiphenyl ether (BDE47) on C. vulgaris was 3.97 μg/L, the 48 hLC50 and 96 hLC50 of BDE47 on D. magna were 1.09 and 0.84 mg/L respectively, and the 96 hLC50 of BDE47 on B. rerio was higher than 56.2 mg/L. According to the grading standard of toxicity evaluation, BDE47 had extremely high toxicity to C. vulgaris, high toxicity to D. magna, and low to medium toxicity to B. rerio. The toxicity of BDE47 to C. vulgaris, D. magna and B. rerio followed the order of C. vulgaris>D. magna>B. rerio. For the water solubility of BDE47 is extremely low (15 μg/L), it could not cause the acute poisoning death of D. magna and B. rerio.
Key words 2,2′,4,4′tetrabromodiphenyl ether; Acute toxicity; Chlorella vulgaris; Dqohnia magna; Barchydanio rerio
Received: May 13, 2019Accepted: August 21, 2019
Supported by Central Publicinterest Scientific Institution Basal Research Fund, Chinese Academy of Fishery Science (2017HYZD0208); China Agricultural Research SystemFreshwater Fish (CARS46).
Shunlong MENG (1982-), male, P. R. China, associate researcher, devoted to research about environmental toxicology, fisheries environmental protection, and aquatic product quality and safety risk assessment.
*Corresponding author. Email: chenjz@ffrc.cn; xup@ffrc.cn.
Polybrominated diphenyl ethers (PBDEs) are brominated aromatic hydrocarbons with the general formula C12H(0-9)Br(1-10) O. According to the number of bromine atoms in polybrominated diphenyl ether molecules, they are divided into 209 homologues. PBDEs are highly flameretardant and are often added to high molecular synthetic materials such as polyurethane, resin, rubber and polystyrene to produce fireproof materials. They are also widely used in coatings, textiles, building materials and electrical equipment[1]. In the 1940s and 1950s, PBDEs were industrially produced and added to various industrial products. In the 1960s and 1970s, PBDEs and their products were widely used due to their excellent flame retardant effects[2]. However, as PBDEs are continuously detected in environmental samples, the environmental problems caused by them are getting more and more attention from the society. Currently PBDEs have been restricted to production and use as new POPs[3]. However, since PBDEs are already present in industrial products and have strong stability, they are released into the environment with the use of industrial products and incineration of waste industrial products. Existing research has confirmed that PBDEs have become an important type of environmental pollutants, which is widely detected in air, water, sediment, soil, plants, wildlife and human body[4]. The concentration of PBDEs in the sediments of the Great Lakes[5-7] in North America, Tokyo Bay[8] in Japan, and the Pearl River Delta[9] in China showed a trend of increasing with time. The concentration of PBDEs in airborne dust around an electronic waste treatment plant is as high as 1.96-340.71 μg/g[10]; the concentration of PBDEs in river sediments near an incineration point in Guangdong is 63 300 ng/g[11]; the content of PBDEs in the sediment of the Pearl River Estuary reaches 0.04-94.7 ng/g [12]; and the content of PBDEs in the water body of the Pearl River estuary reaches 26.1-94.6 pg/L[13]. Moreover, since PBDEs can enter organisms through the food chain, PBDEs are also detected in organisms in some areas and exhibit biomagnification[14].
At present, there are many studies on the toxic effects of PBDEs on mammals, but there are few studies on aquatic organisms, especially from the algaedadoceranfish food chain. Since PBDEs in the environment are mainly tetrabromodiphenyl ether, pentabromodiphenyl ether, hexabromodiphenyl ether and decabromodiphenyl ether[14], in this study, 2,2′,4,4 ′tetrabromodiphenyl ether was selected as a representative PBDE, the acute toxicity of which on representative species including Chlorella vulgaris, Dqohnia magna and Barchydanio rerio in the aquatic food chain was investigated, aiming to provide basic data for the development of residue limits for PBDEs in water bodies and for the protection of aquatic ecosystems.
Materials and Methods
Test organisms
In order to improve the representativeness and comparability of the test results, the alga, cladoceran and fish used in this test used standardized model organisms, namely C. vulgaris, D. magna and B. rerio. The test was carried out by the standardized acute toxicity test method.
C. vulgaris used in the test was purchased from the Institute of Hydrobiology, Chinese Academy of Sciences. It was cultured in BG11 medium at a temperature of (25±1) ℃ under a lightdark ratio at 12 h∶12 h with a light intensity of 4 000 lx and a pH value of 7.0-7.5. The conical flasks were shaken once every 2 h during the light period, and stood in the dark period, and at the same time, the positions of the conical flasks were randomly changed, so that the algal liquids were evenly illuminated. The D. magna was purchased from Jiangsu HOPE Analytech Inc. The D. magna used in the test was a monoclonal strain which had been cultured for more than three generations in the laboratory. The culture method was based on GB/T 16125-2012, and the tap water was filtered through a 0.22 μm filter membrane. The D. magna was fed C. vulgaris, and the culture medium was changed periodically. It was cultured in an incubator at a temperature of (20±1) ℃ and a lightdark ratio of 16 h∶8 h with an illumination intensity of 1 000 lx. At 24 h before the test, the healthy pregnant females were selected and placed in water. The females were picked out 6 h before the test, and healthy individuals were selected from the proliferated D. magna to carry out the test. C. vulgaris was fed during the culture. The BG11 medium was sterilized and introduced into C. vulgaris, which was cultured at (20±1) ℃ under 1 000 lx continuous light condition. The algal liquid in the logarithmic growth phase was collected and centrifuged at 8 000 r/min for 5 min, the algal cells were collected, then suspended in deionized water, and input into a D. magna vessel. The D. magna was not fed 1 d before the test and during the test.
The test B. rerio had an average body length of (2.8±0.3) cm and an average body weight of (0.30 ± 0.06) g (n=10). After domesticated in the laboratory for two weeks, the active individuals were selected for research. The fish was fed timely once a day during domestication. No feed was input 1 d before the test and during the test. There was no dead fish in the last 7 d during the domestication period.
Test water
The test water was dechlorinated tap water aerated for 7 d, which had a temperature of (20±1) ℃ and a pH value of 7.0-7.5, and contained dissolved oxygen 6.5-7.0 mg/L. The test water met the fishery water quality standard (GB 11607-89).
Test drugs
The used 2, 2′,4, 4′tetrabromodiphenyl ether (BDE47) was produced by Wuhan Kaymke Chemical Technology Co., Ltd., and had a purity equal to or higher than 98%. Because BDE47 is slightly soluble in water, according to the sensitivity of the test organisms to cosolvents in relevant literatures, dimethyl sulfoxide (DMSO) was used as a cosolvent for the acute toxicity tests of D. magna and B. rerio, and Ndimethylformamide (DMF) was selected as a cosolvent for acute toxicity test of the alga. A BDE47 mother liquor was prepared and stored in a refrigerator at 4 ℃ for use. During the test, it was diluted with water to a contamination solution with a response mass concentration.
Test methods
The acute toxicity tests of BDE47 against C. vulgaris, D. magna and B. rerio were carried out by the semistatic water contacting method. According to the trial test results and the preparation requirements of medial lethal concentration, the appropriate test concentration range was selected to carry out the acute toxicity tests of the PBDE on C. vulgaris, D. magna and B. rerio.
C. vulgaris
The test was carried out with 250 ml flasks. Before the test, the flasks were immersed in dilute nitric acid for 48 h, rinsed with purified water, and airdried for use. Two control groups were set up in the test, which were the blank control group and the cosolvent control group, and the concentration of the cosolvent in the cosolvent control group was the same as that in the highestdose group. The concentrations of BDE47 in the test were: 0.1, 1.0, 5.0, 10.0 and 50.0 μg/L. C. vulgaris in the exponential growth phase was inoculated into a triangular flask containing 150 ml of BG11 medium, and the initial algal density was 1×105 cells/L. Each of the concentration gradients was done in three parallels. The algal fluid was collected at 24, 48, 72, and 96 h of the test, and microscopically examined under a 400fold microscope, and the cell density of C. vulgaris was calculated.
D. magna
The test was carried out in 100 ml beakers. Before the test, the beakers were soaked in dilute nitric acid for 48 h, rinsed with purified water, and airdried for use. Two control groups were set up in the test, which were the blank control group and the cosolvent control group, and the concentration of the cosolvent in the cosolvent control group was the same as that in the highestdose group. The concentrations of BDE47 were set according to the logarithmic distance of 0.1 as follows: 0.50, 0.63, 0.79, 10.00, 1.26 and 1.58 mg/L, respectively. Each beaker contained 80 ml of BDE47 solution, and was added with 10 individuals. Three parallels were done for each gradient. The test was carried out at a water temperature of (20±1) ℃, a light intensity of 1 000 lx and a lightdark ratio of 16 h∶8 h. No feed was input during the test. At the end of the test, the death of D. magna in the beaker was observed microscopically, with no heartbeat as the criterion for the death of D. magna.
B. rerio
The test was carried out in cuboid aquaria with a length, width and height of 40, 30 and 30 cm, respectively. Before the test, the aquaria were soaked for 48 h with potassium permanganate solution, and rinsed with dechlorinated tap water aerated for 7 d before use. Two control groups were set up in the test, which were the blank control group and the cosolvent control group, and the concentration of the cosolvent in the cosolvent control group was the same as that in the highestdose group. The concentrations of BDE47 were set according to the logarithmic distance of 0.125 as follows: 10.0, 13.3, 17.8, 23.7, 31.6, 42.2 and 56.2 mg/L, respectively. Each aquarium contained 30 L of corresponding PBDE solution, and was added with 10 individuals. Three parallels were done for each gradient. The acute test was observed continuously in the first 8 h, and then observed and recorded at 24, 48, and 96 h, respectively.
Data processing and statistical analysis
C. vulgaris
The 96 hmedian effect concentration (EC50) of BDE47 against C. vulgaris was calculated according to the method specified by the OECD. According to the experimental data, the growth curves of C. vulgaris under the various treatment concentrations of BDE47 at 96 h were constructed. The areas under the growth curves were calculated according to formula (1):
A=(N1-N0)/2×t1 +(N1+N2-2N0)/2×(t2-t1)+…+(Nn-1+Nn-2N0)/2×(tn-tn-1)(1)
In formula (1): A is the area under the growth curve; N0 is the initial cell number at t0 (cells/ml); N1 is the algal number determined at t1 (cells/ml); Nn is the algal number determined at tn (cells/ml); t1 is the time for the first determination at the beginning of the test; and tn is the nth determination after the test is started. The cell growth inhibition percentage (IA) of C. vulgaris was calculated by formula (2):
IA=(Ac-At)/Ac×100(2)
In formula (2): IA is the cell growth inhibition percentage of C. vulgaris in the treatment group (%); Ac is the area under the growth curve of C. vulgaris of the control group; and At is the area under the growth curve of C. vulgaris of the treatment group. The concentrationeffect equation was obtained by the probability unitconcentration logarithm method; and when the probability unit was 5, the 96 hEC50 was calculated.
D. magna and B. rerio
Linear regression was used to analyze the 95% confidence limit of the LC50 of BDE47 to each of D. magna and B. rerio[15]. The safe concentration was estimated based on the medial lethal concentration[15].
Statistical Analysis
The significance of the correlation coefficient of the regression equation was tested by t test. The significance level was α=0.05.
Results and Analysis
Halfeffect concentration of BDE47 on C. vulgaris
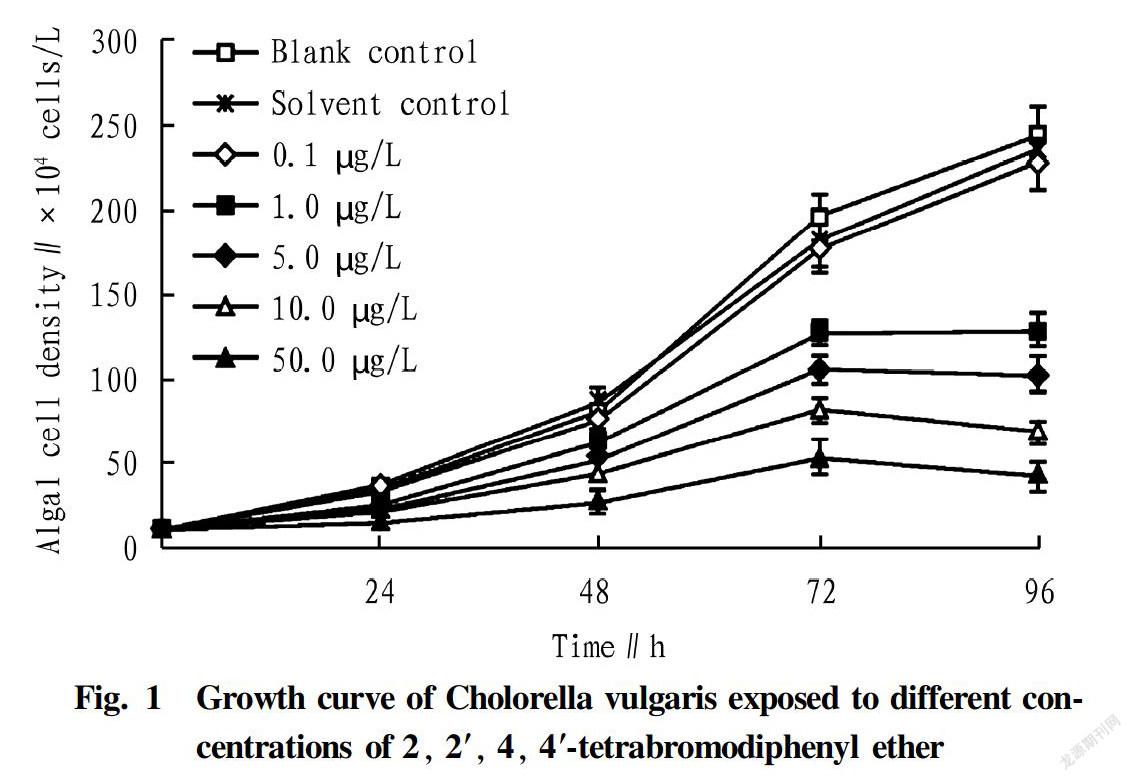
The trends of the C. vulgaris density with culture time at different treatment concentrations of BDE47 are shown in Fig. 1. The test results (Fig. 1) showed that the C. vulgaris of the blank control group and the cosolvent control group showed a rapid growth trend, and good exponential growth characteristics as well. With the advancement of time, the growth trend of C. vulgaris in each BDE47 group was significantly changed compared with the control group. The growth of C. vulgaris in each treatment group was significantly inhibited, and the inhibition was gradually aggravated with the increase of the treatment concentration and the prolongation of the treatment time.
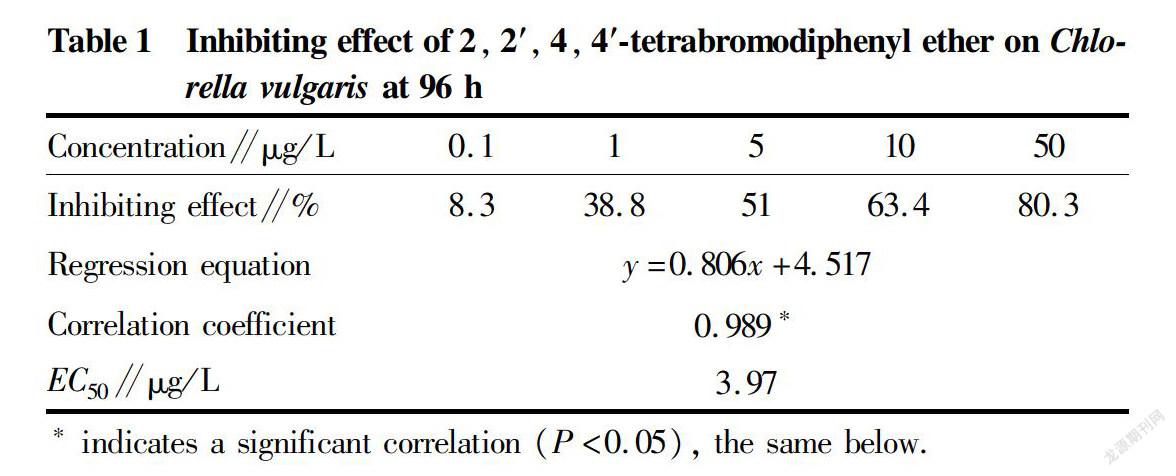
The regression equation, correlation coefficient and EC50 of BDE47 for acute toxicity on C. vulgaris are listed in Table 1. The correlation coefficient at 96 h was 0.989, reaching the significant level (P<0.05). BDE47 had a 96 hEC50 of 3.97 μg/L and a safe concentration of 0.397 μg/L for C. vulgaris.
Medial lethal concentration of BDE47 on D. magna
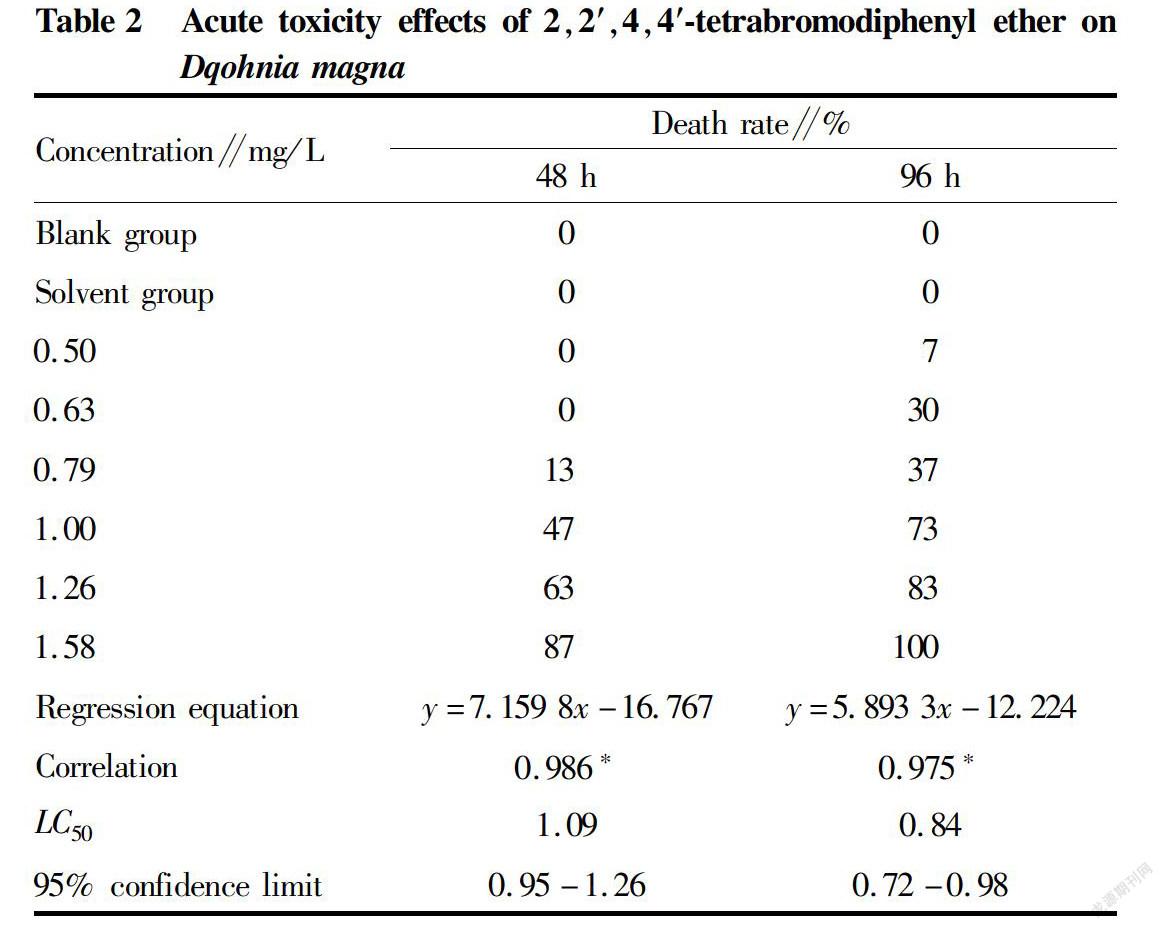
The toxicity test of BDE47 on D. magna showed that in the BDE47 contamination series, the death rate of D. magna increased gradually from low to high in the various concentration groups (Table 2). The correlation coefficient, regression equation, LC50 and 95% confidence limit of BDE47 for the acute toxicity on D. magna are listed in Table 3. The correlation coefficients of BDE47 at 48 and 96 h were 0.986 and 0.975, respectively, both reaching the significant level (P<0.05). The LC50 values of BDE47 on D. magna at 48 and 96 h were 1.09 and 0.84 mg/L, respectively, and the safe concentration was 0.084 mg/L.
Acute toxicity of BDE47 on B. rerio
The acute toxicity test of BDE47 on B. rerio showed that all the individuals in the treatment groups and the control groups did not die. Therefore, the 96 hLC50 of BDE47 on B. rerio was higher than 56.2 mg/L. BDE47 has a very low solubility in water of 15 μg/L, so BDE47 and BDE153 do not cause acute toxicity to B. rerio in the natural environment.
Discussion
In this study, the 96 hEC50 of BDE47 to C. vulgaris was 3.97 μg/L, the 48 hLC50 and 96 hLC50 to D. magna were 1.09 and 0.84 mg/L, respectively, and the 96 hLC50 to B. rerio was higher than 56.2 mg/L. According to the grading standards of toxicity evaluation, BDE47 had extremely high toxicity, high toxicity and low to medium toxicity for C. vulgaris, D. magna and B. rerio, respectively; and the toxicity of BDE47 to aquatic organisms ranked as C. vulgaris>D. magna>B. rerio. Since BDE47 has a very low solubility in water of 15 μg/L, BDE47 does not cause acute poisoning death of D. magna and B. rerio in the natural environment.
The acute toxicity of exogenous chemicals to organisms can be grasped according to acute toxicity tests. Therefore, an acute toxicity test can provide the firsthand information for chemical safety evaluation, and it is generally required in the regulatory procedures of different types of chemicals[16]. Algae are sensitive to PBDEs, and the results of the acute toxicity tests of BDE47 on algae showed that the 48 hEC50 for Skeletonema costatum was 70 μg/L[17] and the 96 hEC50 was 1.99 μg/L[18]; the 96 hEC50 for C. vulgaris, Chaetoceros mulleri and Heterosigma akashiwo were 0.79, 1.52 and 2.25 μg/L, respectively[18]; the 96 hEC50 for Phytophthora salina and Tetraselmis subcordiformis were 119.93 and 113.66 μg/L, respectively[19]; and the 96 hEC50 for Chrysophyceae, H. akashiwo, Karenia mikimotoi and Platymonas helgolandica were 0.016, 0.046, 0.25 and 2 mg/L, respectively[20]. According to the statistics of existing data, the 96 hEC50 of BDE47 varies from 0.016 to 2 mg/L, and the fluctuation range is large. This study showed that the 96 hEC50 of BDE47 against C. vulgaris was 3.97 μg/L, which was within the above 96 hEC50 range of BDE47 for algae. The toxicity effects of BDE47 on different kinds of microalgae differ to different degrees, which might be related to the morphological structure of algae, such as cell wall and extracellular collagen[11]. The presence or absence of cell wall and extracellular collagen both may affect the acute toxicity of contaminant to algae. According to the grading standards for alga growth inhibition toxicity evaluation[21], BDE47 is extremely toxic to algae and can easily lead to acute poisoning death of C. vulgaris.
Similar to algae, zooplankton is also sensitive to PBDEs. The acute toxicity tests of BDE47 on zooplankton showed that the 96 hLC50 of the Nitocra lacustris was 72 μg/L[22]; The 96 hLC50 for Eurytemora pacifica and Tigriopus japonicus were 57 and 851 μg/L, respectively[23]; the 48 hLC50 for D. magna was 1.04 mg/L, with a 95% confidence limit in the range of 0.93-1.16 mg/L[24]; and the 24, 48, 72, and 96 hLC50 for Brachionus plicata were 22, 2.113, 0.376 and 0.163 mg/L, respectively[25]. However, compared with the results of Sha et al.[25], Zhang et al.[26] concluded that the 24 hLC50 of BDE47 against B. plicata was 7.92 mg/L. Statistical analysis of the available data showed that the 96 hLC50 of BDE47 for cladocerans was 57.00-1.04 mg/L, which fluctuated greatly. In this study, the 48 hLC50 and 96 hLC50 of BDE47 for D. magna were 1.09 and 0.84 mg/L, of which the 96 hLC50 of BDE47 for D. magna was within the above 96 hEC50 variation range of BDE47 for cladocerans. According to the grading standards for cladoceran acute toxicity evaluation[21], BDE47 was highly toxic to D. magna. However, since BDE47 has a very low solubility in water of 15 μg/L, BDE47 does not cause acute poisoning death of D. magna in the natural environment.
Unlike phytoplankton and zooplankton, fishes are extremely insensitive to PBDEs. Studies have shown that BDE47 had 24, 48, 72 and 96 hLC50 for adult B. rerio at 250, 250, 250 and 85 mg/L, respectively[27]; the 24, 48, and 96 hLC50 for Takifugu rubripes were 70.01, 18.83 and 9.44 mg/L[28]; and the 96 hLC50 for Xiphophorus hellerii was 2.75 mg/L, with a 95% confidence limit in the range of 1.73-3.45 mg/L, and the maximum nonlethal dose or concentration was 2.2 mg/L[29]. In this study, the 96 hLC50 of BDE47 on B. rerio was higher than 56.2 mg/L, which is similar to the test results of Zhao et al.[27]. According to the grading standards for fish acute toxicity evaluation[21], BDE47 had low to medium toxicity to B. rerio. Since BDE47 has a very low solubility in water of 15 μg/L, BDE47 does not cause acute poisoning death of B. rerio in the natural environment. The toxicity of BDE47 to different types of aquatic organisms varies greatly, and even the toxicity to the same species is different. These differences in acute toxicity tests may be related to the purity of the drug, the health and type of the test organisms, and the physical and chemical characteristics of the test water. Overall, the toxicity of BDE47 to aquatic organisms follows the order of algae>cladocerans>fishes.
From the perspective of the food chain and ecosystem composed of phytoplankton, zooplankton and fishes, algae are an important producer in aquatic ecosystems, a foundation and a key link in the food chain, which can synthesize nutrients in water through photosynthesis into substances necessary for ecosystems and store them in the form of energy to maintain the normal functioning of aquatic ecosystems. Zooplankton is a key group in aquatic ecosystems, which can control the quantity of phytoplankton through predation. Meanwhile, it can be used as a bait for highlevel trophic fish. Its quantity change can directly affect the resources of large aquatic animals such as fish. Zooplankton regulates the structure and function of aquatic ecosystems. Therefore, the extremely high toxicity and high toxicity of BDE47 to C. vulgaris and D. magna show that it has great harm to aquatic ecosystems. It is necessary to establish the residue limit standard of PBDEs in water to better protect aquatic ecosystems.
References
[1] LI J. The bioaccumulation and trophodynamics of heavy metals in the food web of three gorges reservoir[D]. Wuhan: Huazhong Agricultural University, 2015. (in Chinese)
[2] HE LF, GUO ZC. Application study on waterborne inorganic zincrich coating[J]. Surface Technology, 2006, 35(1): 55-59. (in Chinese)
[3] LIANG XW. Bioavailability of a new persistent organic pollutantpolybrominated diphenyl ethers in soil[D]. Tianjin: Nankai University, 2011. (in Chinese)
[4] DE WIT C A. An overview of brominated flame retardants in the environment[J]. Chemosphere, 2002, 46(5): 583-624.
[5] SONG W, FORD JC, LI A, et al. Polybrominated diphenyl ethers in the sediments of the Great Lakes. 1. Lake Superior[J]. Environmental Science and Technology, 2004, 38(12): 3286-3293.
[6] SONG W, FORD JC, LI A, et al. Polybrominated diphenyl ethers in the sediments of the Great Lakes[J]. Environmental Science and Technology, 2005, 39(15): 5600-5605.
[7] SONG W, LI A, FORD JC, et al. Polybrominated diphenyl ethers in the sediments of the Great Lakes. 2. Lakes Michigan and Huron[J]. Environmental Science and Technology, 2005,39(10):3474-3479.
[8] MINH NH, ISOBE T, UENO D, et al. Spatial distribution and vertical profile of polybrominated diphenyl ethers and hexabromocyclododecanes in sediment core from Tokyo Bay, Japan[J]. Environmental Pollution, 2007, 148(2): 409-417.
[9] CHEN SJ, LUO XJ, LIN Z, et al. Time trends of polybrominated diphenyl ethers in sediment cores from the Pearl River Estuary, south China[J]. Environmental Science and Technology, 2007, 41(16): 5595-5600.
[10] ZHAO J. Research progress on the investigation of toxic effects of polybrominated diphenyl ethers in fish[J]. Journal of Shanghai Second Polytechnic University, 2015, 32(3): 177-184. (in Chinese)
[11] LEUNG AO, LUKSEMBURG WJ, WONG AS, et al. Spatial distribution of polybrominated diphenyl ethers and polychlorinated dibenzopdioxins and dibenzofurans in soil and combusted residue at Guiyu, an electronic waste recycling site in southeast China[J]. Environmental Science and Technology, 2007, 41(8): 2730-2737.
[12] MAI BX, CHEN SJ, LUO XJ, et al. Distribution of polybrominated diphenyl ethers in sediments of the Pearl River Delta and adjacent South China Sea[J]. Environmental Science and Technology, 2005, 39(10): 3521-3527.
[13] LUO XJ, YU M, CHEN SJ, et al. Distribution and assignment of polybrominated diphenyl ethers in Pearl River Estuary[J]. Chinese Science Bulletin, 2008, 53(2): 141-146. (in Chinese)
[14] LUO XJ, MAI BX, CHEN SJ. Advances on study of polybrominated diphenyl ethers[J]. Progress in Chemistry, 2009, 21(Z1): 359-368. (in Chinese)
[15] State Environmental Protection Administration. Methods of water and wastewater monitoring and analysis[M]. Beijing: China Environmental Science Press, 1989. (in Chinese)
[16] ZHANG AH, ZHANG H. Experimental course on public health and preventive medicine[M]. Beijing: Science Press, 2012: 252. (in Chinese)
[17] KLLQVIST T, GRUNG M, TOLLEFSEN KE. Chronic toxicity of 2,4,2′,4′tetrabromodiphenyl ether on the marine alga Skeletonemacostatum, and the crustacean Daphnia magna[J]. Environmental Toxicology & Chemistry, 2010, 25(6): 1657-1662.
[18] LI ZN, MENG FP, ZHAO SS, et al. Acute toxic effects of 2,2′,4,4′tetrabromodiphenyl ether (BDE47) on four marine microalgae[J]. Asian Journal of Ecotoxicology, 2009,4(3):435-439. (in Chinese)
[19] HU H, YU T, MENG FP, et al. Acute toxicity of five brominated diphenyl ether congeners to marine baitalgae species: Platymonas subcordiformis and Dunaliella saliva[J]. Marine Environmental Science, 2015, 34(5): 654-660. (in Chinese)
[20] JIANG S. The toxic effects of two kinds of polybrominated biphenyl ethers (PBDEs) on four species of marine microalgae[D]. Qingdao: Ocean University of China, 2011. (in Chinese)
[21] State Environmental Protection Administration. Methods of water and wastewater monitoring and analysis[M]. Beijing: China Environmental Science Press, 2002. (in Chinese)
[22] BREITHOLTZ M, WOLLENBERGER L. Effects of three PBDEs on development, reproduction and population growth rate of the harpacticoid copepod Nitocraspinipes[J]. Aquatic Toxicology, 2003, 64(1): 85-96.
[23] XU FF. Toxic effects of BDE47 on two marine copepods[D]. Qingdao: Ocean University of China, 2013. (in Chinese)
[24] PENG Y, FAN CP, LIAO W, et al. Toxic effects of 2,2′,4,4′tetrabromodiphenyl ether on Daphnia magna[J]. Asian Journal of Ecotoxicology, 2012, 7(1): 79-86. (in Chinese)
[25] SHA JJ. Study on single and joint toxic effects of two PBDEs (BDE47,BDE209) on rotifer Brachionus plicatilis[D]. Qingdao: Ocean University of China, 2015. (in Chinese)
[26] Zhang J. The reproductive and developmental toxicity and mechanism of polybrominated diphenyl ethers in rotifer Brahchiohus Plicatilis[D]. Qingdao:Ocean University of China, 2013. (in Chinese)
[27] ZHAO XS, REN X, YANG CW, et al. Joint effect of acute exposure to 2,2′,4,4′tetrabromodiphenyl ether and perchlorate on adult zebrafish[J]. Jilin Normal University Journal: Natural Science Edition, 2016, 37(2):105-110. (in Chinese)
[28] ZHANG YM. Toxicity and effect on antioxidant enzymes activity of tetrabromodiphenyl ether (BDE47) in marine teleosts[D]. Qingdao: Ocean University of China, 2014. (in Chinese)
[29] FAN CP, WANG Q, LIU XY, et al. Toxicity and effect of tetrabromodiphenyl ether upon the antioxidant defense system of swordtail fish (Xiphophorus helleri)[J]. Acta Scientiae Circumstantiae, 2011, 31(3): 642-648. (in Chinese)
- 农业生物技术(英文版)的其它文章
- Observation on Cardiac Opening of the Inferior Vena Cava in Goat Fetuses
- Evaluation on Application and Spraying Effect of AirAssisted Sprayer in Apple Orchard with Dwarfing Rootstocks
- Problems in the Development of Traditional Chinese Medicinal Materials Planting Industry in Shiyan City and Countermeasures
- Study on Practical Mature Age of Individual Pinus thunbergii×P. densiflora
- Effects of Different Densityreducing Methods on Canopy Microenvironment, Tree Growth and Fruit Quality in Closed Apple Orchard
- Development of Whole Potato Flour Fish Noodles

