罗氏沼虾性别相关基因研究进展及其单性化养殖现状
姜建萍 袁翔 邱庆庆 黄光华 蒋钦杨 杨秀荣 蒋和生

摘要:罗氏沼虾(Macrobrachium rosenbergii)具有生长快、养殖周期短、营养价值高等优点,是我国主要的淡水虾养殖品种,在生长性能方面表现出性别两态性:同龄雌、雄虾个体的大小、生长速度相差悬殊,导致大规模生产罗氏沼虾受到限制,因此迫切需要采用性别控制技术开展罗氏沼虾单性化育苗,培育全雌/全雄罗氏沼虾以提高养殖产量,而实现罗氏沼虾单性化养殖的前提必须明确性别分化和性别决定的分子机制及其关键基因。文章就罗氏沼虾性别相关基因的研究进展及其单性化养殖发展现状进行综述,认为全雄/全雌罗氏沼虾育种的关键技术是伪雌或超雌虾制备。鉴于罗非鱼三系[原系(XX♀)、雄性纯合系(YY♂)和雄性纯合转化系(YY♀)]配套方案的启发,今后可对罗氏沼虾遗传型WW超雌个体进行性逆转,获得伪雄个体(遗传型WW,生理型ZZ),经回交所得后代用于构建超雌虾种质库,即通过性别分化和性别决定机制解析及超雌种质库构建,研发自主的单性化罗氏沼虾制种技术,培育全雄/全雌罗氏沼虾将成为可能。
关键词: 罗氏沼虾;性别控制;性别相关基因;单性化养殖
中图分类号: S966.12 文献标志码: A 文章编号:2095-1191(2019)09-2111-08
Abstract:Giant freshwater prawn(Macrobrachium rosenbergii) as an important freshwater prawn has become one of the leading varieties of freshwater aquaculture in China due to its quicker growth rate, shorter breeding cycle and higher nutrition. The male and female individuals exhibit sexual dimorphism in terms of growth performance:the size and growth rate of the same age was different, which limited the boost production of M. rosenbergii. Thus, it was urgent to adopt sex manipulation to carry out monosex culture, and to cultivate all-female and all-male prawns to increase the aquaculture yield. The prerequisite of the monosex culture was understanding the molecular mechanism and key genes of sex differentiation and sex determination. Hence, in this paper, the research progress of sex-related genes and research status of monosex culture at home and abroad were summarized, and it was considered that the essential for all-female and all-male breeding was the preparation of neo-female and super female. Inspired by the three-line matching scheme of tilapia[original(XX♀), male homozygous(YY♂) and male homozygous transformation(YY♀)], the genetic reversal WW super-female individuals in the future could be sex-reversed, and neo-male individuals(genotype WW, physiologic type ZZ) were obtained. After backcrossing, they were used to construct super female breeding germplasm bank. Notably, it is po-ssible to develop independent parthenogenesis techniques of M. rosenbergii, and to cultivate all-female and all-male prawns through the analysis of the molecular mechanism of sex differentiation and sex determination.
Key words: Macrobrachium rosenbergii; sex control; sex related gene; monosex culture
0 引言
羅氏沼虾(Macrobrachium rosenbergii)属大型淡水虾类,具有生长快、养殖周期短、营养价值高等优点,是我国主要的淡水虾养殖品种。我国于1976年引进罗氏沼虾,至1990年得到快速推广。近年来,随着市场需求的日益增长和人工繁育苗种技术的进步,我国罗氏沼虾养殖呈现方兴未艾之势。据统计,2016年我国罗氏沼虾养殖产量达13.2万t,较2015年增长了2.49%(农业部渔业渔政管理局,2017),其产量已超过全球罗氏沼虾养殖产量(约23.0万t)的1/2。与其他甲壳类动物一样,罗氏沼虾也表现出性别两态性:同龄雌、雄虾个体的大小、生长速度相差悬殊,即在同等养殖条件下,雌虾生长速度较雄虾慢50%~70%,性成熟后的雄虾个体平均体重约是雌虾的2倍(俞炎琴,2013)。这是由于我国传统的罗氏沼虾养殖模式均为雌雄混养,在养殖过程中部分虾性成熟早,并进行交配繁殖而消耗大量能量,进而影响其生长速度和群体均匀度。为改善这一现状,部分养殖户通过人工挑选留大剔小、雌雄分开等方法以提高罗氏沼虾产量,但此法劳动强度大、成本高,且雌雄幼虾区分不明显,导致大规模生产罗氏沼虾受到限制。因此,迫切需要采用性别控制技术开展罗氏沼虾单性化育苗,培育全雌/全雄罗氏沼虾以提高养殖产量,而实现罗氏沼虾单性化养殖的前提必须明确性别分化和性别决定的分子机制及其关键基因。本文就罗氏沼虾性别相关基因的研究进展及其单性化养殖发展现状进行综述,以期为研发罗氏沼虾性别控制技术提供参考,进而有效指导罗氏沼虾单性化养殖。
1 罗氏沼虾性别决定及性别分化
甲壳类动物性别决定类型有遗传决定型、环境决定型及遗传与环境共同决定型(楼允东等,2004;周梦颖,2014)。目前,虽然无直接证据证实罗氏沼虾存在性染色体,但前人的相关研究结果显示,通过移植促雄性腺(Androgenic gland,AG)获得的伪雄罗氏沼虾与正常雌性个体杂交F1代雌雄比例约3∶1,而F1代雌性个体与伪雄罗氏沼虾回交的F2代中雌雄比例为6.63∶1(Malecha,2012);此外,通过摘除促雄性腺获得的伪雌罗氏沼虾与正常雄性个体杂交,获得的F1代性别全为雄性(Aflalo et al.,2006;Rungsin et al.,2006)。因此,根据性逆转及杂交试验的性别比可推测罗氏沼虾属于WZ/ZZ染色体性别决定类型,且表现为雌异(WZ)雄同(ZZ)(Lécher et al.,1995;Benzie et al.,2001;Aflalo et al.,2006;Staelens et al.,2008)。
值得注意的是,甲壳类动物特有的内分泌腺——促雄性腺在雄性甲壳类动物的性别分化、精子发生及雄性第二性征维持中发挥重要作用。尤其对于性别已分化的罗氏沼虾个体来说,通过人为摘除或植入促雄性腺可使其性别发生逆转,从而改变生理性别,因此其分泌的促雄性腺激素(Androgenic gland hormone,AGH)不仅是性别决定因素,还是雄性性别分化的调控因子(楼允东等,2004)。此外,甲壳类动物特殊的进化地位导致其性别分化过程极易受外界环境因素如温度、盐度、pH、食物丰度、光照和水质,以及环境内分泌干扰物(壬基酚)等的影响(吴楠等,2007;朱春华等,2011;周梦颖,2014;戴习林等,2016)。关于罗氏沼虾性别分化的时间,朱春华等(2011)通过外部形态观察结合性腺组织学切片的方法,确定了罗氏沼虾性别分化和性成熟的时间,具体表现为外部形态特征的出现稍早于内部性征分化时间,且早期分化和性成熟分别发生在幼虾变态后21和45 d。
2 罗氏沼虾性别相关候选基因及分子标记
性别控制技术开发是实现罗氏沼虾单性化养殖的基础,而性别控制技术的重点在于挖掘和鉴定性别分化与性别决定的关键基因。近年来,随着分子生物技术的快速发展,已有学者通过cDNA文库构建和转录组测序等方法筛选获得罗氏沼虾性别相关基因(Cao et al.,2006,2007;Jin et al.,2013;Ma et al.,2019);同時,借助AFLP(Amplified fragment length polymorphism)技术鉴定出罗氏沼虾性别特异性分子标记(Ventura et al.,2011;Jiang and Qiu,2013),为实现单性化养殖提供了重要理论依据。
2. 1 性别相关基因
2. 1. 1 Mr-IAG基因 甲壳类雄性动物特异的促雄性腺最先在软甲亚纲雄兰蟹中发现,作为特有的内分泌器官,其分泌的促雄性腺激素在促进雄性性别分化过程中发挥重要作用(Sagi et al.,1997)。近年来,促雄性腺特异性基因(Insulin-like androgenic gland,IAG)相继被发现(Lugo et al.,2006;Manor et al.,2007;Shechter et al.,2008;Rosen et al.,2010)。Ventura等(2009)通过构建罗氏沼虾促雄性腺cDNA消减文库鉴定出Mr-IAG基因,经测序比对分析,发现成熟Mr-IAG肽段的半胱氨酸骨架结构与其他甲壳类动物IAG具有高度的同源性;此外,注射Mr-IAG双链RNA会暂时抑制雄性第二性征的重塑和雄性附肢再生,并伴随蜕皮延后和生长参数下降现象;而沉默Mr-IAG基因表达会引起精子发生阻滞和促雄性腺的肥大增生。鉴于Mr-IAG基因能引起雄性性别分化的逆转,目前已在小龙虾(Curtis and Jones,1995;Parnes and Sagi,2002)、青蟹(Triño et al.,1999)和斑节对虾(Gopal et al.,2010)等甲壳类动物中开展了一系列针对IAG基因的性别控制研究。
2. 1. 2 Mr-IR基因 胰岛素受体(Insulin receptor,IR)属于酪氨酸激酶受体超家族,作为胰岛素家族信号通路中的重要蛋白,在调节胞内和胞间环境的稳态过程中发挥重要作用(Fafalios et al.,2011)。已有研究表明,胰岛素信号通路在性别发育过程中起主导作用(Nef et al.,2003)。Sharabi等(2016)基于多资源信息构建转录本文库鉴定出与脊椎动物IR基因具有较高同源性的Mr-IR基因,并成功克隆获得该基因的cDNA序列,生物信息学分析结果显示其编码1508个氨基酸,包含2个保守跨膜结构域(Transmembrane domain,TM)、2个配体结合结构域、3个纤维蛋白连接素-3结构域及1个酪氨酸激酶结构域。Mr-IR基因在罗氏沼虾成虾的各组织中呈广泛表达模式,但与IR基因在脊椎动物中的表达模式存在差异,如在肌肉组织中不表达(Chen et al.,1996;Steele et al.,1996)。Sharabi等(2016)研究表明,Mr-IR基因沉默并不影响罗氏沼虾的生长,但显著影响促雄性腺的增生,促进Mr-IAG基因表达上调和输精管远端未成熟精细胞数量大幅增加;配体斑点试验结果进一步证实,Mr-IR基因与Mr-IAG基因确实存在配体—受体互作现象。可见,Mr-IR基因主要通过作用于促雄性腺而调控甲壳类动物性别分化。
2. 1. 3 Mar-Mrr和MRPINK基因 Cao等(2006,2007)以罗氏沼虾性腺组织为材料,构建雌性和雄性特异的抑制性消减杂交文库,再通过差异基因表达筛选与测序分析,首次鉴定出与罗氏沼虾性别相关的基因Mar-Mrr(M. rosenbergii male reproduction-related gene)和MRPINK(Male reproduction-related peptidase inhibitor kazal-type gene);Northern blotting检测和半定量RT-PCR结果显示,Mar-Mrr和MRPNIK基因只在雄性生殖系统中特异表达,且以输精管中的表达量较高,提示Mar-Mrr和MRPNIK基因可能参与雄性生殖相关的生理过程。Phoungpetchara等(2012)通过RT-PCR和原位杂交技术检测罗氏沼虾Mar-Mrr基因的时空表达模式,结果也发现Mar-Mrr基因在雄性生殖系统中特异性高表达,与Cao等(2006,2007)的研究结果一致。关于MRPINK基因,Li等(2008)研究发现,MRPINK蛋白对罗氏沼虾精子的水解明胶活性具有明显抑制作用,免疫荧光检测结果显示,MRPINK基因特异性结合在精子的基体边缘部位,通过抑制精子上的类明胶酶而影响精子活性,由此推测其对罗氏沼虾的受精具有调节作用。
2. 1. 4 MroSxl和MroDmrt基因 Sxl基因是果蝇性别决定的关键因子(宋艳等,2009)。俞炎琴(2013)通过简并PCR和cDNA文库构建等方法获得4个罗氏沼虾Sxl异构体(MroSxl1~MroSxl4)。其中,MroSxl1定位于精原细胞中,推测其参与精细胞生成;Mro-Sxl3和MroSxl4在卵巢中特异性高表达,提示其可能参与卵巢发育。Dmrt(Doublesex and mab-3 related transcription factor)是目前发现对性别决定和性别分化起重要作用的基因家族,其家族成员编码蛋虫均包含一个具有DNA结合能力的保守序列——DM结构域(Doublesex和Mab-3)(Zhu et al.,2000;Kopp,2012)。Dmrt基因编码蛋白作为转录调控因子,已被证实与果蝇、水蚤及其他甲壳类动物的性别两态性和性腺发育密切相关(Burtis et al.,1991;Keyes et al.,1992;Salz and Erickson,2010)。俞炎琴(2013)采用简并PCR和RACE扩增成功克隆获得2个罗氏沼虾Dmart基因(MroDmrt11E和MroDmrt99B),其中,MroDmrt11E基因在精巢中高表达,在卵巢中表达量极低;而MroDmrt99B基因特异性低表达于精巢中;此外,随着罗氏沼虾胚胎的发育,MroDmrt11E和MroDmrt99B基因的表达量逐渐增加。
2. 1. 5 其他基因 雌激素相关受体(Estrogen related receptor,ERR)被视为核受体超家族的第三类亚族,参与雌激素受体信号通路。ERR作为真核转录因子,在卵巢发育和精子生产过程中发挥重要作用(Beato et al.,1995;Escriva et al.,2000)。赵苗鑫(2016)成功克隆获得罗氏沼虾ERR基因的cDNA序列,其组织表达谱显示ERR基因在雌虾的卵巢组织中高表达,推测该基因参与调控罗氏沼虾的卵巢发育(赵苗鑫等,2017)。在前期研究的基础上,刘金磊等(2018)通过双链RNA(double-strand RNA,dsRNA)干扰沉默罗氏沼虾ERR基因,并对dsRNA干擾前后的卵巢样本进行转录组测序,结果共获得318269674条Clean reads和96272条Unigenes,差异表达分析得到2490条上调表达Unigenes、2557条下调表达Unigenes,GO功能富集分析和KEGG通路富集分析发现,差异表达的Unigenes被富集到与生殖相关的GO分类条目或KEGG通路上,且发现ERR基因可能通过影响cyclinB、PPP2A和ADCY9基因表达以调控罗氏沼虾的卵巢发育。
随着第二代测序技术的广泛应用,对罗氏沼虾性腺进行转录组测序已成为挖掘性别决定和性别分化候选基因的主要手段。Jung等(2016)以18尾罗氏沼虾的卵巢和精巢组织为材料,基于454测序平台进行转录组测序分析,结果得到超过750000条高质量Clean reads和44407条Contigs,在罗氏沼虾的卵巢和精巢组织分别得到112和270个差异表达基因,提示这些基因可能与性别分化相关。
2. 2 性别相关分子标记
稳定的性别特异分子标记对开展虾蟹类早期性别鉴定及后期单性化养殖至关重要。Ventura等(2011)利用AFLP技术鉴定出一段与罗氏沼虾性别相关、3 kb长的基因组区域,且该片段存在串联序列和逆重复序列。在此基础上,设计特异性引物并将其转换为SCAR(Sequence-characterized amplified region)分子标记,进一步分析发现该段序列在雌、雄罗氏沼虾中存在细微差别:雌虾中,在SCAR上游区域存在3 bp缺失,且存在2个雌性特异的SNP位点,而该位点基因型表现为雌虾杂合、雄虾纯合,即SCAR分子标记能有效区分雌、雄和超雌罗氏沼虾。Jiang和Qiu(2013)利用64对引物组合对罗氏沼虾进行AFLP扩增,结果获得8400条条带,其中13条为雌性特有的条带,将其进行重扩增、克隆及测序分析,成功筛选出2条可靠的SCAR分子标记。上述研究结果同时进一步佐证罗氏沼虾可能为WZ/ZZ染色体性别决定类型。
3 罗氏沼虾单性化养殖
目前,在甲壳类动物中主要采用外源激素处理、温度调控、种间杂交、雌核发育、多倍体诱导和促雄性腺移植或摘除等方法进行性别控制研究。尤其是性别相关关键基因的挖掘与鉴定,为甲壳类动物的性别控制提供了契机,至今罗氏沼虾单性化养殖已取得长足发展。
3. 1 全雄罗氏沼虾
国外关于全雄罗氏沼虾的研究始于1990年。Sagi等(1990)通过摘除早期雄性罗氏沼虾的促雄性腺,发现其雄性个体性逆转为雌性个体,即伪雌(表型WZ,生理型ZZ),将伪雌个体与正常雄性个体杂交即可获得全雄后代。制约该方法推广应用的最主要技术瓶颈是无法准确、有效地判定罗氏沼虾的早期发育阶段。为克服这一难题,Aflalo等(2006)开发两步法进行罗氏沼虾全雄育种,首先摘除雄性亲本的促雄性腺并结合后裔测定法确定获得伪雌个体,然后与正常雄性罗氏沼虾杂交获得F1代,经性逆转后大批量生产伪雌个体,再与正常雄性个体进行回交,而达到大规模生产全雄罗氏沼虾的目的。随着罗氏沼虾性别特异分子标记的成功挖掘,使得其单性化繁殖更便捷,可操作性更强(Ventura et al.,2011)。随后,Ventura等(2012)采用注射Mr-IAG基因的dsRNA干扰方法(图1),成功实现雄性罗氏沼虾的性逆转并获得伪雌个体;与正常雄性个体进行杂交后,利用前期鉴定获得的性别特异分子标记对F1代个体进行验证,发现F1代罗氏沼虾全为雄性个体,该结论为后续规模化全雄繁殖打下了坚实的基础。Lezer等(2015)基于性逆转技术手段进行大规模全雄罗氏沼虾的生产,并对其后代的生长性状表型数据进行统计分析,结果发现罗氏沼虾的性逆转成功率达86%。通常情况下,伪雌个体的体重较正常雌性个体重,生长速度快,养殖产量较雌雄混养模式提高17%,对应经济利润提高60%(吕华当和沙燮雪,2014)。在国内,广东海洋大学朱春华教授从1994年开始着手于罗氏沼虾种苗培育技术研究,近年来其团队基于引进的泰国罗氏沼虾原种,结合群体选育和物理、化学因素的性别控制等手段,成功培育获得全雄罗氏沼虾群体(方琼玟,2017)。
3. 2 全雌罗氏沼虾
尽管雄虾较雌虾生长快、养殖产出量高;但雄虾个体大,性情凶残,且具有明显的侵略行为和领地意识,导致单位养殖面积养殖密度较低,进而严重影响整个罗氏沼虾群体的生长(Mohanakumaran Nair et al.,2006)。相对而言,雌虾具有性情温顺、体型较小及单位面积养殖密度高等优点,更重要的是含有高营养价值且味美如蟹黄的生殖腺(Gopal et al.,2010;Malecha,2012)。由于雄性罗氏沼虾的残杀现象和雌虾的高营养价值,相同养殖条件下雌虾养殖所带来的经济效益远高于雄虾养殖。早在1992年,国外研究学者就提出甲壳类动物全雌养殖极具优越性的理念(Malecha et al.,1992)。
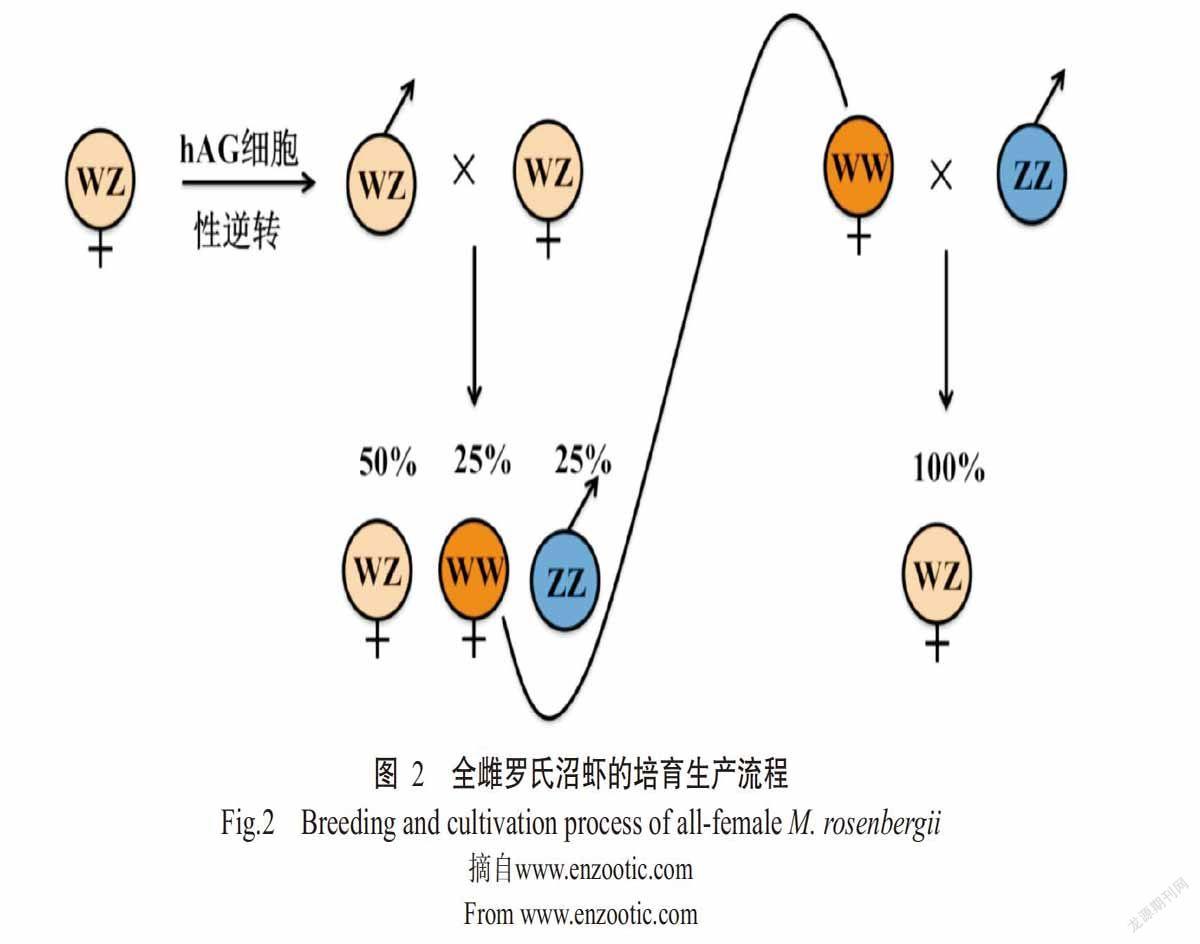
继全雄选育之后,全雌养殖模式将成为一个全新的养殖策略。以色列本·古里安大学的Sgai教授团队率先研发出罗氏沼虾全雌养殖技术(Levy et al.,2016),该技术是采用移植雄性腺体方法替代传统的激素、化学处理或转基因方法,以获得超雌虾群体(WW型),目前已实现大规模的商业化模式运转。罗氏沼虾全雌养殖的理论基础是基于促雄性腺对动物性别分化、精子发生及雄性性征的调控作用,其具体操作流程如下:采用眼柄摘除法去除成熟雄性罗氏沼虾神经内分泌X器官窦腺体复合物,使其促雄性腺肥大增生,然后利用酶解法进行肥大增生促雄性腺(Hypertrophied androgenic gland,hAG)细胞的分离和培养,获得的hAG细胞培养21 d后,以2×103个细胞为单位注射到罗氏沼虾幼体第一腹节的肌肉组织中,结合外部形态特征、组织切片观察及分子标记技术进行伪雄个体鉴定。通过对雌虾注射hAG细胞后能成功诱导其性逆转为新的伪雄个体,与正常雄虾个体杂交后,其子代个体的WW型∶WZ型∶ZZ型比例为1∶2∶1,即获得25%的WW型超雌个体。采用分子标记技术对WW型和WZ型个体进行分离,再与正常雄性个体进行杂交,可获得100%的全雌个体(图2)。大规模养殖结果显示,全雌养殖模式下罗氏沼虾具有更优的生长性能,主要表现为:①在成虾养殖过程中无个体残杀现象,养殖成活率较混养模式提高22%;②养殖群体中无雄性个体,雌性个体不存在抱卵现象,保证在养殖期间持续增重,成虾平均体重达40 g/尾左右;③全雌养殖条件下,罗氏沼虾总产量较混养模式提高36%;④全雌养殖模式显著提高了成虾规格均一性,饲料转化率提高20%。
4 展望
开展性别分化和性别决定相关基因研究是实现罗氏沼虾单性化养殖及提高其产量的前提工作,但至今鲜见罗氏沼虾性别相关基因克隆鉴定的研究报道。随着高通量测序技术的快速发展,利用转录组测序技术挖掘缺乏参考基因组信息的罗氏沼虾性别特异功能基因已成为可能,为揭开罗氏沼虾性别决定和性别分化的分子机制提供了技术保障。基因编辑技术的发展为人类疾病和动、植物复杂性状的改良提供了机遇,目前已在甲壳类动物基因编辑方面展开了大量研究工作(Hiruta et al.,2014;Nakanishi et al.,2014;Naitou et al.,2015;Martin et al.,2016)。中国科学院海洋研究所张继泉博士团队基于CRISPR/Cas9技术成功实现了脊尾白虾的基因组编辑(Gui et al.,2016;Sun et al.,2017),为罗氏沼虾性别控制指明了新方向,可基于鉴定获得的性别决定和性别分化主效基因,借助基因编辑技术实现性别控制。可见,全雄/全雌罗氏沼虾育种的关键技术是伪雌或超雌虾制备。尽管以色列本·古里安大学Sagi教授的团队(Ventura et al.,2011;Lezer et al.,2015)已将全雄罗氏沼虾制种技术公之于众,但具体细节及操作规程仍需进一步摸索完善,加之超雌虾的制备程序繁琐,选育耗时较长,且使用年限有限,极大限制了全雌虾的大规模生产。受罗非鱼三系[原系(XX♀)、雄性纯合系(YY♂)和雄性纯合转化系(YY♀)]配套方案(杨永铨等,1980,2012)的启发,今后可对罗氏沼虾遗传型WW超雌个体进行性逆转,获得伪雄个体(遗传型WW,生理型ZZ),经回交所得后代用于构建超雌虾种质库,即通过性别决定和性别分化机制解析及超雌种质库构建,研发自主的单性化罗氏沼虾制种技术,培育全雄/全雌罗氏沼虾将成为可能。
参考文献:
戴习林,周梦颖,鞠波,过正乾,蒋飞,苏建,丁福江. 2016. 养殖密度对罗氏沼虾生长、性别分化与性腺发育的影响[J]. 水产学报,40(12):1874-1882. [Dai X L,Zhou M Y,Ju B,Guo Z Q,Jiang F,Su J,Ding F J. 2016. Effects of stocking density on growth,sexual differentiation and gonad development of Macrobrachium rosenbergii[J]. Journal of Fisheries of China,40(12):1874-1882.]
方瓊玟. 2017. 朱春华:全雄性罗氏沼虾已选育到第五代[J]. 海洋与渔业,(9):52-53. [Fang Q W. 2017. Zhu Chunhua:All-male prawns(Macrobrachium rosenbergii) have been bred to the fifth generation[J]. Marine and Fisheries,(9):52-53.]
刘金磊,邓思平,江东能,陈华谱,李广丽,吴天利,田昌绪,朱春华. 2018. ERR-dsRNA对罗氏沼虾卵巢中ERR及生殖相关基因表达的影响[J]. 广东海洋大学学报,38(3):8-16. [Liu J L,Deng S P,Jiang D N,Chen H P,Li G L,Wu T L,Tian C X,Zhu C H. 2018. Screening of ovarian genes associated with reproduction in Macrobrachium rosenbergii and their changes in expression pattern in di-fferent development stages after ERR interference[J]. Journal of Guangdong Ocean University,38(3):8-16.]
楼允东,刘艳红,邱高峰. 2004. 虾蟹类性别决定研究进展[J]. 上海水产大学学报,13(2):157-163. [Lou Y D,Liu Y H,Qiu G F. 2004. Advances in sex determination of shrimps (prawns) and crabs[J]. Journal of Shanghai Fishe-ries University,13(2):157-163.]
吕华当,沙燮雪. 2014. “全雄性罗氏沼虾项目”获首届诺伟司全球水产创新奖[J]. 海洋与渔业,(1):17. [Lü H D,Sha Y X. 2014. The project of all-male prawns(Macrobra-chium rosenbergii) won the first novus global aquatic innovation award[J]. Marine and Fisheries,(1):17.]
农业部渔业渔政管理局. 2017. 中国渔业统计年鉴[M]. 北京:中国农业出版社. [Bureau of Fisheries, Ministry of Agriculture. 2017. China fishery statistical yearbook[M]. Beijing:Chinese Agriculture Press.]
宋艳,柳学广,司马杨虎,朱晓苏,徐丽,徐世清. 2009. 野桑蚕Bmand-Sxl基因的克隆及原核表达[J]. 江苏蚕业,(1):14-18. [Song Y,Liu X G,Sima Y H,Zhu X S,Xu L,Xu S Q. 2009. Cloning and prokaryotic expression of Bmand-Sxl gene in Bambyx mandarina[J]. Jiangsu Sericulture,(1):14-18.]
吴楠,张毅,李惠云,张高峰,刘青,魏华. 2007. 壬基酚和雌二醇干扰罗氏沼虾卵黄蛋白原VTG基因表达的效应[J]. 动物学杂志,42(4):1-7. [Wu N,Zhang Y,Li H Y,Zhang G F,Liu Q,Wei H. 2007. Endocrine disruption effects of 4-nonylphenol and estradiol on vitellogenin gene expression in vivo in Macrobrachium rosenbergii[J]. Chinese Journal of Zoology,42(4):1-7.]
杨永铨,张海明,陈远生. 2012. 尼罗超雄鱼的规模化制种与生产应用[J]. 淡水渔业,42(4):75-78. [Yang Y Q,Zhang H M,Chen Y S. 2012. Large-scale breeding production and application of super male Oreochromis niloticus[J]. Freshwater Fisheries,42(4):75-78.]
杨永铨,张中英,林克宏,魏于生,黄二春,高志慧,徐振,柯善春,卫建国. 1980. 应用三系配套途径产生遗传上全雄莫桑比克罗非鱼[J]. 遗传学报,7(3):241-246. [Yang Y Q,Zhang Z Y,Lin K H,Wei Y S,Huang E C,Gao Z H,Xu Z,Ke S C,Wei J G. 1980. Use of three line combination for production of genetic all-male tilapia Mossambica[J]. Journal of Genetics and Genomics,7(3):241-246.]
俞炎琴. 2013. 罗氏沼虾中性别发育相关基因Sxl和Dmrt基因的分子特征和功能研究[D]. 杭州:浙江大学. [Yu Y Q. 2013. The molecular characterization and functional ana-lysis of sexual development related genes Sxl and Dmrt in the prawn,Macrobrachium rosenbergii[D]. Hangzhou:Zhejiang University.]
趙苗鑫. 2016. 罗氏沼虾雌激素相关受体的克隆与表达及壬基酚对其表达的影响[D]. 湛江:广东海洋大学. [Zhao M X. 2016. Molecular cloning and expression of the estrogen related receptor in Macrobrachium rosenbergii and the effect of nonylphenol on its gene expresstion[D]. Zhanjiang:Guangdong Ocean University.]
赵苗鑫,陈华谱,刘金磊,邓思平,李广丽,朱春华,洪宇聪. 2017. 罗氏沼虾雌激素相关受体(ERR)基因原核表达与纯化[J]. 广东海洋大学学报,37(1):108-112. [Zhao M X,Chen H P,Liu J L,Deng S P,Li G L,Zhu C H,Hong Y C. 2017. Prokaryotic expression and purification of estrogen related receptor(ERR) gene from Macrobrachium rosenbergii[J]. Journal of Guangdong Ocean University,37(1):108-112.]
周夢颖. 2014. 养殖密度、盐度和温度对罗氏沼虾性别分化和早期性腺发育的影响[D]. 上海:上海海洋大学. [Zhou M Y. 2014. Effect of stocking density,salinity and temperature on sex differention and early gonadal development on Macrobrachium rosenbergii[D]. Shanghai:Shanghai Ocean University.]
朱春华,薛海波,李郁娇,黄国钟,刘易洋,李广丽. 2011. 壬基酚(NP)对罗氏沼虾幼虾生长和性别分化的影响[J]. 水产学报,35(3):365-371. [Zhu C H,Xue H B,Li Y J,Huang G Z,Liu Y Y,Li G L. 2011. Effects of 4-nonylphenol on growth and sex differentiation in Macrobra-chium rosenbergii[J]. Journal of Fisheries of China,35(3):365-371.]
Aflalo E D,Hoang T T,Nguyen V H,Lam Q,Nguyen D M,Trinh Q S,Raviv S,Sagi A. 2006. A novel two-step procedure for mass production of all-male populations of the giant freshwater prawn Macrobrachium rosenbergii[J]. Aquaculture,256(1-4):468-478.
Beato M,Herrlich P,Schütz G. 1995. Steroid hormone receptors:Many actors in search of a plot[J]. Cell,83(6):851-857.
Benzie J A,Kenway M,Ballment E. 2001. Growth of Penaeus monodon×Penaeus esculentus tiger prawn hybrids relative to the parental species[J]. Aquaculture,193(3-4):227-237.
Burtis K C,Coschigano K T,Baker B S,Wensink P C. 1991. The doublesex proteins of Drosophila melanogaster bind directly to a sex-specific yolk protein gene enhancer[J]. The EMBO Journal,10(9):2577-2582.
Cao J X,Dai J Q,Dai Z M,Yin G L,Yang W J. 2007. A male reproduction-related Kazal-type peptidase inhibitor gene in the prawn,Macrobrachium rosenbergii:Molecular characterization and expression patterns[J]. Marine Biotechnology,9(1):45-55.
Cao J X,Yin G L,Yang W J. 2006. Identification of a novel male reproduction-related gene and its regulated expre-ssion patterns in the prawn,Macrobrachium rosenbergii[J]. Peptides,27(4):728-735.
Chen C,Jack J,Garofalo R S. 1996. The drosophila insulin receptor is required for normal growth[J]. Endocrinology,137(3):846-856.
Curtis M C,Jones C M. 1995. Observations on monosex culture of redclaw crayfish Cherax quadricarinatus von Martens(Decapoda:Parastacidae) in earthen ponds[J]. Journal of the World Aquaculture Society,26(2):154-159.
Escriva H,Delaunay F,Laudet V. 2000. Ligand binding and nuclear receptor evolution[J]. BioEssays,22(8):717-727.
Fafalios A,Ma J,Tan X,Stoops J,Luo J,Defrances M C,Zarnegar R. 2011. A hepatocyte growth factor receptor (Met)-insulin receptor hybrid governs hepatic glucose metabolism[J]. Nature Medicine,17(12):1577-1584.
Gopal C,Gopikrishna G,Krishna G,Jahageerdar S S,Rye M,Hayes B J,Paulpandi S,Kiran R P,Pillai S M,Ravichandran P,Ponniah A G,Kumar D. 2010. Weight and time of onset of female-superior sexual dimorphism in pond reared Penaeus monodon[J]. Aquaculture,300(1-4):237-239.
Gui T S,Zhang J Q,Song F G,Sun Y Y,Xie S J,Yu K J,Xiang J H. 2016. CRISPR/Cas9-mediated genome edi-ting and mutagenesis of EcChi4 in Exopalaemon carinicauda[J]. G3(Bethesda),6(11):3757-3764.
Hiruta C,Ogino Y,Sakuma T,Toyota K,Miyagawa S,Yamamoto T,Iguchi T. 2014. Targeted gene disruption by use of transcription activator-like effector nuclease(TALEN) in the water flea Daphnia pulex[J]. BMC Biotechnology,14:95. doi:10.1186/s12896-014-0095-7.
Jiang X H,Qiu G F. 2013. Female-only sex-linked amplified fragment length polymorphism markers support ZW/ZZ sex determination in the giant freshwater prawn Macrobrachium rosenbergii[J]. Animal Genetics,44(6):782-785.
Jin S B,Fu H T,Zhou Q,Sun S M,Jiang S F,Xiong Y W,Gong Y S,Qiao H,Zhang W Y. 2013. Transcriptome analysis of androgenic gland for discovery of novel genes from the oriental river prawn,Macrobrachium nippo-nense,using Illumina Hiseq 2000[J]. PLoS One,8(10):e76840.
Jung H T,Yoon B H,Kim W J,Kim D W,Hurwood D A,Lyons R E,Salin K R,Kim H S,Baek I,Chand V,Mather P B. 2016. Optimizing hybrid de novo transcriptome assembly and extending genomic resources for giant freshwater prawns(Macrobrachium rosenbergii):The identification of genes and markers associated with reproduction[J]. International Journal of Molecular Sciences,17(5):690. doi:10.3390/ijms17050690.
Keyes L N,Cline T W,Schedl P. 1992. The primary sex determination signal of Drosophila acts at the level of transcription[J]. Cell,68(5):933-943.
Kopp A. 2012. Dmrt genes in the development and evolution of sexual dimorphism[J]. Trends in Genetics,28(4):175-184.
Lécher P,Defaye D,Noel P. 1995. Chromosomes and nuclear-DNA of crustacea[J]. Invertebrate Reproduction & Development,27(2):85-114.
Levy T,Rosen O,Eilam B,Azulay D,Aflalo E D,Manor R,Shechter A,Sagi A. 2016. A single injection of hypertrophied androgenic gland cells produces all-female aquaculture[J]. Marine Biotechnology,18(5):554-563.
Lezer Y,Sagi A,Aflalo E D,Abilevich L K,Sharabi O,Manor R. 2015. On the safety of RNAi usage in aquaculture:The case of all-male prawn stocks generated through manipulation of the insulin-like androgenic gland hormone[J]. Aquaculture,435:157-166.
Li Y,Ma W M,Dai J Q,Feng C Z,Yang F,Ohira T,Nagasawa H,Yang W J. 2008. Inhibition of a novel sperm gelatinase in prawn sperm by the male reproduction-related kazal-type peptidase inhibitor[J]. Molecular Reproduction and Development,75(8):1327-1337.
Lugo J M,Morera Y,Rodríguez T,Huberman A,Ramos L,Estrada M P. 2006. Molecular cloning and characterization of the crustacean hyperglycemic hormone cDNA from Litopenaeus schmitti. Functional analysis by double-stranded RNA interference technique[J]. The FEBS Journal,273(24):5669-5677.
Ma K Y,Yu S H,Du Y X,Feng S Q,Qiu L J,Ke D Y,Luo M Z,Qiu G F. 2019. Construction of a genomic bacterial artificial chromosome(BAC) library for the prawn Macrobrachium rosenbergii and initial analysis of ZW chromosome-derived BAC inserts[J]. Marine Biotechnology,21(2):206-216.
Malecha S R,Nevin P A,Ha P,Barck L E,Lamadrid-Rose Y,Masuno S,Hedgecock D. 1992. Sex-ratios and sex-determination in progeny from crosses of surgically sex-reversed freshwater prawns,Macrobrachium rosenbergii[J]. Aquaculture,105(3-4):201-218.
Malecha S. 2012. The case for all-female freshwater prawn,Macrobrachium rosenbergii (De Man),culture[J]. Aquaculture Research,43(7):1038-1048.
Manor R,Weil S,Oren S,Glazer L,Aflalo E D,Ventura T,Chalifa-Caspi V,Lapidot M,Sagi A.2007. Insulin and gender:An insulin-like gene expressed exclusively in the androgenic gland of the male crayfish[J]. General and Comparative Endocrinology,150(2):326-336.
Martin A,Serano J M,Jarvis E,Bruce H S,Wang J,Ray S,Barker C A,O'Connell L C,Patel N H. 2016. CRISPR/Cas9 mutagenesis reveals versatile roles of Hox genes in crustacean limb specification and evolution[J]. Current Biology,26(1):14-26.
Mohanakumaran Nair C,Salin K R,Raju M S,Sebastian M. 2006. Economic analysis of monosex culture of giant freshwater prawn(Macrobrachium rosenbergii De Man):A case study[J]. Aquaculture Research,37(9):949-954.
Naitou A,Kato Y,Nakanishi T,Matsuura T,Watanabe H. 2015. Heterodimeric TALENs induce targeted heritable mutations in the crustacean Daphnia magna[J]. Biology Open,4(3):364-369.
Nakanishi T,Kato Y,Matsuura T,Watanabe H. 2014. CRISPR/Cas-mediated targeted mutagenesis in Daphnia magna[J]. PLoS One,9(5):e98363.
Nef S,Verma-Kurvari S,Merenmies J,Vassalli J D,Efstratiadis A,Accili D,Parada L F. 2003. Testis determination requires insulin receptor family function in mice[J]. Nature,426(6964):291-295.
Parnes S,Sagi A. 2002. Intensification of redclaw crayfish Cherax quadricarinatus culture I.:Growout in a separate cell system[J]. Aquacultural Engineering,26(4):251-262.
Phoungpetchara I,Tinikul Y,Poljaroen J,Changklungmoa N,Siangcham T,Sroyraya M,Chotwiwatthanakun C,Vanichviriyakit R,Hanna P J,Sobhon P. 2012. Expression of the male reproduction-related gene(Mar-Mrr) in the spermatic duct of the giant freshwater prawn,Macrobra-chium rosenbergii[J]. Cell and Tissue Research,348(3):609-623.
Rosen O,Manor R,Weil S,Gafni O,Linial A,Aflalo E D,Ventura T,Sagi A. 2010. A sexual shift induced by silen-cing of a single insulin-like gene in crayfish:Ovarian upregulation and testicular degeneration[J]. PLoS One,5(12):e15281.
Rungsin W,Paankhao N,Na-Nakorn U. 2006. Production of all-male stock by neofemale technology of the thai strain of freshwater prawn,Macrobrachium rosenbergii[J]. Aquaculture,259(1-4):88-94.
Sagi A,Cohen D,Milner Y. 1990. Effect of androgenic gland ablation on morphotypic differentiation and sexual chara-cteristics of male freshwater prawns,Macrobrachium rosenbergii[J]. General and Comparative Endocrinology,77(1):15-22.
Sagi A,Snir E,Khalaila I. 1997. Sexual differentiation in decapod crustaceans:Role of the androgenic gland[J]. Invertebrate Reproduction & Development,31(1-3):55-61.
Salz H K,Erickson J W. 2010. Sex determination in Drosophi-la:The view from the top[J]. Fly(Austin),4(1):60-70.
Sharabi O,Manor R,Weil S,Aflalo E D,Lezer Y,Levy T,Aizen J,Ventura T,Mather P B,Khalaila I,Sagi A. 2016. Identification and characterization of an insulin-like receptor involved in crustacean reproduction[J]. Endocrinology,157(2):928-941.
Shechter A,Glazer L,Cheled S,Mor E,Weil S,Berman A,Bentov S,Aflalo E D,Khalaila I,Sagi A. 2008. A gastrolith protein serving a dual role in the formation of an amorphous mineral containing extracellular matrix[J]. Proceedings of the National Academy of Sciences of the United States of America,105(20):7129-7134.
Staelens J,Rombaut D,Vercauteren I,Argue B,Benzie J,Vuylsteke M. 2008. High-density linkage maps and sex-linked markers for the black tiger shrimp(Penaeus mono-don)[J]. Genetics,179(2):917-925.
Steele R E,Lieu P,Mai N H,Shenk M A,Sarras M P Jr. 1996. Response to insulin and the expression pattern of a gene encoding an insulin receptor homologue suggest a role for an insulin-like molecule in regulating growth and patterning in Hydra[J]. Development Genes and Evolution,206(4):247-259.
Sun Y Y,Zhang J Q,Xiang J H. 2017. A CRISPR/Cas9-media-ted mutation in chitinase changes immune response to bacteria in Exopalaemon carinicauda[J]. Fish & Shellfish Immunology,71:43-49.
Triño A T,Millamena O M,Keenan C. 1999. Commercial evaluation of monosex pond culture of the mud crab Scy-lla species at three stocking densities in the Philippines[J]. Aquaculture,174(1-2):109-118.
Ventura T,Aflalo E D,Weil S,Kashkush K,Sagi A. 2011. Isolation and characterization of a female-specific DNA marker in the giant freshwater prawn Macrobrachium rosenbergii[J]. Heredity,107(5):456-461.
Ventura T,Manor R,Aflalo E D,Weil S,Raviv S,Glazer L,Sagi A. 2009. Temporal silencing of an androgenic gland-specific insulin-like gene affecting phenotypical gender differences and spermatogenesis[J]. Endocrinology,150(3):1278-1286.
Ventura T,Manor R,Aflalo E D,Weil S,Rosen O,Sagi A. 2012. Timing sexual differentiation:Full functional sex reversal achieved through silencing of a single insulin-like gene in the prawn,Macrobrachium rosenbergii[J]. Biology of Reproduction,86(3):90. doi:10.1095/biolreprod.111.097261.
Zhu L,Wilken J,Phillips N B,Narendra U,Chan Q,Stratton S M,Kent S B,Weiss M A. 2000. Sexual dimorphism in diverse metazoans is regulated by a novel class of intertwined zinc fingers[J]. Genes & Development,14(14):1750-1764.
(責任编辑 兰宗宝)

