Longitudinal observation of ten family members with idiopathic basal ganglia calcification:A case report
Seiju Kobayashi,Kumiko Utsumi,Masaru Tateno,Tomo Iwamoto,Tomonori Murayama,Hitoshi Sohma,Wataru Ukai,Eri Hashimoto,Chiaki Kawanishi
Abstract
Key words: Idiopathic basal ganglia calcification; Fahr's disease; SLC20A2; Diffuse neurofibrillary tangles with calcification; Single-photon emission computed tomography;Case report
INTRODUCTION
Idiopathic basal ganglia calcification (IBGC),which is also known as Fahr’s disease,is a relatively rare neurological disease characterized by symmetrical calcification in the basal ganglia,cerebellar dentate nucleus,and subcortical brain white matter.Clinical manifestations range widely from asymptomatic to variable symptoms including movement disorders,dementia,and behavioural abnormalities[1].The diagnosis of IBGC relies mainly on the visualization of bilateral calcification in the basal ganglia through neuroimaging and the absence of metabolic,infectious,toxic,or traumatic causes[2].The prevalence of IBGC is unknown,but an incidence of basal ganglia calcification ranging from 0.3% to 1.9% has been reported in routine radiological examinations[3-4].Primary familial brain calcification is usually inherited in an autosomal dominant manner; thus far,mutations in three genes have been found to cause the disease:SLC20A2,PDGFB,andPDGFRB.These mutations are implicated in phosphate homeostasis in IBGC[5].
The aim of this study is to report a rare case of familial idiopathic basal ganglia calcification (FIBGC) with cognitive and behavioural impairments presenting at onset only.Since patients with FIBGC show variability in clinical manifestations,even among the families,we should accumulate and report as many cases as possible.There are few clinical reports that precisely evaluate patients not only neuropsychologically but also neuroradiologically with computed tomography (CT),magnetic resonance imaging (MRI),and brain perfusion Single-Photon Emission Computed Tomography (SPECT).Additionally,there are no previous reports of FIBGC with as many as 10 related patients (spanning 3 generations),with DNA information and CT findings that have been observed longitudinally for over ten years.After we briefly reported on the female proband and her relatives with FIBGC in Neurology[6],additional symmetrical calcification in the basal ganglia and the same gene mutation(SLC20A2:c.344C>T) were found in her son (III-1 in the pedigree in Figure 1).Furthermore,we describe manifestations in the proband and her son,who we recently had contact with,in more detail.
CASE PRESENTATION
Chief complaints
Forgetfulness.

Figure1 Pedigree of the Sunagawa family.
History of present illness
The proband was a 76-year-old woman (II-1 in the pedigree in Figure 1).She was admitted to a hospital at the age of 65 because of forgetfulness that had been present since she was 60 years old.She could make only a simple meal,repeated the same conversations,and bought the same things many times.Her MMSE (mini mental state examination) score and HDS-R (Revised Hasegawa Dementia Scale) scores were 19 and 20,respectively,which indicated a possibility of dementia (MMSE score below 24,HDS-R score below 21).The coefficient of correlation of the HDS-R to the MMSE was as high as 0.94,which suggested the HDS-R was valid in terms of compatibility with the established dementia screening test[7].Her Wechsler adult intelligence scalerevised (WIAS-R) total intelligence quotient (IQ) was 87,verbal IQ was 83 and performance IQ was 92.
History of past illness
42 year:Uterine myoma.
58 year:Cerebral aneurysm clipping surgery.
Personal and family history
The proband graduated from a junior high school,married at the age of 21,and had been employed in farming,for a construction,and food service,etc.
She had a positive family history of brain calcification,as shown in Figure 1.Her brother had calcification in the brain (Figure 1-II-7,Figure 6-C) as well as mental retardation,and another brother (Figure 1-II-8,Figure 6-D) presented with alcoholism.Her parents had no clinical symptoms and lived a normal life as far as we know,and they had no dementia.Although we do not know the details,her father died of heart disease and her mother died of stroke.
Physical examination upon admission
No pyramidal or extrapyramidal signs were observed.The Albright sign was negative.
Laboratory examinations
Biochemical examination showed that the levels of thyroid hormones,parathyroid hormone (PT),serum calcium,serum phosphate and cerebrospinal fluid (CSF) were all in the range of normal values.Additionally,theTreponema pallidumhaemagglutination assay test was negative.
Imaging examinations
Symmetrical calcifications in the globus pallidus,pulvinar thalami,subcortical area in the right frontal lobe,and border area of the cortex and white matter in the occipital lobe were found in CT scanning (Figure 2).A T1-weighted MRI revealed small patchy hypersignals in the globus pallidus and pulvinar thalami (Figure 2).A T2-weighted MRI revealed small patchy hyposignals in the globus pallidus (Figure 2).However,it is obscure in MRI scans compared to CT scans.Her brain perfusion SPECT images showed decreased perfusion in the bilateral basal ganglia and thalamus as well as the right frontal lobe (Figure 3).

Figure2 Computed tomography,magnetic resonance imaging-T1,and magnetic resonance imaging-T2 images of the proband (65 yr).
FINAL DIAGNOSIS
The initial clinical diagnosis had been diffuse neurofibrillary tangles with calcification(DNTC)[8]; to the best of our knowledge,familial cases of DNTC have not been reported.Therefore,the patients were diagnosed as FIBGC.
TREATMENT
There is no causal treatment for FIBGC,so we only have the options of symptomatic treatment or observation.
OUTCOME AND FOLLOW-UP
The change in the MMSE and HDS-R scores of the proband is summarized in Table 1.At the one-year follow-up (66 years old),although she could not communicate very well and recognize the expiration date of food,however,there weren’t substantial changes in her overall cognitive function (MMSE:21/30,HDS-R:20).
At the two-year follow-up (67 years old),the patient presented with further decreased short-term memory and disorientation.She performed misplaced acts of kindness such as delivering the same things to neighbours many times.Her daughter recognized that her memory impairment was gradually progressing,which was confirmed by her HDS-R score.The MMSE and HDS-R scores were 23/30 and 16/30,respectively.At the four-year follow-up (69 years old),she started losing memory daily and presented aggressive and restless behaviours that required antipsychotic medication.The MMSE and HDS-R scores were 21/30 and 13/30,respectively.The decreased score of HDS-R indicated deteriorated memory disturbance.She entered a nursing home at the age of 70 due to personality changes,such as increased irritability and displaying aggression to her family.Brain atrophy of frontotemporal lobe was slightly seen compared to her results at 65 years old (Figure 4,5).At the six-year follow-up (71 years old),a gradual progression of cognitive dysfunction was found.The MMSE and HDS-R scores were 19/30 and 12/30,respectively.At the nine-year follow-up (74 years old),though she showed signs of excessive meddling with other patients,only a slight progression in dementia was found.The MMSE and HDS-R scores were 19/30 and 12/30,respectively.When she was 75 years old,she suffered from acute Stanford an aortic dissection and multiple cerebral infarction as a result.Further brain atrophy of frontotemporal lobe was seen compared to her results at 65 and 69 years old (Figure 4,5).Although she received an operation that saved her life,her disordered consciousness remained.Therefore,she moved to a recuperation hospital away from our advanced treatment hospital at the age of 76.

Figure3 Brain perfusion single photon emission computed tomography (easy Z-score imaging system) of the proband (65 yr).
She had a positive family history of brain calcification,as shown in Figure 1.Demographic information,clinical features,and instrumental data of all the patients are summarized in Table 2.Among the two children of the patient,her son (III-1)showed evidence of brain calcification; however,brain CT scans of her daughter (III-2)did not reveal the same finding.Her son,a 49-year-old male,had no remarkable history of illness until 47 years of age.He worked at a machine production manufacturing company for ten years.When he moved to another department of the company,he started to be confounded by unfamiliar tasks and would sometimes make some mistakes.Although he had managed to continue working,he became depressed and anxious.Finally,he decided to visit our hospital and requested treatment.He was diagnosed with an adjustment disorder based on his history.Neither parkinsonism nor cerebellar ataxia were recognized.A biochemical examination revealed that levels of PTH,serum calcium,serum phosphate and CSF were all within normal ranges.The Albright sign was negative.There was no evidence of hypoparathyroidism and pseudohypoparathyroidism.A cranial CT revealed distinct,symmetrical calcification of the basal ganglia,primarily in the caudate nucleus,globus pallidus,putamen,and pulvinar thalami (Figure 6-F).Since we recognized by chance that he was the proband’s son,informed consent for genic analysis of FIBGC was obtained from him.TheSLC20A2mutation was found in his blood sample.
The proband’s brother had calcification in the brain (Figure 1-II-7,Figure 6-C) as well as mental retardation,and another brother (Figure 1-II-8,Figure 6-D) presented with alcoholism.He died of descending colon cancer and frequent cerebral infarctions at the age of 62,four years after we checked his brain CT and blood sample.The three other relatives with calcification (Figure 1-II-5,II-9,and III-3) were basically asymptomatic,although the proband’s sister (Figure 1-II-5) only had headaches.The degree of calcification in these families was relatively mild compared to the calcification observed in other families we examined.
The symptomatic patients (Figure 6A,C,and D) showed more apparent brain atrophy than the others (Figure 6B,E,F,and G).The individuals with calcification on the CT images (II-1,II-5,II-7,II-8,II-9,III-1,and III-3) had the same mutation in exon 3 inSLC20A2(c.344C>T).However,the individuals with no calcification (III-2,III-5,and IV-1) revealed no mutation inSLC20A2.In summary,7 patients had calcification among the 10 individuals who were examined by CT scan in the family,and all of the patients carrying theSLC20A2mutation exhibited similar calcification in their CT images.However,individuals without the mutation did not show calcification.
For other family members outside of the 10 included in this study,mutational analysis and CT scan were not performed due to death (II-3) and lack of consent (II-4).
DISCUSSION
This is a rare case of FIBGC solely presenting cognitive and behavioural impairments.Indeed,to the best of our knowledge,only a few cases with the same clinical features have been described so far[9].In the “Fahr’s Disease Registry,” the most common manifestation was movement disorders (55%),in particular parkinsonism,while hyperkinetic movement disorders accounted for the rest[9].Other manifestations are described,including memory disturbance,hallucination,delusions and personality change,depression[10-13],and stereotypical behaviours[12],which may be accompanied by extrapyramidal signs,such as parkinsonism and paroxysmal non-kinesigenic dyskinesia[14,15].Thus,patients who met the criteria for IBGC[2]have diverse manifestations.Patients with FIBGC show variability in clinical manifestations,even among families.Therefore,we should accumulate and report as many cases as possible.In our familial cases,the proband has dementia followed by personality changes,such as irritability and aggression.Her cognitive function gradually worsened according to her history and HDS-R.Compared with MMSE,the relative weight ofHDS-R for memory was strengthened,and a measure for language was added[16].The study by Kim[16]indicated that the HDS-R did better than MMSE because of the larger AUC (area under the curve) as well as the higher sensitivity and specificity for dementia regardless of severity and the educational level of the subjects.One of the proband’s brothers (Figure 1-II-7) has mental retardation and another one (Figure 1-II-8) had alcoholism.Although the association between these symptoms and calcification is unclear,3 symptomatic patients had signs of brain atrophy,especially in the frontal lobe in CT images.
Table1 The changes in mini mental state examination and revised hasegawa dementia scale scores of the proband
Considering the gradual progressive frontotemporal atrophy of the proband (II-1)as well,the differential diagnosis of DNTC is needed[8]; however,to the best of our knowledge,familial cases of DNTC have not been reported.We hope to perform a pathological diagnosis in the future.
The proband’s son (Figure 1-III-1) has adjustment disorder instead of depression.Although it is difficult to judge whether this disorder is related to calcification or not,it is possible that depression is one of the symptoms of IBGC.At least vulnerability to stress may be associated with IBGC.Interestingly,none of our patients with calcification showed neurological deficits.The non-existence of calcification in the cerebellum may be able to explain why there was no ataxia.On the other hand,the association between parkinsonism and calcification in portions of the brain is unclear.Though the proband’s son (III-1) has calcification in the bilateral striatum,but there is no sign of a movement disorder such as parkinsonism.Additionally,we did not find a correlation between clinical severity and the extent of brain calcification.
With vague criteria and an unknown aetiology,Fahr’s disease presents a blind spot in medical care.The discovery of the mutations in the geneSLC20A2that cause IBGC3 was a turning point in understanding the disease’s pathophysiology.In our familial cases,all of the individuals carrying theSLC20A2mutation exhibited similar calcification in their CT images.However,individuals without the mutation did not show calcification.
In the proband,the bilateral basal ganglia and thalamus as well as the right dominant frontal lobe hypoperfusion were observed (Figure 3).The hypoperfusion presumably results from a disruption of pathways interconnecting the basal ganglia to frontal areas as well as calcification.
We acknowledge some limitations to our report.We have not confirmed the diagnosis through neuropathological means.However,we strongly believe that a detailed history combined with careful physical,neuro-psychological cognitive tests,neuroimaging tools (CT,MRI and brain perfusion SPECT),and genetic tests can significantly increase the precision of clinical diagnosis.Since the members of our memory clinic include psychiatrists,a neurologist,a neurosurgeon,a clinical psychologist and radiological technicians,team collaboration also contributed to providing accurate diagnoses.
CONCLUSION
In summary,the patients in this study showed heterogeneity in terms of their manifestations and different severity in their symptoms,even within the same family.More case reports and further studies related to the manifestations of FIBGC are needed.The elucidation of the molecular basis underlying IBGC will contribute to the development of therapeutic measures for patients with calcification in their brains.

Table2 Demographic information,clinical features,and instrumental data for all patient
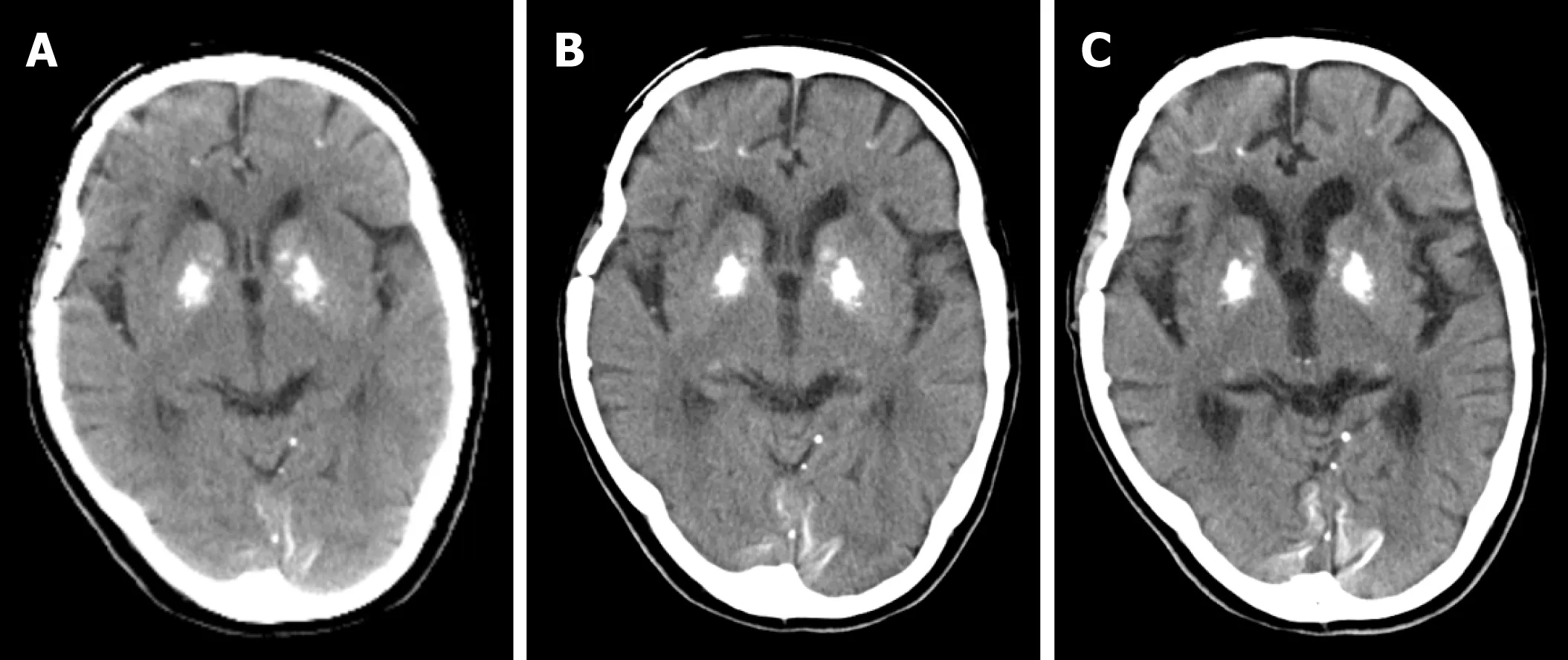
Figure4 Computed tomography images of the proband.

Figure5 Magnetic resonance imaging-coronal images of the proband.
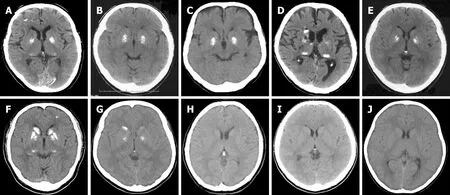
Figure6 Computed tomography images of the family members.
ACKNOWLEDGEMENTS
The authors thank the patients and their families who supported this research.
We also thank the involved doctors (Dr.Kenjirou Kamiguchi,Sunagawa Jikeikai Hospital,Dr.Megumi Yamada and Dr.Isao Hozumi,Laboratory of Medical Therapeutics and Molecular Therapeutics,Gifu Pharmaceutical University).
 World Journal of Clinical Cases2019年12期
World Journal of Clinical Cases2019年12期
- World Journal of Clinical Cases的其它文章
- Biomarkers vs imaging in the early detection of hepatocellular carcinoma and prognosis
- Study on gene expression patterns and functional pathways of peripheral blood monocytes reveals potential molecular mechanism of surgical treatment for periodontitis
- Feasibility of prostatectomy without prostate biopsy in the era of new imaging technology and minimally invasive techniques
- Safety and efficacy of transfemoral intrahepatic portosystemic shunt for portal hypertension:A single-center retrospective study
- Impact of gastroesophageal reflux disease on the quality of life of Polish patients
- Non-albicans Candida prosthetic joint infections:A systematic review of treatment
