Cytotoxic effect of methanolic extracts of Fritillaria imperialis bulbs and Eryngium caucasicum leaves on hepatoma and colon cancer cells
Mostafa Kardan, Zahra Yazdani, Zaher Morsaljahan, Mohammad Ali Ebrahimzadeh, Alireza Rafiei✉
1Student Research Committee, Department of Immunology, Molecular and cell Biology Research Center, School of Medicine, Mazandaran University of Medical Sciences, Sari, Iran
2Department of Immunology, School of Medicine, Mazandaran University of Medical Sciences, Sari, Iran
3Pharmaceutical Sciences Research Center, Department of Medicinal Chemistry, Faculty of Pharmacy, Mazandaran University of Medical Sciences,Sari, Iran
Keywords:Fritillaria imperialis Eryngium caucasicum Cytotoxity Colon cancer Hepatoma cancer
ABSTRACT Objective: To evaluate antitumor activities of Fritillaria imperialis and Eryngium caucasicum methanolic extracts on human hepatoma (HepG2) and colon cancer (HCT116) cell lines in comparison to human foreskin fibroblasts as the normal cells.Methods: Methanolic extracts of Fritillaria imperialis and Eryngium caucasicum were prepared by the maceration method. The effect of the extracts at various concentrations (100, 200,400, 600, and 800 μg/mL) on cell survival was evaluated using the MTT method. Besides,fluorescence staining was used to evaluate death patterns of the cells.Results: MTT assay showed that Fritillaria imperialis significantly decreased the viability of all cell lines after 24 and 48 hours of treatments. However, Eryngium caucasicum extract did not show any significant cytotoxicity effect on the cell lines. Fluorescence staining revealed that Fritillaria imperialis induced apoptosis of HCT116 cells at 550 μg/mL.Conclusions: Fritillaria imperialis extract has antiproliferative and cytotoxic effects on HCT116 and HepG2 cancer cells and therefore, may serve as an anticancer agent.
1. Introduction
Colorectal and hepatoma cancers are the leading causes of cancerrelated deaths worldwide. Their prevalence is increasing, with over 8 million people reported to be infected in 2017[1]. Currently,several approaches are used to treat cancer such as chemotherapy,radiotherapy, hormone therapy and immunotherapy[2]. However, the side effects of these methods are often one of the most important concerns of scientists.
Many herbal compounds have been identified to have anticancer properties. Medicinal plants, as natural sources, have greater compatibility with living organisms and thus cause fewer side effects on live organisms such as human body, compared to chemotherapy drugs[3,4]. Hence, the researchers intended to find more effective plants possessing antiproliferative activities on in vitro cancer models[5,6]. Cell culture techniques allow researchers to evaluate the effect of plant compounds under controlled conditions and to verify their effect on cell lines[7,8].
The medicinal herb Fritillaria is a spring plant, a member of Liliales order and obtained from monocot plants. Currently, almost one hundred species of this genus exist in the world with fourteen of them grown in Iran[9]. Fritillaria imperialis (F. imperialis) that is locally identified as Ashk-e-maryam, is a member of Fritillaria family. It grows in many regions of Iran. Morphologically, this species has a big and perennial bulb, a thick cylindrical stem and sword like, sharp and shiny green leaves, which are located lower down on the stem. A batch of leaves grow at the end of the stem and have five to eight flowers like bulbs. Flowers are orange to red and rarely yellow[9-11]. Bulb of Fritillaria is very important. It is traditionally used as an anti-cough and spasmodic drug, and for treatment of various diseases such as asthma, bronchitis, Alzheimer,sciatica and wound healing[11-13]. It has been shown that the bulb of some species of Fritillaria has alkaloid compounds such as verticine,vericinone, imperialine and ebeiedine, and the main compounds have anticancer properties and inhibitory function on solid tumors growth in vitro and in vivo[5,14-18]. Eryngium caucasicum (E. caucasicum) is a vegetable and a member of Eryngium genus which naturally grows in the north of Iran. The leaves of this plant are used in a variety of local foods. Recent studies have proven that the leaves of this plant have antioxidant and proxidant properties[19-22].
Based on the search results in Scopus, ISI, ISC and SID databases until August 2018, there were no studies conducted to investigate the anticancer effect of F. imperialis and E. caucasicum extracts on HepG2 and HCT116 cell lines. Therefore, in this study, the inhibitory effects of methanolic extracts of F. imperialis bulbs and E.caucasicum leaves on the growth of HCT116 and HepG2 cancer cells were investigated in comparison to human foreskin fibroblasts (HFF)cells.
2. Materials and methods
2.1. Chemicals and reagents
Roswell Park Memorial Institute (RPMI)-1640 medium, Dulbecco's Modified Eagle's medium and fetal bovine serum were purchased from PANbiotech (Germany), Trypsin and penicillin-streptomycin were purchased from Capricorn (Germany), 3-(4, 5-dimethylthiazol-2-yl)-diphenyl tetrazolium bromide (MTT), dimethyl sulphoxide were purchased from Sigma Aldrich, Inc. (USA). Ethidium bromide(EB) and acridine orange (AO) were provided by Merck (Germany),Sterilized cell culture materials, such as syringe filter, 15 mL and 50 mL tubes, 96-well plates, and pipettes were purchased from SPL(South Korea). Other chemicals and reagents used were of analytical grade and commercially available.
2.2. Extraction and isolation
In June 2017, the plants F. imperialis and E. caucasicum were collected from mountains in Naghde, West Azarbaijan province and Sari, Mazandaran province, respectively, in Iran and identified by Dr. Bahman Eslami. Vouchers have been deposited in the Sari School of Pharmacy herbarium. The bulbs of F. imperialis and leaves of E. caucasicum were separated from the remaining parts of the each plant, and dried under dark conditions at room temperature and then ground. Then following steps were conducted for each plant:50 g of dried-powder sample was macerated for 24 h with 300 mL of methanol. Extraction was repeated three times, and the extracts were concentrated by a rotary evaporator until a solid sample was obtained. The solid sample was extracted in a Soxhlet extractor with methanol for 24 h and the crude solid extracts were freeze-dried for complete removal of the solvent. Subsequently, the samples were extracted with methanol in an ultrasonic bath at a frequency of 100 kHz for 1 h (3 × 20 min) to yield ultrasonic extracts. Then,concentrated extract was resolved in 1 mL dimethyl sulphoxide and diluted at concentrations of 100, 200, 400, 600 and 800 microgram per milliliter (μg/mL)[22].
2.3. Cell culture
HepG2, HCT116 cell lines and HFF cells were purchased from the Pasteur Institute (Tehran, Iran). HepG2 and HCT116 cell lines were cultured in RPMI-1640 containing 10% fetal bovine serum and 1% penicillin and streptomycin. HFF cells were cultured in low-glucose Dulbecco's Modified Eagle's medium containing 10%fetal bovine serum and 1% penicillin and streptomycin. All cell lines were maintained in a humidified incubator at 37 ℃ in 5% CO2atmosphere[23-25].
2.4. Cell growth and viability assay
Assessment of cell growth and viability was performed using MTT assay. In this method, HepG2, HCT116, and HFF cells (1×104cells/well) were cultured in 96 well tissue culture plates and incubated for 24 h. Then the cell supernatant was replaced by new enriched medium including various concentration of the methanolic extract (100, 200, 400, 600, and 800 μg/mL) of F. imperialis or E.caucasicum, and continued to incubate for up to 24 and 48 h. All experiments were conducted in triplicate.
Doxorubicin was used as a positive control in all experiments.At the end of the incubation, 20 μL of MTT (5 mg/mL) was added to the wells to allow formation of MTT formazan crystals for 4 h.The medium was then removed, and the crystals were completely solubilized by adding of 200 μL of dimethyl sulphoxide. Finally, the absorbance of each well was measured at 570 nm[26,27].
2.5. Apoptotic activity
In this method, 1×104cells of HCT116, HepG2 and HFF were seeded in a 96 well tissue culture plate and incubated for 24 h at 37 ℃in a humidified and 5% CO2atmosphere. Subsequently, the cells were treated with 550 μg/mL of methanolic extract of F. imperialis as MTT assay showed that the lowest IC50was 550 μg/mL for this extract at 48 h. All experiments were done in triplicate. After incubation, cells were detached with 0.25% trypsin-EDTA and washed twice with phosphate buffer saline. A dye mixture of 10 μL of AO (50 μg/mL) and 10 μL of EB (50 μg/mL) was used for staining treated- and untreated-cells for 10 min. Thereafter, the cells were washed twice with phosphate buffer saline and visualized under a fluorescence microscope at 100×magnification[26,28].
2.6. Statistical analysis
Statistical analysis was performed using SPSS version 23.Quantitative variables were presented as mean ± SD. Any difference among study groups was determined by Student t test or one way analysis of variance (ANOVA) followed by post hoc test. In all comparisons, P value less than 0.000 1 was considered statistically significant.
3. Results
3.1. Viability of the cells treated with methanolic extracts of F. imperialis and E. caucasicum
After 24 hours of treatment with F. imperialis bulb extract, the viability of HCT116 cells was significantly decreased in a dosedependent manner (Figure 1A). Increasing exposure time from 24 to 48 h resulted in a significantly reduction in cell viability in all cell lines (Figure 1B). F. imperialis bulb extract showed marked cytotoxicity against HCT116 cell line than HFF cells at concentrations of 400-800 μg/mL. IC50values of this extract at 24 and 48 h are shown in Table 1, and compared with the IC50values of doxorubicin on HepG2 and HCT116 cell lines. The methanolic extract of E. caucasicum leaves did not show any significant cytotoxity effect on the cancer cell lines or normal cells (Figure 2).
3.2. Apoptotic activity by EB/AO staining
EB/AO staining showed no changes in morphology or green color appearance of untreated cells. Treatment with methanolic extract of F. imperialis resulted in membrane blebbing, chromatin condensation, and cell shrinkage and apoptotic formation suggesting cell death. On the other hand, as shown in Figure 3, these changes in color and morphology were more significant in HCT116 compared to HepG2 and HFF cells.

Figure 1. Effects of methanolic extract of Fritillaria imperialis bulbs on viability of tumor cell lines (HepG2 and HCT116) and normal cells (HFF)at 24 h (A) and 48 h (B). HCT116: Human colon cancer cell line, HepG2:Hepatoma cancer cell line, HFF: Human foreskin fibroblasts.

Table 1. IC50 values of Fritillaria imperialis extract and doxorubicin on HCT116, HepG2 and HFF at 24 and 48 h (μg/mL).

Figure 2. Effects of methanolic extract of Eryngium caucasicum leaves on viability of tumor cell lines (HepG2 and HCT116) and normal cells (HFF)at 24 h (A) and 48 h (B). HCT116: Human colon cancer cell line, HepG2:Hepatoma cancer cell line and HFF: Human foreskin fibroblasts.
4. Discussion
The present study investigates the effect of methanolic extracts of the F. imperialis bulb and E. caucasicum leaves on HCT116 and HepG2 cell lines in comparison with HFF cells. The results showed potent cytotoxicity of the crude methanolic extract of F. imperialis on colon cancer cell line (HCT116), with IC50of 550 μg/mL after 48 hours of exposure while less therapeutic effect on the hepatoma cell line (HepG2) with IC50of 999.2 μg/mL. A significant difference was found between the cytotoxic effect of F. imperialis extract and doxorubicin on the tumor cell lines (P<0.000 1). This difference can be based on the nature of the two therapeutic agents. Doxorubicin is a pure chemical compound[29]whereas, F. imperialis extract contains numerous compounds that have antioxidant and anticancer properties[16-18]. Despite the good efficiency of doxorubicin, for many reasons including drug toxicity, low systemic stability and degradation of the drug by degrading enzymes, the use of this drug is limited[30]. Therefore, many studies may seek to substitute or supplement it with more effective and safer new drugs. In this regard,medicinal plants are more affordable.
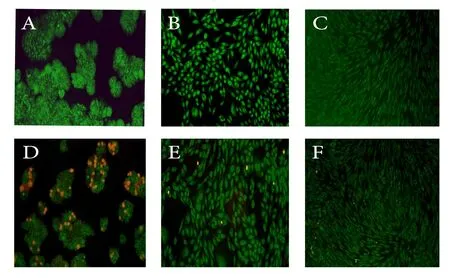
Figure 3. Apoptotic activity of HCT116, HepG2 and HFF cell lines treated with Fritillaria imperialis extract after 24 h by ethidium bromide and acridine orange staining. A, B, C: Untreated HCT-116, HepG2 and HFF cells, respectively and D, E, F: HCT116, HepG2 and HFF cells respectively treated with Fritillaria imperialis extract.
To better distinguish the death pattern following treatment with methanolic extracts, a fluorescence-based cell viability assay was adopted to study the apoptotic activity of F. imperialis bulb extract on HepG2, HCT116 and HFF cells. We applied a combination of EB/AO staining dyes, where viable cells are indicated by green and dead cells by orange fluorescence. Methanolic extract of F. imperialis at the lowest IC50concentration induced morphological changes indicating apoptosis in HCT116 cell line. These changes included nuclear condensation and apoptotic body formation. However, the extracts did not show a significant effect on the apoptotic activity in the HepG2 cell line and HFF cells after 24 hours of exposure.Therefore, as a result of further studies, the extract of F. imperialis with high efficiency and less side effects is a suitable alternative to chemotherapy drugs such as doxorubicin. No significant effect of the methanolic extract of E. caucasicum was shown on the cancer cell lines. This finding is inconsistent with previous reports which revealed anticancer activity of this plant due to the presence of high amounts of antioxidant compounds[23,24,30].
In recent years, several studies have been done on the anticancer effects of Fritillaria bulb, in which alkaloid compounds were found in the bulb of various Fritillaria species such as verticine,vericinone, imperialine, ebeiedine, zhebeinine and puqiedinone.These compounds have inhibitory effects and toxicity on growth of the various types of cancer cells[5,15-19,31], therefore, extracts of different Fritillaria species have cytotoxity effects on cancer cell lines. In line with this claim, some reports recently demonstrated the anticancer activity of the Fritillaria species. Zhang et al reported that two compounds from Fritillaria hupehensis against Hela and HepG2 cancer cell lines had IC50values similar to that of 5-FU[19]. Wang et al also proved that extracts and steroidal alkaloids from Fritillaria ussuriensis had efficient antitumor activities[15]. Ping et al reported that the bulb extract of Fritillaria ebeiensis inhibited the growth of the solid type of hepatoma in mice[17]. Wang et al indicated that the bulbs of Fritillaria cirrhosae showed cytotoxity on Lewis lung carcinoma cells[18].
In conclusion, the methanolic extract of F. imperialis revealed cytotoxic activity in HCT116 and HepG2 cell lines in a dosedependent manner, and may have the potential to be used as an anticancer drug. It is therefore recommended to evaluate its other extracts such as aqueous, acetone, ethyl acetate etc in future studies,to fi nd the best therapeutic formula in various cell lines.
Conflict of interest statement
The authors declare that there is no con fl ict of interest.
Acknowledgments
Research reported in this publication was supported by deputy of Research and Technology, Mazandaran University of Medical Sciences. We thank Sara Tahaghoghi -Hajighorbani for editing the manuscript. We are grateful to the staff of biologic laboratory of Molecular and Cell Biology Research Center who facilitate and provide interactive environment for us to do all experiments.
Funding
This work was supported by deputy of Research and Technology,Mazandaran University of Medical Sciences.
 Asian Pacific Journal of Tropical Biomedicine2019年8期
Asian Pacific Journal of Tropical Biomedicine2019年8期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- Development of new lateral-flow immunochromatographic strip using colloidal gold and mesoporous silica nanoparticles for rapid diagnosis of active schistosomiasis
- Antioxidant, anti-quorum sensing and anti-biofilm potential of ethanolic leaf extract of Phrynium capitatum and Dryptes indica
- Synergistic antioxidant interactions between green tea and Ocimum gratissimum
- Proapoptotic activities of Oroxylum indicum leave extract in HeLa cells
- Cladogynos orientalis Zipp. extracts inhibit cell culture-derived hepatitis C virus genotype 2a replication in Huh-7 cells through NS5B inhibition
