Magnetic Chitosan/ZnS∶Fe Nanocomposite:Preparation and Application in Malachite Green Removal
XIA Hong,PENG Mao-min,LIU Li
(Institute of Quality Standard &Testing Technology for Agro-Products,Hubei Academy of Agricultural Sciences,Wuhan 430064,China)
Abstract:Magnetic chitosan/ZnS∶Fe(M-CS/ZnS∶Fe)nanocomposite was prepared and applied to the photodegradation of malachite green(MG).The synthesized M-CS/ZnS∶Fe exhibits excellent superparamagnetism and photocatalytic activity.In photodegradation studies,the effect of main experimental parameters on the decolorization efficiency was studied and optimized.The maximum decolorization efficiency of 95.3% was obtained when the initial MG concentration,M-CS/ZnS∶Fe dosage,and contact time were optimally set as 10 mg/L,100 mg/L,and 60 min,respectively.The process kinetics can be successfully fitted to the first order kinetics of Langmuir-Hinshelwood model.Moreover,M-CS/ZnS∶Fe could be easily separated under an external magnetic field.After five consecutive runs,a small and gradual decrease from 95.3% to 80.8% in the decolorization ration was observed.
Key words:magnetic chitosan;ZnS∶Fe;quantum dots;malachite green;photodegradation
1 Introduction
Malachite green (MG),a N-methylated diaminotriaryl-methane cationic dye,is highly effective against protozoal and fungal infections,has been extensively used as fungicide,ectoparasiticide and disinfectant in the aquaculture and dye industries over the world[1-2].However,scientific evidence indicates that MG has genotoxic and carcinogenic properties.It will affect the aquatic life and may cause irritation to the gastrointestinal tract upon ingestion[3].MG has been removed from aqueous systemviaadsorption[4-8],photocatalytic degradation[9-13],and ultrasonic irradiation methods[14-15].Among these methods,magnetic adsorbents based adsorption method is supported by their excellent magnetic properties,ease of separation by an external magnetic field,and reusability[16].Quantum dots (QDs)based photocatalytic degradation method is supported by some advantages such as no formation of secondary pollutant,fast degradation,high degradation efficiency,and degradation of residual dye in the low concentration[17-19].However,the removal of MG by combination magnetic adsorbents and QDs has not been studied yet.
Chitosan (β-(1→4)-2-amino-2-deoxy-D-glucose,CS),a natural hydrophilic and cationic polymer prepared from deacetylation of chitin,the next most abound natural polysaccharide,has been considered as an excellent ad-sorbent for removing pollutants from contaminated water,due to the presence of high content of amino and hydroxyl groups.The magnetic chitosan (M-CS)nanoparticles have been applied for the removal of dyes in the recent years[20].Due to the high chemical stability,non-toxicity and high surface area-to-volume ratio to their bulk counterparts,zinc sulfide (ZnS)QDs have been extensively focused for photocatalytic applications.In particular,the doping of ZnS QDs with transition metals such as Mn,Cu,Co and Fe is of interest for research the effects of dopants on the photocatalytic properties of the QDs[17].
In present work,magnetic chitosan/ZnS∶Fe (M-CS/ZnS∶Fe)nanocomposite combined M-CS nano-particles and ZnS∶Fe QDs were synthesized and used as photocatalyst for the removal of MG.At first,the M-CS nanoparticles were prepared based on co-precipitation of Fe2+and Fe3+ions in CS solution.Afterwards,Zn2+and Fe3+ions were added and chelated on the surface of the obtained M-CS nanoparticles.M-CS/ZnS∶Fe nanocomposite was obtained by hydrothermal method with the introduction of S2-.The as-prepared M-CS/ZnS∶Fe nanocomposite exhibits excellent superparamagnetism,adsorptive and photocatalytic activity.After characterizations,the effect of different experimental parameters,such as initial MG concentration,dosage of M-CS/ZnS∶Fe nanocomposite,and time on the performance was investigated according to their efficiencies for the removal of MG.The reproducibility behavior of M-CS/ZnS∶Fe nanocomposite as nanophotocatalyst in photodecolorization of MG was also studied.A photo-catalytic decolorization process based on the magnetic chitosan/quantum dots nanocomposite was presented as an efficient and green strategy for the removal of MG.
2 Experiments
2.1 Materials
CS (molecular weight of 600 000 g/mol,degree of deacetylation 87%),ferric chioride hexahydrate (FeCl3·6H2O),ferrous chloride tetrahydrate (FeCl2·4H2O),zinc chloride (ZnCl2·H2O),sodium sulfide (Na2S·9H2O),acetic acid (HAc),and ammonium hydroxide (NH3·H2O,28%)were obtained from Sinopharm Chemical Reagent Co.,Ltd.,China.
All chemicals were of analytical reagent grade and used without purification.
2.2 Synthesis of M-CS/ZnS∶Fe Nanocomposite
Firstly,M-CS nanoparticles were synthesized by hydro-thermal method as previously reported[21].Typically,0.5 g CS was dissolved in 200 mL 0.25%(V/V)HAc aqueous solution,then 44 mL aqueous solution containing 0.7 g FeCl3·6H2O and 0.3 g FeCl2·4H2O was added and stirred for 1 h at 40 ℃ under nitrogen atmosphere.Subsequently,20 mL ammonium hydroxide was added drop by drop by tempestuous stirring.The mixture was heated to 60 ℃ and maintained for 3 h.After cooled to room temperature,the black produce was washed with ultrapure water and HAc aqueous solution,then dried in an oven at 70 ℃ for 12 h and kept in a vacuum desiccator.
The preparation procedure of M-CS/ZnS∶Fe nanocomposite was briefed as follows.Firstly,M-CS solution was prepared by dissolving 0.01 g M-CS nanoparticles into 200 mL 0.25%(V/V)HAc aqueous solution under stirring for 3 h at room temperature.Then,20 mL aqueous solution containing 0.28 g ZnCl2·4H2O and 0.03 g FeCl3·6H2O was added and the mixture was stirred continually for 30 min at 40 ℃.The pH of the precursor solution was adjusted to 7.0 with NaOH.Afterwards,20 mL 0.48 g fresh Na2S·9H2O solution was dropped into the mixture.The temperature was rose to about 100 ℃ and remained constant until for 3 h.The precipitate was collected using a magnet,and washed consecutively with ultrapure water.
2.3 Evaluation of Photocatalytic Activity
For the photocatalytic degradation process,aqueous dye solutions of MG (15 mg/L)with M-CS/ZnS∶Fe nanocomposite (100 mg/L)were taken in separate 10 mL colorimeter tube and simultaneously irradiated with a 365 nm UV lamp (6 W).The samples were collected at regular time intervals and filtered using a 0.45 μm water filter.Absorbance was measured atλmax=618 nm using a UV-Vis spectrophotometer to follow the decolourization of MG.
The decolorization efficiency was calculated as:
(1)
Where,C0andCeare the concentration of MG before and after photocatalytic reaction,respectively.Saturated adsorption experiments were carried out on all samples.The saturated adsorption capacity of the sample is 0.007 5 g/g.
2.4 Characterizations
The samples were subjected to powder X-ray diffraction (XRD)analysis for structural characterization using Bruker D8-advance X-ray diffractometer with Cu Ka radiation (λ=0.154 06 nm).The data were collected with a step size of 4° and step time of 1 s.Ultraviolet-visible (UV-Vis)absorbance was recorded on a DU800 spectrophotometer.Magnetic properties of the prepared MFNPs were studied by JDAW-2000B vibrating sample magnetometer (VSM).
3 Results and Discussion
3.1 Characterization of M-CS/ZnS∶Fe
The prepared M-CS/ZnS∶Fe was characterized by X-ray diffraction (XRD)(Fig.1(a)).The spectra were matched for various diffraction peaks at 2θvalues of 29.03°,48.02°,and 57.21°corresponding to the diffraction planes (111),(220),and (311),respectively,for cubic ZnS.The peaks were also found to match at 35.5°,43.1°,and 62.8° corresponding to the cubic inverse spinel Fe3O4[22].From XRD patterns,it can be concluded that,no additional phase such as FeS is observed.The dopant ions(Fe3+)occupy the lattice sites of the cubic close packed ZnS host matrix.
Fig.1(b)showed the magnetization of M-CS/ZnS∶Fe as a function of applied magnetic field at 300 K.It can be seen that,the corresponding magnetization of the M-CS/ZnS∶Fe increased with an increase in the magnetic fields,the saturated magnetization (M)of M-CS/ZnS∶Fe was 15.88 A·m2·kg-1.M-CS/ZnS∶Fe kept intrinsic magnetic properties of M-CS nanoparticles.

Fig.1 (a)XRD pattern.(b)Magnetic hysteresis curves of M-CS/ZnS∶Fe nanocomposite.
UV-Vis absorbance spectra of freshly prepared colloidal particles of M-CS/ZnS∶Fe and ZnS are shown in Fig.2.As can be detected in the figure,the absorption edge displayed a red shift compared to the ZnS due to emergence of extra energy levels between VB and CB from dopant ions[23].

Fig.2 Optical absorbance spectra of ZnS and M-CS/ZnS∶Fe
3.2 Performance of Photocatalytic Degradation of MG
Our aim of this study is to test photodegradation ability of M-CS/ZnS∶Fe to MG.The photodegradation tests are studied under different conditions,such as (1)effect of initial MG concentration,(2)effect of M-CS/ZnS∶Fe dosages,and (3)effect of irradiation time.Spectrophotometric analyses of the samples before and after irradiation were used to monitor the photodegradation process by measuring the absorption intensity atλmax=618 nm.
3.2.1 Effect of Initial MG Concentration
The systematic influence of initial MG concentration ([MG]=5-25 mg/L)on the photocatalytic degradation was studied at 100 mg/L M-CS/ZnS∶Fe (Fig.3).The decolorization efficiencies of MG increase with increasing the initial MG concentration,and reached a maximum when the initial MG concentration was set at 15 mg/L.Then,by increasing the initial MG concentration,the efficiencies of MFNPs for the removal of MG was decreased.CS has a medium affinity for the MG molecules[7].At a high initial MG concentration,a significant amount of MG may be absorbed on M-CS/ZnS∶Fe surface,MG would have acted as a filter for the incident light and thus reduce the efficiency of the photocatalytic reaction.Similar results have been reported by other researchers[24-25].At a low initial MG concentration,some reactants might be reduced to Leuco-MG during pre-saturated adsorption process.Degradation of such products requires a greater consumption of reactive oxygen species.This is why the degradation effect is worse when the MG concentration is low.

Fig.3 Effect of initial MG concentration on the decolorization efficiency E%
3.2.2 Effect of M-CS/ZnS∶Fe Dosages
Effect of M-CS/ZnS∶Fe dosages on the photocatalytic degradation of MG (15 mg/L)was investigated.As shown in Fig.4,the degradation efficiency of MG was found to increase then decrease with the increase in the M-CS/ZnS∶Fe dosages.The most effective decomposition of MG was observed with 100 mg/L M-CS/ZnS∶Fe.In principle,the dosage of M-CS/ZnS∶Fe is proportional to the number of the adsorbed photons which in turn increased the number of active radical,decolorization the adsorbed MG rapidly.With an increase in the dosages,the aggregation decreases the total active sites of M-CS/ZnS∶Fe for adsorption of MG.Moreover,high dosages of M-CS/ZnS∶Fe can create turbidity which causes a deactivation of activated molecules due to collision with the ground state molecules[9,26].

Fig.4 Effect of M-CS/ZnS∶Fe dosages on the decolorization efficiency E%
3.2.3 Effect of Irradiation Time
Fig.5 showed the relationship between the decolorization efficiency of MG and irradiation time in decolorization process.It was found that the decolorization efficiencies of MG increased rapidly in the first 60 min,followed by the gradually increased trends with regard to irradiation time.

Fig.5 Effect of irradiation time on the decolorization efficiency E%
3.3 Kinetic Model and Possible Mechanism
The photodecolorization of MG (10 mg/L)in the presence of M-CS/ZnS∶Fe nanocomposite was investigated under conditions:at 100 mg/L M-CS/ZnS∶Fe,after 120 min irradiation (Fig.6).The obtained data at various times were analyzed and fitted with pseudo first order kinetics of Langmuir-Hinshelwood model,as:
(2)

Fig.6 Fitted kinetic model for the photodecolorization of MG by M-CS/ZnS∶Fe nanocomposite

Tab.1Pseudofirstorderrateconstants(kobs)withcorrespondingcorrelationcoefficients(R2),maximumMGdecolorizationforthephotocatalyticdecolorizationofMGinthepresentofM-CS/ZnS∶Fenanocomposite
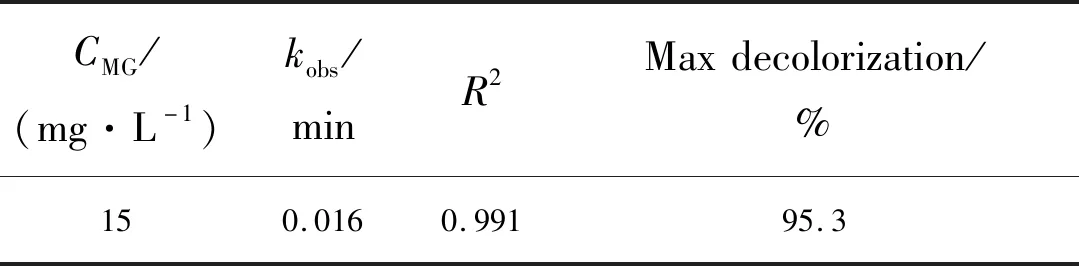
CMG/(mg·L-1)kobs/minR2Maxdecolorization/%150.0160.99195.3
Application of the synthesis M-CS/ZnS∶Fe nanocomposite for the removal of MG was shown in Fig.7.The possible mechanism by M-CS/ZnS∶Fe based photodegradation processes may be described by the following stages[27]:
Step 1:Diffusion and adsorption of MG onto the surface of M-CS/ZnS∶Fe,
Step 2:Photoexcitation of M-CS/ZnS∶Fe and generation of electron-hole pair on the surface,
Step 3:Formation of hydroxyl radical by reaction of the hole with surface-bound hydroxyl ion or by the decomposition of water,
Step 4:Degradation of MG,
M-CS/ZnS∶Fe+hν→M-CS/ZnS∶Fe(h+/e-),
M-CS/ZnS∶Fe(h+)+H2O→HO·+H+,
M-CS/ZnS∶Fe(h+)+OH-→HO·,
HO·+MG→Degradation of MG molecules.

Fig.7 Application of the synthesis M-CS/ZnS∶Fe nanocomposite for the removal of MG
3.4 Reproducibility of M-CS/ZnS∶Fe Nanophotocatalyst
The reusability of the prepared M-CS/ZnS∶Fe for the decolorization of MG was examined during five cycle experiments,under optimum conditions as:10 mL of 10 mg/L MG solution,in the presence of a fixed amount (100 mg/L)of M-CS/ZnS∶Fe.After irradiated for 60 min,the M-CS/ZnS∶Fe was isolated by magnetic separation,and washed with ultrapure water and HAc aqueous solution,the MG concentration was adjusted back to its initial value (10 mg/L).The results are presented in Fig.8.It can be seen that after five consecutive runs,only a
small and gradual decrease in the decolorization ration was observed (from 95.3% to 80.8%).The photocatalytic decolorization process of MG is associated with the adsorption on M-CS/ZnS∶Fe surface,after several cycles,the active sites of M-CS/ZnS∶Fe were occupied by the adsorbed MG molecules,a decrease in the photocatalytic activity was observed[12].The good reproducibility suggested that M-CS/ZnS∶Fe could be used as an economic and recyclable magnetic photocatalyst for MG treatment.

Fig.8 Reproducibility of M-CS/ZnS∶Fe nanophotocatalyst
A comparison between the efficiency of the prepared M-CS/ZnS∶Fe such as amount of sorbent,initial MG concentration,time,and maximun decolorization with the previously reported revealed that,M-CS/ZnS∶Fe exhibited good photocatalytic degradation performance (Tab.2).
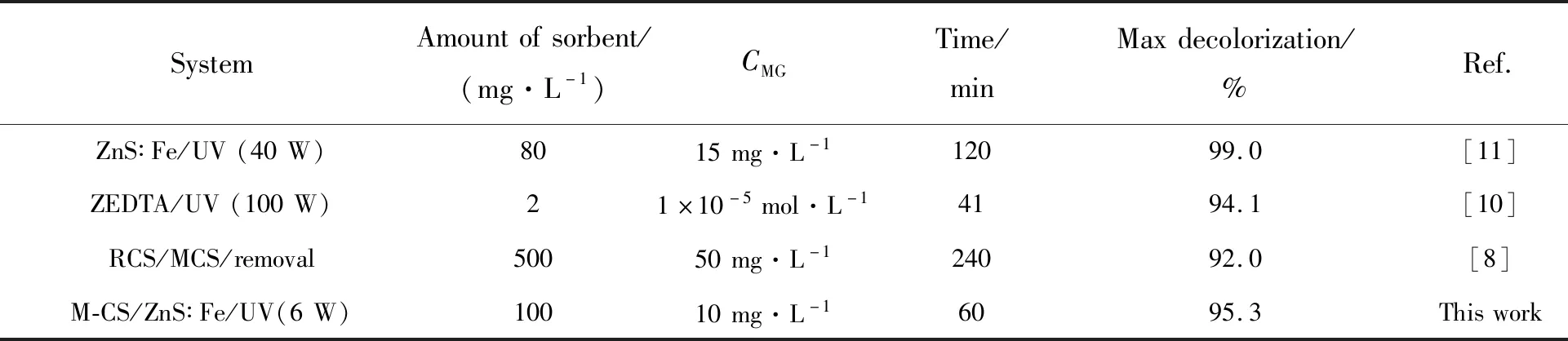
Tab.2 Characteristical performance of some reported methods for removal of MG
4 Conclusion
In summary,M-CS/ZnS∶Fe nanocomposite was successfully synthesized and applied for MG photodegradation.The influences of experimental parameters,such as initial MG concentration,dosage of M-CS/ZnS∶Fe,and the reusability of M-CS/ZnS∶Fe on the MG removal percentage were investigated by batch experiments.The result demonstrated that the combination of photodecolorization method with M-CS/ZnS∶Fe nanocomposite is an efficient,fast and sensitive method for the removal of MG.The process kinetics can be successfully fitted to the first order kinetic model.

