The potential for estrogen disrupting chemicals to contribute to migration, invasion and metastasis of human breast cancer cells
Philippa D. Darbre
School of Biological Sciences, University of Reading, Reading RG6 6UB, UK.
Abstract Estrogen disrupting chemicals are environmental compounds which mimic, antagonize or interfere in the action of physiological estrogens. They occur naturally (plant phytoestrogens) but the majority are man-made compounds,which, through their use in agricultural, industrial and consumer products, have become widely present in human tissues including breast tissue. Since exposure to estrogen is a risk factor for breast cancer, estrogen disrupting chemicals may also contribute to breast cancer development. This review discusses evidence implicating estrogen disrupting chemicals in increasing migratory and invasive activity of breast epithelial cells, in epithelial-tomesenchymal transition, and in growth of breast tumours at metastatic sites as well as the primary site. Mechanisms may be through the ability of such chemicals to bind to estrogen receptors, but unlike for proliferation, effects on cell migration and invasion are not limited to estrogen receptor-mediated mechanisms. Furthermore, whilst effects on proliferation can be measured within hours/days of adding an estrogen disrupting chemical to estrogen-responsive breast cancer cells, effects on cell migration occur after longer times (weeks). Most studies have focused on individual chemicals, but there is now a need to consider the environmentally relevant effects of long-term, lowdose exposure to complex mixtures of estrogen disrupting chemicals on mechanisms of metastasis.
Keywords: Aluminium, breast cancer, estrogen disrupting chemicals, invasion, metastasis, migration, paraben,personal care products, UV filter.
INTRODUCTION
Estrogen disrupting chemicals are exogenous environmental compounds that can interfere in the action of endogenous estrogens. THrough their ability to bind to estrogen receptors, they may be able to mimic or antagonize the cellular actions of physiological estrogens[1]. THey may also influence synthesis, transport,metabolism and clearance of physiological estrogens[1]. Some are found naturally in plants (phytoestrogens)or fungi (mycoestrogens), but the majority are man-made compounds to which the human population is widely exposed through their use in agricultural, industrial and consumer products[1]. Such compounds are used as components of pesticides and herbicides in agricultural, urban and domestic environments.They are widely used in industrial applications and occur as byproducts of combustion from vehicles,aircraft and ships. THese compounds are found as components in plastics, with applications from building materials to food/water containers, in detergents used for cleaning in industrial and domestic settings, and as flame retardants in soft furnishings. A range of components added to personal care products are also now known to possess estrogen disrupting activity[1]. Some more specific examples are shown in Table 1.Intake to the human body may be by the oral route (food/water) or inhalation (air pollution) or dermal absorption (products applied to skin or present in the air). So ubiquitous are the many estrogen disrupting chemicals in modern life that exposure is almost never from a single source but rather from multiple sources reflecting not only occupational exposures but also personal lifestyle choices.
Since exposure to estrogens is an established risk factor for breast cancer[2], it is thought that estrogen disrupting chemicals may also contribute to breast cancer development if they are present in human breast tissue at sufficient concentrations[1]. Development of cancer is a lengthy and complex process, that originates from a loss of growth control, and culminates in metastatic tumour spread. Both genetic and environmental influences interact to enable the associated hallmarks of cancer to develop[3]. THe hallmark of sustained cell proliferation in breast cancer is well established as involving estrogens[4]and probably also estrogen disrupting chemicals[5]. Since metabolism of some endogenous estrogens gives rise to genotoxic compounds[6]and since some estrogen disrupting chemicals are also genotoxic[1], a role has been recognized in generating genomic instability which is an underlying enabling characteristic of cancer[3]. However,the role of endogenous and exogenous estrogens in contributing to the processes of metastatic tumour spread are less well established. THis is especially important in the context of breast cancer where the main cause of mortality arises from growth of metastatic tumours[2,7]. THis review will discuss current evidence suggesting that endogenous and exogenous estrogens can contribute to increasing migratory and invasive activity of breast epithelial cells which are hallmarks of cancer required for the metastatic process[3].
ENDOGENOUS ESTROGENS AND METASTASIS
Cellular actions of estrogens are mediated through binding to specific receptor proteins[8]. THere are two types of nuclear estrogen receptors, estrogen receptor α (ERα) and estrogen receptor β (ERβ) which exist as multiple isoforms and which function as ligand-activated transcription factors to influence patterns of gene expression[8]. Final outcomes are dependent on the affinity of ligand binding to receptor, the concentrations of receptors in the target cells and the presence of co-acting factors/transcription factors[8]. In addition,estrogenic ligands can also act through binding to membrane-associated molecules such as the G-proteincoupled estrogen receptors[8,9].
Metastasis involves a complex series of events whereby the malignant cells break away from the primary tumour, invade through local tissue, infiltrate circulatory vessels (blood and/or lymph) and at distant sites exit the vessels to from new foci of cancer cell growths (colonization)[7]. Endogenous estrogens can influence all these processes including immune evasion and the angiogenesis needed for effective metastatic colonization[10]. In their capacity to regulate growth of estrogen-responsive human breast cancer cells[2,4], estrogens can influence metastatic tumour growth at both the primary and secondary sites[2].However, endogenous estrogens are also now known to be able to influence cell motility, cell migration and invasive behavior of human breast cancer cells through altering expression of proteins and transcription factors key to the process of epithelial to mesenchymal transition (EMT). EMT is a process in which the epithelial cells lose their polarity and strong cell-cell adhesion properties in order to assume a more mesenchymal phenotype lacking the polarization and the strong cell-cell interactions. THe cell adhesion junctions of epithelial cells are reliant on high levels of E-cadherin which is a transmembrane protein linked to the cytoskeleton by α- and β-catenin, and EMT is typically associated with reduction in levels of these adhesion proteins. It is also associated with altered levels of transcription factors (such as slug and snail) which control expression of the adhesion proteins[7]. THis allows the cells to break away from neighbouring cells and to become generally more motile. Human breast cancer cell lines which possess ERα (ERα+) tend to be less invasive, less metastatic and possess higher levels of E-cadherin than those which lack ERα (ERα-)[11]. Knockdown of ERα in the ERα+ cell lines has been shown to result in decreased E-cadherin and increased slug expression[12]. Conversely, transfected overexpression of ERα in the ERα- cell lines has been shown to enable estrogen-mediated increase in E-cadherin and decrease in slug[12]. In the ERα+ MCF-7 human breast cancer cell line, estradiol can increase EMT whilst the antiestrogen tamoxifen can reduce EMT[13]suggesting that the mechanism is ER-dependent. However, there appear to be multiple molecular mechanisms involving not only genomic alterations to levels of E-cadherin and associated transcription factors such as slug[12]but also non-genomic alterations via c-src and phosphorylation of focal adhesion kinase[14,15].
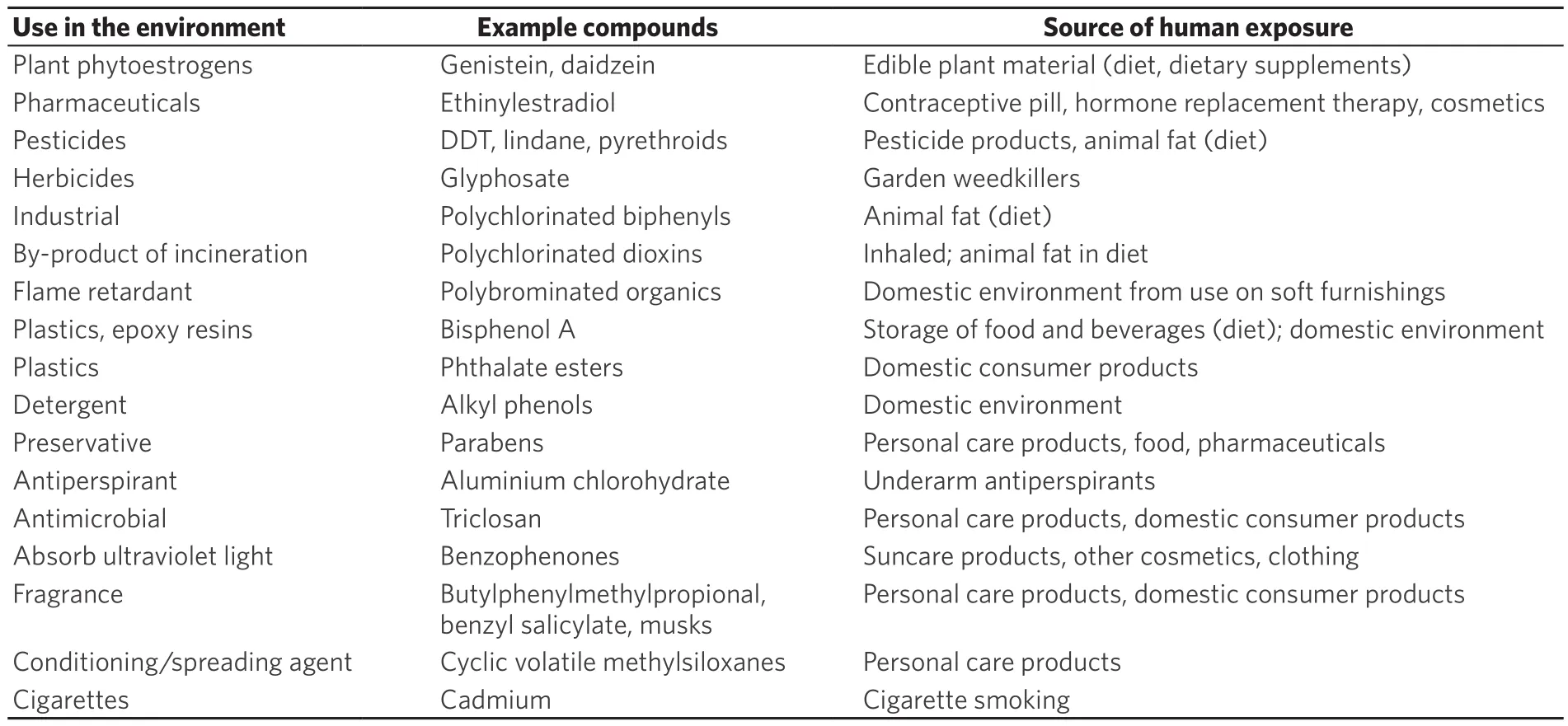
Table 1. Estrogen disrupting chemicals: example compounds, uses in the environment, and sources of human exposure
In order to facilitate invasion through local tissue, the cells need also to secrete greater levels of extracellular proteases which aid in the digestion of the surrounding extracellular matrix (ECM). Some of the extracellular proteases identified specifically in human breast cancer cells are matrix metalloproteinases(MMPs)[16,17]and cathepsin D[18]. It is notable that one of the first estrogen-regulated genes to be identified was cathepsin D[19]and studies now also show effects of estrogen on expression of MMPs[16]. THese studies attest to the role of estrogen in altering proteases which can degrade the ECM.
Estrogens are also known to play a role in the processes of angiogenesis[10,20]. Vascular endothelial growth factor (VEGF) is a key angiogenesis promoting factor and theVEGFgene has been shown to possess functional estrogen response elements indicating it is an estrogen-responsive gene[21]. Estrogen treatment has also been reported to increase intra-tumoural angiogenesis[22]and VEGF levels[23]in mouse models of breast cancer.
ESTROGEN DISRUPTING CHEMICALS AND METASTASIS
On the basis that endogenous estrogens can influence processes necessary for metastasis, it would seem plausible that estrogen disrupting chemicals will also contribute if they can enter breast tissue and target metastatic tissues at sufficient concentrations. Whilst endogenous estrogens are subject to physiological regulation in terms of both concentration and timing, it is noteworthy that the estrogen disrupting chemicals are not regulated in this manner. The presence of estrogen disrupting chemicals in the body will follow rather intake from environmental exposures balanced by rates of elimination and ability of individual chemicals to bioaccumulate. Many estrogen disrupting chemicals tend to be lipophilic accumulating in body fat over the years such that body burdens increase with age[1]. In this context it may be significant that 80% of breast cancers occur in women over the age of 50[24]at a time when endogenous estrogens are reduced following menopause but exogenous estrogen disrupting chemical body burdens would be continuing to increase.
Estrogen disrupting chemicals and metastatic colonisation
Metastasis of breast cancer cells begins with local spread within the breast and to the underarm lymph nodes. THe pattern of more distant dissemination is less predictable with preferred sites of colonization being brain, bone, lungs and liver[7]. Stephen Paget’s original “seed and soil” hypothesis proposed that preferred sites of metastasis would reflect a favourable organ microenvironment in which the metastatic breast cancer cells could grow[25]. His hypothesis was based on the tenet that “when a plant goes to seed, its seeds are carried in all directions, but they can only live and grow if they fall on congenial soil”. For breast cancer cells, one important favourable element for cell growth would be an “estrogenic” environment and it is in this context that estrogen disrupting chemicals may influence sites of metastatic spread. Estrogen disrupting chemicals have been widely measured in human populations across the globe in body fluids such as blood and urine and in various body tissues[1]. With such widespread contamination of the human body, it is becoming accepted that there are ramifications for human health including effects on male and female reproduction and on cancers in reproductive tissues[1]. The presence of estrogen disrupting chemicals across many body tissues could act to change a previously non-favourable location into a favourable environment for colonization and growth of human breast cancer cells into metastatic tumours.
Evidence for effects of estrogen disrupting chemicals on the metastatic process
There is now mounting evidence for effects of the parabens, ultraviolet (UV) filters, aluminium salts,triclosan, phthalates, bisphenol A (BPA), dioxins and phytoestrogens in the processes of EMT, motility,migration and invasion, and relevant studies of breast cells are discussed below. Most studies to date have been based in vitro but there are some animal models which demonstrate measurable effects also in vivo.Many of the mechanisms may involve mimicking the action of endogenous estrogens through the ability of these estrogen disrupting chemicals to bind to estrogen receptors, but unlike for proliferation, the effects on migration and invasion do not seem to be limited to ER-mediated mechanisms. Furthermore, whilst effects on proliferation can be measured within hours/days of adding an estrogen disrupting chemical to estrogen-responsive breast cells, effects on migration tend to occur after a longer time frame of weeks. THe reasons why the time taken is longer remain unknown but would be important to determine in future research.
Parabens
The alkyl esters of p-hydroxybenzoic acid (parabens) are added as preservatives to personal care products, pharmaceuticals and foods[1]. Of the five commonly used esters (methylparaben, ethylparaben,n-propylparaben, n-butylparaben and isobutylparaben) 99% of the human breast tissues were found to contain at least one of the esters, and 60% contained all five esters[26]. Although the parabens bind more weakly to estrogen receptors than do the endogenous estrogens, their efficacy is not weak provided sufficient concentration is present[27]. It is therefore noteworthy that the concentrations of parabens measurable to human breast tissues are considerably higher (in the micromolar range)[26]than the levels of endogenous 17β-estradiol (in the nanomolar range)[2,28]. Previous work has demonstrated that parabens can increase proliferation of estrogen-responsive human breast cancer cells in cell culture at concentrations measurable in some human breast tissue samples[29]through estrogen receptor-mediated mechanisms[27].However, parabens have now been found to also increase migratory and invasive activity of human breast cancer cells in culture[30]. Migratory activity was measured using scratch assays, time-lapse microscopy and xCELLigence technology[30]. Invasive activity was measured using matrix degradation assays and invasion through matrigel on the xCELLigence system[30]. Unlike the proliferative effects which were measurable within days of addition of the parabens[27,29], the increased migratory and invasive properties only developed in the cells after long-term exposure (20 weeks)[30]. Western immunoblotting showed an associated downregulation of E-cadherin and β-catenin in the long-term paraben-exposed cells, which could be consistent with a mechanism involving EMT[30].
One technology which has been particularly useful in determining adhesion, migration and invasion of breast cells following exposure to estrogen disrupting chemicals has been the ACEA BioSciences xCELLigence technology, and the increased cell migration following long-term exposure of MCF-7 human breast cancer cells to paraben which was measured using this technology is shown in Figure 1A. This technology works on the basis of a modified Boyden chamber in which two chambers are separated by a membrane. Migration of cells through the membrane on the base of the upper chamber can be monitored in real time at any pre-determined time interval as the cells move onto the undersurface of this membrane located at the top of the lower chamber. The CIM-plate-16 contains 16 wells which can be monitored independently but simultaneously to measure cell migration/invasion in real time through 8 μm pores in the membrane onto gold electrodes on the underside of the membrane using the ACEA BioSciences xCELLigence analyser system. Cell movement onto the gold electrodes is measured as electrical impedance(cell index). Collated traces of cell index are shown in Figure 1A for cell migration through uncoated membranes following 20 weeks of prior treatment with or without 5 × 10-4M methylparaben or 10-5M n-butylparaben (conditions as published in reference 30).
UV filters
Chemicals which can absorb UV light (UVA and/or UVB) are added to consumer products either to protect the skin of the user from UV damage (sunscreen products) or to protect the product itself from UV damage during storage[31]. THey are also used in textiles marketed as UV protective clothing[32]. Widespread use of benzophenone-3 (BP-3), octylmethoxycinnamate (OMC) and 4-methylbenzilidenecamphor (4-MBC)has led to their detection in environmental water and soil samples[33]and they have been recently also measured as present in human breast tissues[34]. One or more of these UV filters were quantifiable in 84% of the breast tissue samples and in at least one breast region of 95% of the women[34]. BP-3, OMC and 4-MBC all possess estrogenic activity in reporter gene assays in estrogen-responsive MCF-7 human breast cancer cells[31,35]. Long-term exposure (23 weeks) to any one of these three compounds has now also been found to increase migratory activity in MCF-7 cells using scratch assays, time-lapse microscopy and xCELLigence technology, and to increase invasion through matrigel as measured using xCELLigence technology[35].THe increased cell migration following long-term treatment (23 weeks) of the MCF-7 cells with or without 10-5M of BP-3 or OMC is shown in Figure 1B using xCELLigence technology (conditions as published in reference 35). However, increased motility of estrogen-unresponsive MDA-MB-231 human breast cancer cells was also observed after long-term exposure to each of the three compounds, implying the increased migratory activity was not confined to estrogen responsive cells[35]. Furthermore, molecular mechanisms differed between compounds and cell lines, with a noted loss of β-catenin only after long-term exposure to OMC in the MCF-7 cells and an increase in MMP-2 after long-term exposure to OMC and 4-MBC in the MDA-MB-231 cells[35].

Figure 1. Increases in migration of MCF-7 human breast cancer cells following prior long-term exposure to several estrogen disrupting chemicals contained in personal care products. Cell migration was measured in real-time as electrical impedance (cell index) using xCELLigence technology with uncoated CIM-16 plates. Prior to the assay, cells had been grown. A: for 20 weeks with no addition, 5 × 10-4 M methylparaben or 10-5 M n-butylparaben; B: for 23 weeks with no addition, 10-8 M 17β-estradiol, 10-5 M octylmethoxycinnamate or 10-5 M benzophenone-3; C: for 32 weeks with or without 10-4 M aluminium chlorohydrate; D: for 9 weeks with or without 10-7 M triclosan. Conditions were as published for the parabens[30], UV filters[35], and aluminium chlorohydrate[42].Data for triclosan are unpublished personal results. UV: ultraviolet
Aluminium-based antiperspirant salts
Aluminium-based salts are used as antiperspirant in underarm cosmetics and dermal absorption of aluminium from this use has been implicated in the development of breast cancer[36]. Aluminium has been measured in human breast tissue[37]and breast cyst fluid[38]at higher levels than in blood, and in nipple aspirate fluid at higher levels in samples taken from women with than without breast cancer[39].Aluminium, as well as several other metal ions, is a metalloestrogen[40]and in the form of the antiperspirant salts aluminium chloride or aluminium chlorohydrate it can displace radiolabeled 17β-estradiol from estrogen receptors and regulate estrogen-responsive gene expression[41]. However, aluminium has also been shown to increase migratory and invasive activity of human breast cancer cells, although since effects were found not only in estrogen-responsive MCF-7[42]but also estrogen-unresponsive MDA-MB-231[43]cells,estrogen-independent mechanisms of action must also exist. The increased cell migration following longterm (32 weeks) treatment of MCF-7 cells with or without 10-4M aluminium chlorohydrate are illustrated in Figure 1C using xCELLigence technology (conditions as published in reference 42). More recent animal model research has shown that non-transformed murine mammary gland (NMuMG) epithelial cells exposed to aluminium chloride in vitro were transformed as judged by soft agar assay, and then when injected into three mouse strains with increasing immunodeficiency formed tumours and metastasised in vivo[44].Untreated cells formed tumours and metastasized to a limited extent in the highly immunodeficient and natural killer (NK) cell deficient NSG mouse strain but not in the less permissive and NK cell competent NOD SKID strain or nude strains. In contrast, NMuMG cells transformed in vitro by the aluminium chloride formed large tumours and metastasized in all three mouse models[44].
Triclosan
Triclosan [5-chloro-2-(2,4-dichlorophenoxy)phenol] is a chlorinated aromatic compound which is added as a broadspectrum antimicrobial agent to consumer goods. It was first used as a hospital scrub but is now added widely to personal care products and homeware products[45]. It possesses estrogenic activity[46]and has been detected in human milk[45]implying its presence in the human breast. It can increase proliferation of estrogen-responsive human breast cancer cells[45]. More recently, exposure of MCF-7 human breast cancer cells to triclosan has been shown to increase migration and development of EMT associated with downregulation of E-cadherin and upregulation of N-cadherin, snail and slug[47]. The increased cell migration observed in our laboratory following long-term (9 weeks) exposure of MCF-7 cells to 10-7M triclosan is shown in Figure 1D using xCELLigence technology (personal unpublished data).
Phthalates
Esters of phthalic acid (phthalates) are used as plasticisers in the manufacture of plastics. However, in addition to plastic goods, they are also found in many personal care products, adhesives, paints, air fresheners, food products, pharmaceuticals and textiles[1]. Many of the esters are listed by the Organisation for Economic Cooperation and Development (OECD) as high production volume chemicals[48], with some of the most used being diethylphthalate (DEP), dibutylphthalate (DBP), benzylbutylphthalate (BBP) and di-(2-ethylhexyl)phthalate (DEHP). Metabolites of these esters are widely detected in human urine samples of the US population[49]and can be measured in human milk[50]implying their presence in the human breast.Many of these widely used esters and their metabolites possess estrogenic activity and can increase growth of estrogen-responsive human breast cancer cells[51,52]. Using a stem cell-derived human breast epithelial cell line R2d, DBP and BBP were found to induce EMT through an ER-mediated increase in mesenchymal markers and decrease in epithelial markers[53]. Furthermore, BBP has been reported to increase growth of tumours and lung-derived metastases in an in vivo mouse xenograft model using breast cancer stem cells.THe mechanism was linked to an arylhydrocarbon receptor-mediated increase in sphingosine-1-phosphate receptor 3 (S1PR3) signaling since downregulation of this pathway reduced both the tumour growth and the lung metastasis but the role of estrogen in this remains unknown[54].
Bisphenol A
BPA is used in the manufacture of polycarbonate plastics and epoxy resins which are found ubiquitously in building materials and consumer products[55]. It is used to line food storage containers and water bottles from which it can leach out when heated[56]. It is listed by the OECD as a high production volume chemical[48]. A large literature links BPA to adverse health effects in animals and humans[55], and it is measurable in human body tissues including breast milk[57]which implies its presence in the human breast. Through its estrogenic activity, it is known to stimulate the proliferation of estrogen-responsive human breast cancer cells[56]. Animal models have revealed that prenatal exposure can enhance breast carcinogenesis in the chemically induced DMBA-mouse model[58]and alone can also cause disruption to the mouse mammary tissue increasing susceptibility to breast cancer[59]. However, chronic exposure to BPA in drinking water during adulthood was also shown to increase tumour burden and incidence of metastasis in a transgenic mouse model that spontaneously develops tumors through overexpression of erbB2[60]. More recent in vitro experiments with human breast cancer cells are beginning to suggest that BPA may increase cell migration and invasion. Exposure of SkBr3 human breast cancer cells to BPA (10-8M)increased cell motility and downregulated the transcription factor FOXA1[61]. FOXA1 repression is thought to be characteristic of EMT because it is an activator of the E-cadherin gene[62]. Exposure of the human breast cancer cell lines MDA-MB-231 and BT-549 led to increased invasiveness with an associated increased expression of MMP-2 and MMP-9[63].
Dioxins
Polychlorinated dibenzodioxins (PCDDs) (dioxins) are highly toxic, bioaccumulative environmental pollutants generated as byproducts of combustion[64]. They are carried by air, washed off the land by rainwater into rivers and lakes and then pass up the food chain dissolved in animal fat. THey are measured ubiquitously in human tissues[64]. THere are 75 congeners of which the most toxic is 2,3,7,8-tetrachlorodi benzodioxin (TCDD)[64]and has been shown to exert molecular actions both through ER-mediated and AhR-mediated mechanisms[65]. Gene targets of the AhR include activation of slug and some MMPs[66]. In the ER+, AhR+ MCF-7 human breast cancer cells, TCDD has been found to downregulate E-cadherin and reduce cell-cell contacts in a JNK-dependent mechanism[67]. However, antiestrogenic actions of TCDD have been repeatedly reported over the years[65], and so it is noteworthy that TCDD has been reported to inhibit mammary tumour metastasis in vivo[68,69].
Phytoestrogens
Phytoestrogens are found in over 300 plant species and can be ingested by humans through consumption of plant material either in diet or as dietary supplements[70]. Flavonoids include genistein and daidzein found in soybeans and other legumes, coumestans in clover and young sprouting legumes, prenylflavonoids in hops. THe most prevalent non flavonoids are lignans in cereals, fruits and vegetables. Although many purified phytoestrogens display estrogenic activity in vitro, the estrogenic actions on cell proliferation are concentration-dependent with only the lower doses increasing proliferation of estrogen-responsive human breast cancer cells, whilst higher doses inhibit[71], and many phytoestrogen-containing plant products are considered to have anti-tumour activity[70]. It is interesting therefore that emerging data seem to suggest that several phytoestrogens can act to reduce breast cancer cell migration and invasion including lignans[72],soy-derived daidzein[73], and formononetin[74]. Triclosan-induced EMT can be reversed by kaempferol[47].However, one mouse model of breast cancer has shown that consumption of soy isoflavones increased growth of metastatic tumours in bone and lung[75].
THE ISSUE OF LOW-DOSE MIXTURES
The studies discussed above provide evidence that some individual estrogen disrupting chemicals can influence components of EMT and processes of migration and invasion in human breast cancer cells.However, since many hundreds of estrogen disrupting chemicals have now been measured in the human breast[1], the environmental reality is that, in today’s world, human breast cells in vivo are not exposed to single chemicals but to complex mixtures of pollutant chemicals. THis is especially poignant in view of many estrogen disrupting chemicals being lipophilic and the human breast being a fatty organ. It is also highly relevant in the context of the many non-monotonic responses which have been reported for environmental endocrine disrupting chemicals in general, often with stronger responses at the relatively lower doses tested[76]. THere are therefore outstanding questions as to the effects of exposure to complex mixtures of estrogen disrupting chemicals where each is present at low dose. For proliferation, it has been shown that mixtures of such chemicals can increase cell proliferation where the same concentrations of the estrogen disrupting chemicals individually do not, in what has now been nicknamed “the something from nothing” effect[77]. Even mixtures of the different esters of parabens can add together to give increased cell proliferation[29]. This is relevant environmentally where human breast tissue samples were found to contain differing levels of the individual paraben esters[26]and implies that the same outcomes can arise from different mixtures of estrogen disrupting chemicals[29]. Clearly in ER-mediated mechanisms, such as in increasing cell proliferation, each chemical will act according to its relative estrogen receptor binding affinity but low doses of different chemicals can add together until a maximum proliferative signal is achieved. As yet, there seems to be no published information as to whether the same effect of mixtures can be achieved on development of EMT or on increasing cell migration/invasion. Furthermore, since these studies are technically long and labour-intensive, the range of concentrations are often lacking in order to determine the extent to which non-monotonic responses may be occurring.

Figure 2. Diagrammatic representation of how estrogen disrupting chemicals may influence metastasis of breast cancer cells. Through their estrogenic activity, they may increase growth of cells at the primary site in the breast or at metastatic sites. They may enable EMT, increase cell migration and cell invasion. Through their widespread presence in the human body, they may provide a favourable microenvironment for colonization and metastatic tumour growth
CONCLUSIONS
It can be concluded that there is mounting evidence for a role of estrogen disrupting chemicals in contributing to the processes of metastatic tumour spread and this is summarized in Figure 2. Estrogen disrupting chemicals may contribute to loss of cell-cell adhesion, development of EMT and increased secretion of ECM-degrading proteases leading to increased cell motility, migration and invasion. However,estrogen disrupting chemicals are also likely to play a role in creating a favourable microenvironment for colonization and growth of metastatic tumours at distant sites. Unpredictable awakening from dormancy[7]could result from altered environmental exposures or indeed sudden release of estrogen disrupting chemicals from fat stores such as at times of weight loss. It is well established that estrogen disrupting chemicals are passed from mother to child in breast milk as they are mobilized with the fat in the milk[78].Such detoxification of the the mother’s breast may provide an alternative explanation for the protective effect of breast feeding on incidence of breast cancer[79]. Likewise, release of estrogen disrupting chemicals from storage could change the microenvironment for “dormant niches”[7]causing renewed proliferation.
Research is now needed to answer outstanding questions concerning the effects of long-term exposure to low doses of chemicals and the effects of complex mixtures of chemicals[80]. Since estrogen disrupting chemicals are widely measurable in human tissues and some can bioaccumulate with age, then it follows that breast cells are exposed long term which requires long term cell culture modelling for further understanding. This is especially poignant in the acquisition of increased migratory and invasive properties described above which generally took weeks rather than days to develop. THe effects of mixtures of chemicals each at low dose will be more difficult to resolve because personal lifestyle choices will inevitably result in differing chemical contents between individuals. Furthermore, due to the additive and complementary mechanisms of estrogen disrupting chemicals[81], different mixtures may have the same outcomes which makes tracing individual culprit chemicals impossible. However, if specific environmental exposures can be uncovered, then avoidance would be a good strategy for prevention. In 2001, I proposed a hypothesis that regular application of constituents of underarm cosmetics might play a role in the rising incidence of breast cancer if sufficient of the constituent chemicals were absorbed either from long-term exposure on the skin (these products are not washed off) or from damaging the skin by shaving prior to cosmetic application[82,83]. THis was proposed partly due to the disproportionately high incidence of breast cancer in the upper outer quadrant of the breast which is coincidentally also the site of application of these chemicals. Many years on, it is now known that many of the constituent chemicals can be absorbed through even intact skin after a single application[84,85]and that they are measurable in human breast tissue or human milk (see above for references). Published studies have linked the levels of some of these estrogen disrupting chemicals in the body tissues with personal care product usage[86,87]. However, it is also clear that these chemicals are getting into many body tissues other than breast and therefore could be expected to create distant estrogenic microenvironments which could influence not only the growth of the primary tumour but also growth of metastatic tumours. If excessive use of personal care products is involved in the development of metastatic breast cancer, then reduction or cessation in use could provide a prevention strategy.
DECLARATIONS
Authors’ contributions
This review was written solely by the author and reflects the author’s views.
Availability of data and materials
Not applicable.
Financial support and sponsorship
None.
Conflicts of interest
THe author declares that there are no con flicts of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
© THe Author(s) 2019.
 Journal of Cancer Metastasis and Treatment2019年7期
Journal of Cancer Metastasis and Treatment2019年7期
- Journal of Cancer Metastasis and Treatment的其它文章
- Radiotherapy of brain metastases from small-cell lung cancer: standards and controversies
- Diagnosis and management of brain metastases:an updated review from a radiation oncology perspective
- Peptide nucleic acid-based targeting of microRNAs:possible therapeutic applications for glioblastoma
- Determination of cytokine regulated glycan expression by using molecularly imprinted polymers targeting sialic acid
- Monoclonal antibodies to the exon 18 encoded moiety of NCAM
- Syndrome of inappropriate antidiuresis in prostate adenocarcinoma with neuroendocrine differentiation: a case report and literature review
