Eosinophils in the gastrointestinal tract and their role in the pathogenesis of major colorectal disorders
Alexandre Loktionov
Abstract Eosinophils are currently regarded as versatile mobile cells controlling and regulating multiple biological pathways and responses in health and disease.These cells store in their specific granules numerous biologically active substances (cytotoxic cationic proteins, cytokines, growth factors, chemokines,enzymes) ready for rapid release. The human gut is the main destination of eosinophils that are produced and matured in the bone marrow and then transferred to target tissues through the circulation. In health the most important functions of gut-residing eosinophils comprise their participation in the maintenance of the protective mucosal barrier and interactions with other immune cells in providing immunity to microbiota of the gut lumen. Eosinophils are closely involved in the development of inflammatory bowel disease (IBD),when their cytotoxic granule proteins cause damage to host tissues. However,their roles in Crohn's disease and ulcerative colitis appear to follow different immune response patterns. Eosinophils in IBD are especially important in altering the structure and protective functions of the mucosal barrier and modulating massive neutrophil influx to the lamina propria followed by transepithelial migration to colorectal mucus. IBD-associated inflammatory process involving eosinophils then appears to expand to the mucus overlaying the internal gut surface. The author hypothesises that immune responses within colorectal mucus as well as ETosis exerted by both neutrophils and eosinophils on the both sides of the colonic epithelial barrier act as additional pathogenetic factors in IBD. Literature analysis also shows an association between elevated eosinophil levels and better colorectal cancer (CRC) prognosis, but mechanisms behind this effect remain to be elucidated. In conclusion, the author emphasises the importance of investigating colorectal mucus in IBD and CRC patients as a previously unexplored milieu of disease-related inflammatory responses.
Key words: Eosinophils; Eosinophilopoiesis; Gut lamina propria; Colorectal mucus;Normal human small intestine; Normal human colon; Inflammatory bowel disease;Crohn's disease; Ulcerative colitis; Colorectal cancer S-Editor: Ma RY L-Editor: A E-Editor: Liu JH
INTRODUCTION
The eosinophil was first described in the middle of XIX century when these granulocytes displaying a strong affinity to acidophilic dyes producing red staining of their granules were first identified. The honour of discovering and characterising eosinophils belongs to Paul Ehrlich, who also made first assumptions regarding their biological significance[1]. For many decades eosinophils were regarded as terminally differentiated end-stage cells of innate immunity exerting cytotoxic effector defence against parasitic helminths, but also capable of causing damage to host tissues in allergic conditions[2-5]. That simplistic paradigm had to be fundamentally revised once recent research advances revealed numerous previously unknown eosinophil functions, comprising inflammation control, epithelial barrier maintenance,participation in tissue remodelling and linking innate and adaptive immunity[2-5].Nonetheless, the whole range of roles played by these granulocytes in health and disease remains to be elucidated.
This review focuses on eosinophil action in the distal gastrointestinal tract, both in the normal physiological conditions and in pathology, especially in the context of inflammatory bowel disease (IBD) and colorectal cancer (CRC). The declared task looks timely since eosinophils, despite being recognised as important players in both gut homeostasis maintenance and colorectal disorder pathogenesis, are often overlooked when disease mechanisms are considered. It is remarkable that none of recent comprehensive reviews on the pathogenesis of either IBD[6-10]or CRC[11-15]even mentions possible eosinophil involvement in these complex processes. The author decided to focus on analysing literature describing research in humans, but experimental modelling, especially the use of genetically modified mice is one of the key sources of new information in the field. For this reason, selected experimental studies are considered as well, but caution is always required when results obtained in murine models are extrapolated to humans.
EOSINOPHIL DEVELOPMENT AND MATURATION
Eosinophils are continuously generated in the bone marrow from pluripotent CD34+stem cells, and it was presumed that their development proceeds through the granulocyte/macrophage progenitor (GMP) in mice and the common myeloid progenitor (CMP) in humans[2,16-19]. However, using a mouse model, Drissen et al[20]have recently identified a distinct myeloid differentiation pathway characterised by GATA1 expression and giving rise to eosinophils, mast cells, megakaryocytes and erythroid cells, but not to monocytes, neutrophils and lymphocytes. In any case, it is generally accepted that the control of eosinophilopoiesis is exerted on the one hand by transcription factors produced internally by the developing cells of the eosinophil lineage, but on the other hand by cytokines secreted predominantly by other immune cells (Figure 1).
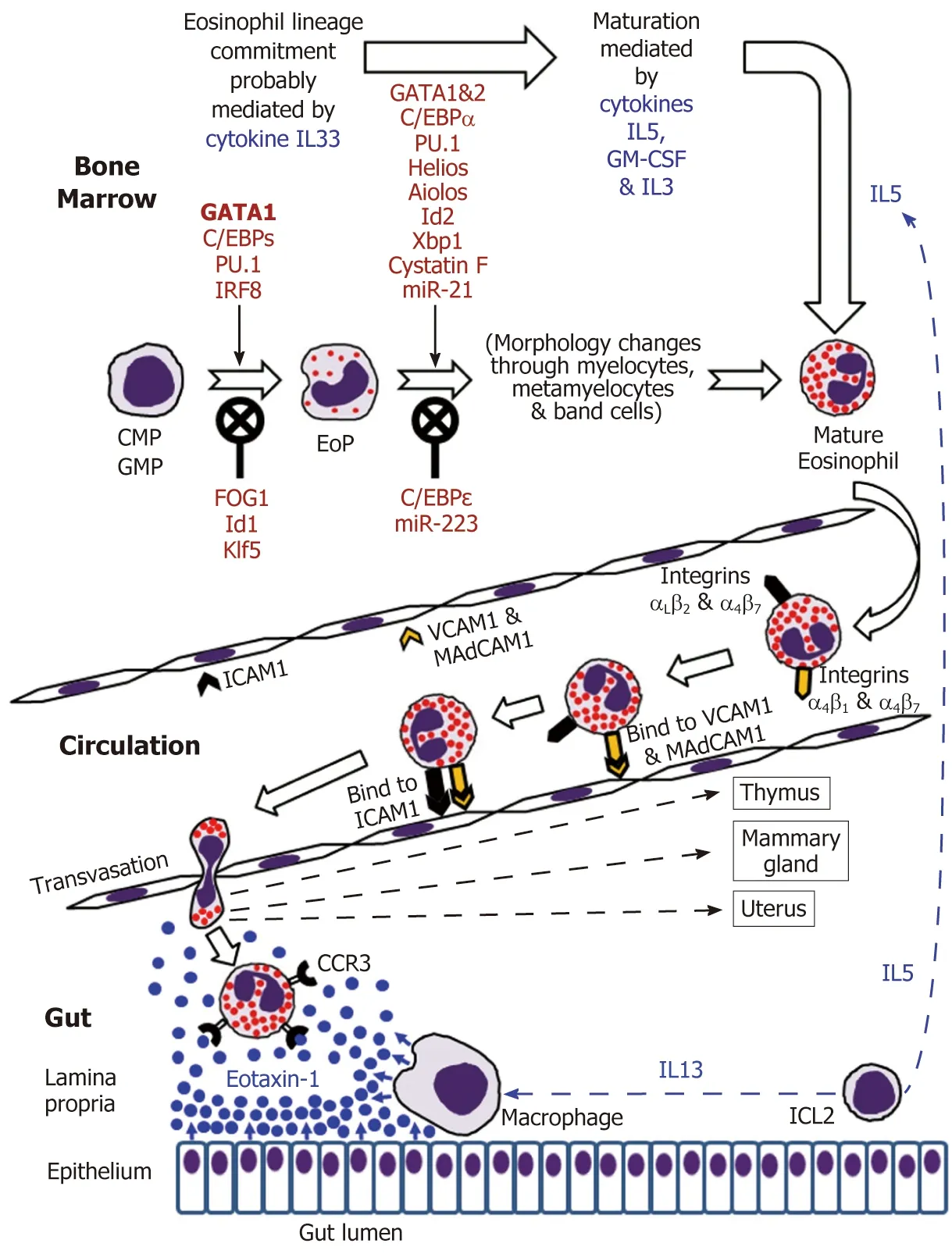
Figure 1 Eosinophil development in the bone marrow followed by mature eosinophil appearance in the circulation and eventual migration to the gut in the normal conditions.
The analysis of cell transcriptome changes during eosinophil lineage commitment followed by maturation has revealed a marked development-related increase in the number of expressed genes[21]. Early stages of this process appear to be regulated by cytokine IL33[22]and are driven at the cellular level by a few transcription factors,GATA1 being essential for eosinophil lineage commitment. Figure 1 illustrates the necessity of the interplay between pro-differentiation transcription factors including GATA1 (the key element), C/EBPs, PU.1 and IRF-8[16-19]and inhibitory influences of FOG1, Id1 and Klf5 in generating eosinophil-restricted precursor (EoP) cells[16-19,23,24].Importantly, the acquisition of surface receptor IL5Rα occurs at the EoP stage[25], thus making EoP cells responsive to the external signalling by IL5, an eosinophil-targeting cytokine secreted by type 2 innate immune cells (ILC2)[26]. Indeed, IL5, IL3 and GMCSF are recognised as the three cytokines regulating eosinophil proliferation,maturation and functional activities in homeostasis and pathology[2,16,18]. Their stimulating effect during eosinophil maturation is modulated by influences of several transcription factors as well as protease activity regulator cystatin F and regulatory miRNAs (Figure 1)[16-19,21,23,27,28]. It is notable that terminally differentiated mature eosinophils produced in the bone marrow have fully formed specific granules essential for the functions of these cells (see below).
DISTINCTIVE FEATURES OF MATURE EOSINOPHILS
Morphological characteristics
Mature human eosinophils can be easily morphologically distinguished by their bilobed nuclei and the presence of specific cytoplasmic granules stained red by eosin.These specific (also called crystalloid, secretory or “secondary”) granules are unique organelles that consist of a dense central crystalloid core and an outer matrix surrounded by a trilaminar membrane[4,29,30]. Although earlier publications sometimes mention the existence of both large “primary” granules lacking the crystalloid core and rich in Charcot-Leyden crystal protein and “small” granules, recent studies suggest that the coreless large granules are simply immature specific granules,whereas the “small” granules are membrane-bound vesiculotubular structures associated with eosinophil secretory activity[31]. Thus, there is only one granule type present in human eosinophils, and these specific granules store pre-formed biologically active substances comprising cytotoxic cationic proteins, cytokines,growth factors, chemokines and enzymes[4,32,33]. Other eosinophil-specific organelles are lipid bodies[34]charged with the synthesis of lipid mediators of inflammation(cysteinyl leukotrienes, thromboxanes and prostaglandins) and pleiomorphic vesiculotubular carriers (eosinophil sombrero vesicles)[35]. Like all somatic cells,eosinophils also have mitochondria, endoplasmic reticulum and Golgi bodies.
Arsenal of secretory substances and cell surface markers expressed by eosinophils
Specific granule formation and maturation associated with the accumulation of cationic granule proteins is essential for eosinophil development, function and survival[4,5,32,33]. Table 1 presents key biomolecules secreted by human eosinophils, and it is evident that, in addition to the well-known cationic proteins, these cells contain pre-formed stores of numerous regulatory molecules including cytokines, chemokines, growth factors and enzymes. Studies in murine models also demonstrated that parallel de novo synthesis of cytokines occurs in mature eosinophils[40-42], however the relationship between pre-formed and de novo synthesised regulatory factors remains to be elucidated.
In addition to secretory substances, eosinophils express a considerable number of surface markers comprising receptors for adhesion molecules, cytokines, chemokines,growth factors, lipid mediators as well as pattern recognition receptors (PRRs) and Fc receptors. Table 2 lists hitherto identified eosinophil surface markers. Notably, stores of receptor molecules were also identified within the specific granules[55], and membrane receptors for cytokines and chemokines located on the surface of these granules enable them to act as receptor-mediated secretory organelles even extracellularly[56,57]. The abundance of receptors and cell surface-associated molecules defines eosinophil ability of participating in an extremely wide range of physiological and pathological processes. More details on eosinophil surface markers can be found in a few recent reviews (Rosenberg et al[5], Ravin et al[43]and Gangwar et al[44]).
Types of secretion/degranulation observed in human eosinophils in relation to their functional activities
The current view on the versatility of eosinophil action in health and disease was concisely formulated by Lee et al[3]in their LIAR (Local Immunity And/or Remodelling/Repair) hypothesis. Expanding this notion, eosinophil functions can be classified into the following four categories: (1) Terminal effector functions; (2) Maintaining homeostasis and supporting tissue repair/remodelling; (3) Immunomodulatory role; and (4) Cooperative interactions with other immune cells[4]. Essentially,all functional activities of eosinophils in both normal and pathological conditions are exerted through the secretion of their specific products. Although classical or compound exocytosis implying granule fusion with the plasma membrane followed by extracellular release of entire granule contents[58]may be occasionally employed by eosinophils attacking multicellular helminths[33,59], this secretory mechanism is rarely observed during inflammatory and allergic responses, when immediate but selective release of pre-formed granule-stored factors is required. Extensive investigation of eosinophil degranulation modes has shown that either piecemeal degranulation(PMD) or cytolysis followed by whole granule release clearly prevail in most situations associated with human disease[33,59,60].
PMD is characterised by stimulus-dependent (receptor-mediated) differential packaging of selected granule-derived proteins into secretory vesicles that are then transported to the cell surface and expelled through it. During this process specific granules are partially emptied but otherwise remain intact[60]. Being highly selective and rapid, PMD is typically employed for cytokine secretion by eosinophils. It is the most common secretion type observed in the context of inflammation and allergy in humans[5,33,59,60].
Recent reports imply that cytolysis or primary lysis of human eosinophils[61]is the second most frequently observed degranulation mode in inflammatory and allergic conditions[33,60,61]. Cytolysis is now regarded as a regulated mechanism of rapid cell death (distinct from apoptosis and necrosis) that is characterised by chromatin decondensation and dissolution of nuclear and plasma membranes as well as the releaseof intact specific granules retaining their functionality[33,56,57,59]. It was later demonstrated that eosinophil cytolysis is often accompanied by the formation of“extracellular traps”, i.e., web-like nets composed of extruded histone-coated strands of DNA and specific granules released from the same lysed cells[62-64]. The latter phenomenon is similar to the formation of bactericidal NETs (neutrophil extracellular traps) by neutrophils first described in 2004 by Brinkmann et al[65]and often defined as a unique form of cell death initially called NETosis[66]. It is, however, more appropriate to call this phenomenon ETosis since it is not neutrophil-specific and was observed in eosinophils[62-64], mast cells[67], basophils[68], monocytes[69], macrophages[70]and even B and T lymphocytes[71]. Moreover, an alternative, “non-lethal”, mechanism of extracellular DNA trap generation by eosinophils was described by Yousefi et al[72],who observed a catapult-like release of mitochondrial DNA from eosinophils remaining alive. The released DNA formed extracellular traps that also contained eosinophil granule proteins apparently secreted through PMD[72]. In the context of eosinophil effector functions, the association of released (by either mechanism) DNA
fibres and functional specific granules (or their proteins) certainly provides a formidable defensive weapon that can be effectively used against infective agents(bacteria, viruses, fungi) or multicellular parasites (helminths)[64]. However,extracellular traps generated by eosinophils are highly cytotoxic and can damage host tissues. Both defensive and host-damaging effects of eosinophils are especially important for barrier tissues including the gut and will be discussed in more detail in further sections of this review.
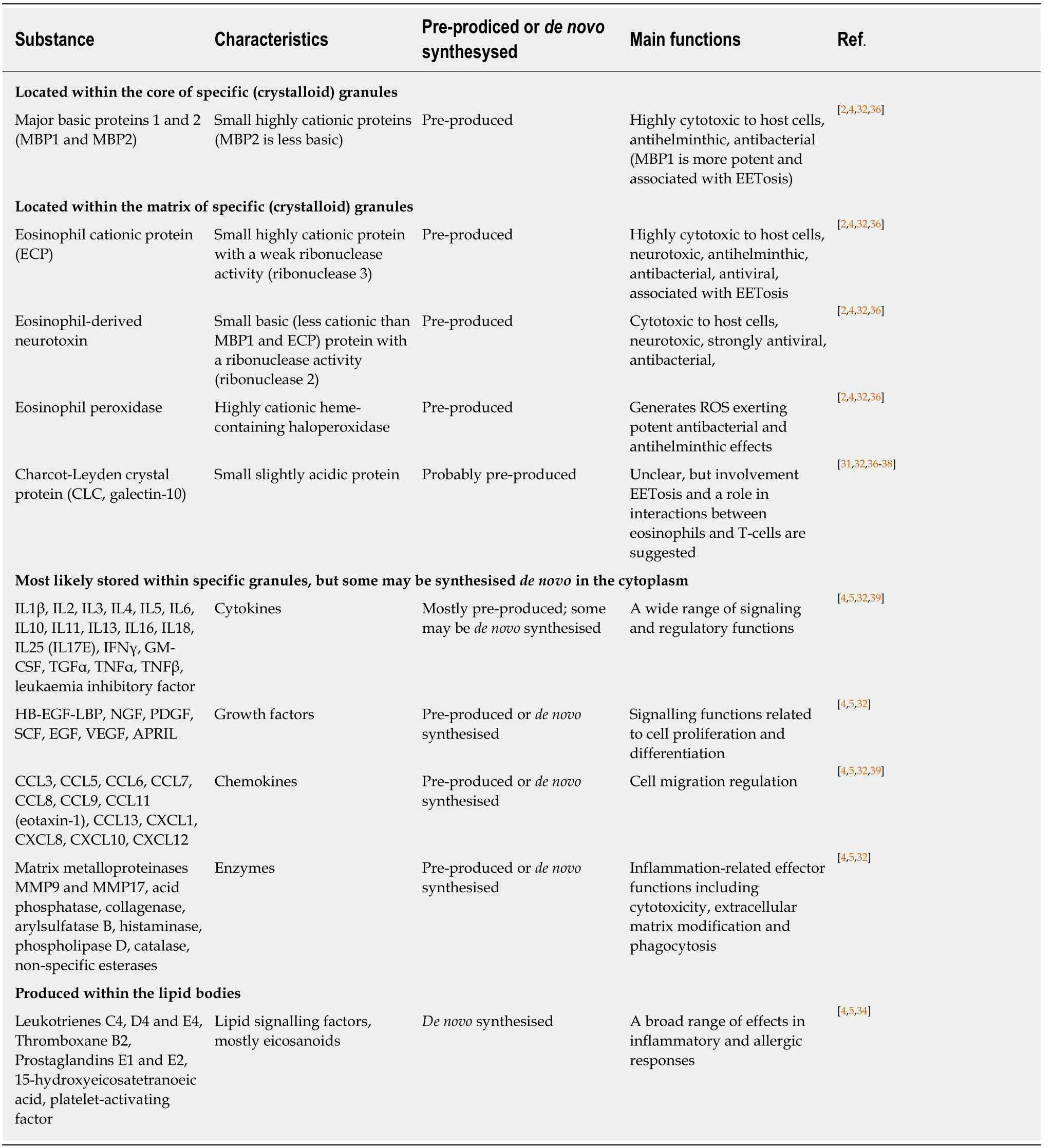
Table 1 Secretory substances produced by eosinophils

Table 2 Cell surface markers expressed by eosinophils
MIGRATION OF MATURE EOSINOPHILS TO THE GUT
According to generally accepted views, upon maturation in the bone marrow eosinophils enter the circulation and migrate to the gastrointestinal tract, their accumulation in the gut mucosa commencing during embryonal development[73,74].
IL5 and possibly GM-CSF stimulate the release of eosinophils from the bone marrow into the circulation[2,16,32]. Mouse eosinophils can stay in circulation for up to 36 h[75], and this estimate has later been shown to be close to their average 25-h intravascular presence in humans[76]. Eosinophil trafficking from the circulation to the gut and other peripheral target tissues (such as thymus, uterus and mammary gland in the normal conditions) is a complex multi-step sequence of events. As for all leukocytes, it comprises tethering, rolling, adhesion and transendothelial migration followed by polarisation and amoeboid movement in the interstitial space[73,77-81].Alongside chemokine and cytokine signalling, the interaction between adhesion molecule receptors expressed by eosinophils and corresponding adhesion proteins of endothelial cells is crucially important for efficient eosinophil trafficking. Although leukocyte extravasation is better studied in inflammatory conditions, it can be assumed that in health the initial adherence (tethering) of eosinophils to endothelial cells and the initiation of rolling motion are selectin-dependent, whereas further rolling, firm adhesion and transendothelial migration are mediated by integrins[77-79].In the context of eosinophil homing in the intestinal lamina propria (schematically shown in Figure 1), binding of the integrins α4β7and α4β1expressed by eosinophils to endothelial adhesion molecules MAdCAM1 and VCAM1 respectively is especially important, and interactions of αLβ2and αMβ2with ICAM1 appear to lead to the eventual transmigration from capillaries[48,82,83]. Further interstitial migration of eosinophils to their destination in the lamina propria is believed to be governed mostly by chemokine eotaxin-1 interacting with its CCR3 receptor on the surface of eosinophils[2,74]. Eotaxin-2 and eotaxin-3 may also contribute to the chemotaxis, but appear to be less eosinophil-specific[2,74]. In the human colon eotaxin-1 concentration gradient directing eosinophil migration depends on the secretion of this chemokine by intestinal macrophages and epithelial cells[84-86], while IL13 produced by ILC2 cells[26]stimulates macrophage eotaxin-1 expression (Figure 1).
EOSINOPHILS IN HEALTHY GASTROINTESTINAL TRACT
Mouse experiments demonstrated that eosinophil homing in the gut occurs in the foetal life, i.e., independently of later intestinal colonisation by microbiota[73]. In the normal human gastrointestinal tract the presence of eosinophils increases in the distal direction (oesophagus < stomach < small intestine < colon) reaching its peak in the caecum and ascending colon[87-89]. As already mentioned, in the normal gut eosinophils are primarily located in the lamina propria of the mucosa rather than in the surface epithelium[82,90].
Unfortunately, functions of human gastrointestinal eosinophils in health remain poorly investigated, mouse models being the main source of available information.Still, recent progress in this complex field allows making some generalisations.
Eosinophil presence in the gut was previously believed to simply constitute an effector element of the innate host defensive barrier, but it is now becoming clear that resident intestinal eosinophils continuously monitor and modulate complex immune responses and tissue remodelling throughout the huge surface of the gastrointestinal tract[3]. It is remarkable that, unlike their circulating counterparts, gastrointestinal eosinophils exhibit an activated phenotype suggesting permanent functional activity[4,91]. Participation in the following physiological mechanisms is currently attributed to these gut-dwelling cells: (1) Maintenance of gastrointestinal mucosal barrier function; (2) Provision of immunity to pathogens present in the gut lumen; (3)Interactions with the enteric nervous system; (4) Linking innate and adaptive immunity[4]. These functions are discussed in more detail hereafter.
Eosinophil participation in the maintenance of gastrointestinal mucosal barrier function
The intestinal epithelium acts as a uniquely important body interface with the environment. In addition to its physical barrier function that prevents underlying tissues from contacting harmful microbiota and dietary antigens of the gut lumen, this epithelium regulates the selective absorption of nutrients, water and electrolytes[92].The epithelial surface throughout the gut is covered by the glycocalyx of enterocytes and colonocytes[93]and abundant mucus rich in mucin 2 (MUC2) produced by goblet cells. The utmost importance of this mucus became evident only recently, and it is now established that the structure of the mucus layer throughout the intestine is not uniform. The small intestine has a single and relatively loose layer of mucus populated by microbiota, whereas the colon has a two-layered system with a dense inner layer firmly attached to the mucosal surface and an outer layer permeable for bacteria (Figure 2)[94,95]. It is remarkable that this structure essentially excludes any direct contact of bacteria with the colonic epithelium. The protective role of the mucus is further enhanced by the presence of antibacterial substances, such as α-defensins and lysozyme secreted by Paneth cells of small intestinal crypts[96]and IgA produced by plasma cells of the lamina propria[97,98].
Interestingly, it has recently been shown that intestinal mucus layer maintenance depends on eosinophil presence in the lamina propria since eosinophil-deficient mice had significantly decreased numbers of mucus-secreting goblet cells in the small intestine[98]. This is not surprising since eosinophils are known to directly induce mucin production in airway epithelial cells by activating EGFR cascade[99]. Moreover,although possible interactions between eosinophils and Paneth cells remain obscure,the transcription factor Xbp1 is important for the development of the both cell types[27], and, like Paneth cells, human eosinophils were demonstrated to produce αdefensins[100]. It was also reported that eosinophils are required for maintaining mucosal IgA production by plasma cells in the lamina propria[97,98,101], probably through mechanisms involving IL1β[98]and TGFβ[97,100]signalling.
Taken together, this information suggests that eosinophils are intimately involved in the maintenance of the protective intestinal mucus in the normal conditions.
Eosinophil participation in the provision of immunity to pathogens in the gut lumen
The immune system of the gut is an extremely complex entity, analysis of which is beyond the scope of this review, but eosinophil impacts are highlighted herein. It is obvious that in the normal homeostatic conditions immune surveillance focused on sampling and assessing luminal antigens occasionally contacting the mucosal surface is of utmost importance. The gut-associated lymphoid tissue (GALT) is believed to be responsible for luminal antigen sampling. In the small intestine this function is primarily associated with microfold (M) cells[102-104]located in the epithelium overlaying Peyer's patches (Figure 2) and capable of communicating with immune cells comprising B lymphocytes, T lymphocytes, macrophages and dendritic cells. Mcells are also believed to be present in the epithelium overlaying lymphoid follicles in the colon[103], but their location and functions in the normal human colon remain very poorly investigated. M-cells exert luminal antigen transcytosis followed by antigen transfer to the relevant immune cells of the lamina propria, but alternative antigen capture mechanisms appear to exist as well. Dendritic cells were shown to sample bacterial antigens by extending their dendrites to penetrate the epithelium and reach the lumen[105,106]. In addition, goblet cells can deliver low molecular weight intestinal antigens to the underlying dendritic cells of the lamina propria[107]. The latter mechanism shown for the normal small intestine[107]may be especially important for the colon, where it becomes activated only when mucus barrier, luminal microbiota balance or microbial sensing by goblet cells are disturbed[108]. Given that the stratified mucus layer securely protects colonic epithelium from any contact with gut contents in the normal conditions, it is likely that immune cells located in the colonic lamina propria may be completely unaware of luminal antigens until the protective barrier is damaged[108]. Although the role of eosinophils in luminal antigen recognition and presentation at baseline remains poorly investigated, it should be noted that these granulocytes promote the development of Peyer's patches[98]and are known to be closely involved in regulating dendritic cell activation and migration[109]. In addition,intestinal eosinophils can express antigen presentation-associated markers, including MHCII and CD80[91]as well as activating receptor FcγRIII[110]. Their possible antigenpresenting function has not been demonstrated in the normal gut and remains to be investigated.

Figure 2 Schematic representation of eosinophil interactions with other cells and tissues in the small intestine (A) and colon (B). Black asterisks indicate gut microbiota. Although one M-cell is shown in the colon, little is known about the presence of M-cells in healthy human colon.DC: Dendritic lells; E: Eosinophils; Fb:Fibroblasts; GobC: Goblet cells; L: Lymphocytes; M: M-cells; Mcr: Macrophages; Ne: Nerves; PanC: Paneth cells; PC: Plasma cells.
Eosinophil role in the provision of interactions with the enteric nervous system
Eosinophil influences on the nervous system were extensively studied in the context of asthma and allergic respiratory inflammation[111,112]. It is also known that human eosinophils can produce nerve growth factor in abundance[113]. Eosinophil-nerve interactions in the gut were demonstrated experimentally[114], and accumulating evidence indicates that eosinophil impact on the enteric nervous system may often be exerted through mast cells residing in the lamina propria[115]. Indeed, eosinophils and mast cells are often found in close proximity to each other. Physical interaction between them can induce a hyperactivation state accompanied by soluble mediator release[116,117]. Mast cells and eosinophils in the gut are often located near sensory nerve fibres and are known to be involved in the pathogenesis of functional gastrointestinal disorders accompanied by motility changes, hyperalgesia and diarrhoea[115,118].Concerted action of eosinophils and mast cells can produce multiple effects in addition to interactions with the nervous system, being among major factors in allergy and responses to infections[119], which are beyond the scope of this review.
Eosinophil role in linking innate and adaptive immunity in the intestine
It is now recognised that eosinophils can modulate T cell-mediated immune responses, owing to their ability to rapidly produce cytokines, chemokines and growth factors[3-5], but little is known about their regulatory functions in the normal intestine. Although eosinophils are able to secrete cytokines associated with both Th1 and Th2 cells[86], in the gut they appear to be primarily associated with Th2 immunity,being producers of Th2-inducing cytokines IL4 and IL13[120]. It is remarkable that IL13 was detectable in a considerable fraction of these cells in the normal human duodenal lamina propria[121]. Recent demonstration of significantly increased Th1 cell presence in the gut of eosinophil-deficient transgenic mice corroborates organ-specific Th1 immunity suppression by these granulocytes[122].
It is assumed that eosinophils may regulate the magnitude and Th2 polarisation of immune responses through interactions with B and T lymphocytes[86]. However, there are many unanswered questions regarding types of cell-mediated immune responses and corresponding cytokine profiles, and a view advocating the existence of three rather than two types of effector immunity has recently emerged[123]. Only further intense research in this dynamic area will lead to better understanding of eosinophil interactions with other immune cells in the human gut.
Clearly, the four topics highlighted above do not entirely cover all activities of intestinal eosinophils. For instance, experimental studies have revealed eosinophil influences on smooth muscle cells[74,124], fibroblasts[74,125]and capillary endothelium[74],but these roles remain to be investigated for the normal human gut.
EOSINOPHIL IMPACT IN THE PATHOGENESIS OF MAJOR COLORECTAL DISEASES (IBD and CRC)
The author opted not to address eosinophil-associated gastrointestinal disorders(EGID) comprising eosinophilic oesophagitis, eosinophilic gastritis, eosinophilic enteritis and eosinophilic colitis as well as gastrointestinal manifestations of hypereosinophilic syndromes. These relatively rare conditions were extensively reviewed elsewhere[83,86,126,127]. The concluding part of this review is focused on two major colorectal diseases: IBD and CRC.
Eosinophils in the pathogenesis of IBD
IBD is represented mainly by two major conditions: ulcerative colitis (UC), which affects exclusively colonic mucosa[10], and Crohn's disease (CD), a transmural asymmetrical inflammation that can involve the entire gastrointestinal tract[9]. These diseases are highly prevalent all over the world[128-131]. Both UC and CD are chronic relapsing disorders with complex and not entirely understood mechanisms of development. IBD etiopathogenesis is, however, believed to include genetic predisposition, environmental/microbial impacts, intestinal barrier dysfunction and dysregulation of the mucosal immune system of the gut[6-10,132]. The latter two pathogenetic components can certainly be influenced by eosinophils, the abundance of which, correlating with disease severity, is well documented in the gut mucosa of patients with UC[133-140]and some CD cases[135,137,141,142]. The increased presence of eosinophils in the mucosa of these patients apparently results from an enhanced production of eotaxin 1 in the lamina propria[84,143-145]by colonocytes[84,136], macrophages[84,145]or B lymphocytes[144]. Furthermore, Manousou et al[146]described an elevated expression of eotaxin receptor CCR3 in colonic biopsy samples from UC rather than CD patients. Although the authors interpreted this finding as a sign of the accumulation of CCR3-expressing T cells, it is likely that lamina propria eosinophils expressing the same receptor could be at least equally responsible for this UCassociated change. Conversely, serum eotaxin levels were reported to surge in active IBD[141,147,148], but the increase looked more pronounced in CD patients compared to UC cases[147,148]. The contrast between UC and CD becomes even more evident in view of different patterns of colonic eosinophil activation described by Lampinen et al[84,136,137,145,170,186,188]and indicating that in disease remission activated eosinophils persisted in the lamina propria of UC, but not CD patients[136,137].
The observed differences in colonic eosinophil presence and activity between the two IBD types are not surprising because it is traditionally accepted that CD pathogenesis, which has a stronger genetic component[6], is dominated by Th1 cytokine profile (combined with Th17 influences)[149-152], whereas Th2 immune response tends to characterise UC[150-153]. Given recent advances in the understanding of immune response types, involved cell populations and cytokine profiles[123,154,155],this straightforward division may now look simplistic, but the association of eosinophils with Th2 immunity is well proven[120]. Therefore, their role in UC pathogenesis looks more evident and easier to explain.
Before further discussing the role of eosinophils in IBD development it will be useful to briefly address gut barrier-related aspects of disease onset. Although numerous factors admittedly contribute to IBD pathogenesis[6-8,156], the precise mechanism of disease initiation remains elusive. Multiple lines of evidence indicate that in most cases IBD can be triggered by an initial contact of the gut microbiota with the mucosal immune cells followed by the development of inadequate immune responses. It is probable that preconditions for this initial contact are associated with functional deficiencies of the protective barrier of the gut mucosa, and they are likely to differ between CD and UC. Indeed, the loose mucus layer of the small intestine may be easily penetrated by microorganisms that then directly contact the epithelium,particularly mucosal M-cells[102-104]of the ileum. Such events can be facilitated by αdefensin deficiency caused by Paneth cell dysfunction that is frequently observed in CD patients, including those genetically predisposed to the disease[157,158]. In contrast,M-cells in the normal colonic mucosa can hardly be reached by microbiota since two layers of mucus, especially the dense inner one[93-95], exclude any bacterial contact with the epithelium (Figure 2B). The protective role of the inner mucus layer is well illustrated by spontaneous colitis development in MUC2-deficient mice[159,160]. Bacterial penetration through the inner mucus layer was observed in UC patients[161], but it is not entirely clear what triggers the initial change of inner mucus layer properties.Mouse models suggest MUC2 secretion deficiency[159,160]or goblet cell depletion[162]as probable causes, and goblet cell numbers in UC patients, indeed, tend to be reduced[163]. Some authors believe that unresolved endoplasmic reticulum stress and the unfolded protein response are early events leading to goblet cell disfunction and mucus layer impairment in UC[164,165]. Altered eosinophil behaviour may well be associated with these phenomena, but this possibility remains to be investigated.Alternatively, the inner colonic mucus layer can be primarily damaged by mucusdegrading bacteria of the gut lumen[166]. The latter process may potentially be modulated by dietary factors since it was experimentally demonstrated that dietary fibre deficiency leads to switching of the gut microbiota on using mucus glycoproteins as a nutrient source and eventual erosion of the mucus barrier[167]. All the pathogenetic components discussed above may contribute to IBD initiation in humans, but further research in the area is obviously needed.
Whatever scenario causes IBD initiation, there is little doubt that bacterial antigen interaction with the gut-associated lymphoid tissue triggers complex cascades of inadequate immune responses leading to disease development. In these circumstances, activated eosinophils present in the lamina propria predominantly start acting as effector cells, excessive protective response of which can cause serious damage to the host through several mechanisms. Activated eosinophils accumulating in the gut of IBD patients[133-142]have an extended lifespan[168], and their degranulation leads to a massive release of both cytotoxic granule proteins and pro-inflammatory cytokines.Gut epithelium is one of the key targets of cytotoxic eosinophil proteins as it was demonstrated that MBP alters colonic epithelium barrier function[169]. Another mechanism of eosinophil contribution to colonic barrier dysfunction in UC involves muscarinic receptors expressed by these granulocytes. Wallon et al[170]showed that cholinergic signals received by the muscarinic receptors caused corticotropinreleasing factor (CRF) production by eosinophils, and CRF induced degranulation of neighbouring mast cells that led to an increase in mucosal barrier permeability.Concerted action of eosinophils and mast cells may have multiple endpoints in IBD as the both types of cells are important sources of cytokines comprising TNFα that,intriguingly, induces M-cell appearance in the mouse colon during inflammation[171].Moreover, cooperation between eosinophils and mast cells was demonstrated in CDassociated fibrosis development at later stages of the disease[172]. Inflammation intensity can also be aggravated by human gut eosinophils through blocking antiinflammatory interleukin 22 (IL22)[173]by overproducing IL22-binding protein in both CD and UC patients[174].
Interactions of gut eosinophils with other immune cells, especially lymphocytes,are very complex and poorly investigated in IBD patients. There is no room for discussing all of them here, but crosstalk between eosinophils and neutrophils deserves to be mentioned. Neutrophils are not present in the lamina propria in the normal conditions but are rapidly attracted there when inflammatory response develops (Figure 3). It is believed that chemokines, especially CXCL8, produced by gut epithelium in inflammation trigger neutrophil chemotaxis[175]. Interestingly,eosinophil impact in this process is now becoming evident since they can synthesise CXCL8[176]. Also, eosinophils were demonstrated to cooperate with colonic epithelium in producing a wider range of neutrophil chemoattractants[177]. In conclusion of discussing the role of lamina propria eosinophils in IBD it needs to be noted that the presented facts are mostly related to human disease. There is a considerable body of additional information obtained in murine models which has been reviewed elsewhere[178].
Until recently, pathogenetic mechanisms of IBD were considered only at the level of events occurring within the gut wall. However, it is now becoming evident that gut mucus layer presents another, poorly investigated but potentially highly important,battlefield for innate immune responses. Although massive neutrophil influx to the mucosa, often leading to crypt abscess formation, is recognised as a hallmark feature of IBD, the importance and mechanisms of immune cell migration through mucosal epithelia remain poorly understood and insufficiently investigated[179,180].Transepithelial neutrophil migration involving a chain of molecular events that include initial attachment to the basal surface of epithelial cells, movement through paracellular space (passing by desmosomes, adherens junctions and tight junctions)and eventual contact with the apical membrane of the epithelium is relatively well understood[179,180]. Moreover, it is known that following transmigration neutrophils release MMP9-rich microparticles that disrupt epithelial junctions facilitating further transmigration[182]. Rapid eosinophil migration through tracheobronchial epithelium in experimental conditions was also reported[181], but remains unexplored in the gut.The process is, thus, well-defined, but the final destination of cells crossing the epithelial barrier remains obscure, being often indicated simply as “gut lumen”[179,180].Neutrophil-associated markers of inflammation are, indeed, easily detectable in faeces of most patients with active UC and CD, as the popularity of faecal diagnostic tests,particularly stool calprotectin, proves[183,184]. Similarly, the presence of eosinophil markers in stool or colorectal perfusion fluid of IBD patients was repeatedly reported[184-188]. It is, however, apparent that any cells or biomolecules leaving gut epithelium surface should first enter mucus barrier already discussed above, and only its occasionally separated fragments can be incorporated into the faecal matter. The author of the present review previously hypothesised that colorectal mucus retains these highly informative cells and molecules and can be conveniently used for diagnosing colorectal diseases[189]. Studies of our group convincingly demonstrated the abundance of inflammatory cells, predominantly neutrophils, in colorectal mucus collected from IBD patients either intrarectally[190,191]or non-invasively[192,193]. Common eosinophil presence in this material was also noted[191,193], especially in UC patients[193],and dramatically increased EDN levels were determined[191,194], typically with higher values in UC compared to CD[194]. Detailed cytological analysis of these samples demonstrated that colorectal mucus from patients with active IBD commonly contains not only huge amounts of neutrophils, but also eosinophils, macrophages,erythrocytes as well as occasional plasma cells, lymphocytes and basophils[193]. Most of these cells are viable and functionally active as our frequent observations of phagocytosis by neutrophils and macrophages indicate[193]. Furthermore, it appears that signs of ETosis were also present in colorectal mucus from IBD patients[193]. These findings allow hypothesising that gut mucus acts in IBD as a unique additional milieu, where immune responses expand from the mucosa. It is apparent that the abundance of active cells, especially granulocytes releasing contents of their granules,considerably loosens the inner mucus layer in the colon, thus making it both permeable for gut microbiota and facilitating further immune cell transmigration and movement through the mucus. These circumstances should favour antibacterial activity of the effector cells, but extensive collateral damage of host epithelium is highly likely. ETosis exerted by neutrophils, eosinophils and other immune cells[62-72]may be especially important in this context. Figure 3 reflects the author's opinion on the extent of inflammatory process in the human gut.
Protection from invading microbiota appears to be the main biological aim of ETosis since histones enveloping released DNA are antibacterial[195], and the addition of DNA strands may increase mucus viscosity, thus mechanically compensating for MUC2 degradation. Further antibacterial action is provided by granule proteins of both neutrophils[196,197]and eosinophils (Table 1). However, ETosis in the mucus,especially combined with the release of intact eosinophil granules, can seriously damage enterocytes or colonocytes. Eosinophil-derived extracellular DNA traps have already been shown to injure airway epithelium in chronic obstructive pulmonary disease[198]and chronic rhinosinusitis[199], where MBPs released from specific granules were especially toxic[200]. This phenomenon is still poorly investigated in relation to UC and CD, but interest in IBD-associated ETosis is emerging. In addition to our results discussed above, NET presence has been demonstrated in biopsy samples from IBD patients[201-203], notably within crypt abscesses[201], i.e., beyond the mucosa.Although the significance of immune responses occurring within colorectal mucus remains to be elucidated, it is impossible to exclude that eosinophil-generated extracellular DNA threads, loaded with entrapped specific granules that release cytotoxic cationic proteins, can cause a continuing colonocyte damage leading to sustained ulceration. This so far unexplored mechanism may constitute an important pathogenetic factor in UC and, to some extent, in colonic CD. On the other hand, it should not be forgotten that transepithelial migration of immune cells is a major factor in mucosal disease resolution, as demonstrated for airway diseases[204,205].Indeed, the presence of both inflammatory cells and biomarkers associated with them significantly decreased in colorectal mucus samples from successfully treated IBD patients[194]. It is, therefore, probable that gradual distal movement of colorectal mucus[189]creates favourable conditions for eliminating dead or obsolete immune cells from the surface of colonic epithelium if inflammation is successfully resolved.

Figure 3 Schematic representation of human colonic mucosa and overlaying mucus during inflammatory bowel disease (ulcerative colitis) flare-ups. Rapid influx of neutrophils results in severe neutrophil infiltration of the lamina propria. Further massive transepithelial migration of neutrophils and other immune cells (especially eosinophils in ulcerative colitis) eliminates mucus layer structure, enables bacterial contact with the epithelium,causes epithelial cell death, ulcer formation and bleeding. Mucus infiltration with neutrophils and eosinophils is accompanied by abundant ETosis and release of both granule proteins and free eosinophil granules. Active inflammation also induces M-cell appearance in the epithelium overlaying lymphoid follicles[171]. Cell images correspond to those used in Figure 2. Erythrocytes are presented by red circles. Small red dots correspond to free eosinophil granules.
The presented analysis of literature on eosinophil impact in IBD pathogenesis reveals that eosinophils are closely involved in this process through regulatory activities, interactions with other cells and tissues and effector functions that can often be excessive and damage the host. The impact of eosinophils appears to be especially important in altering the structure and protective functions of the mucosal barrier.The author also tried highlighting an interesting new research direction related to exploring poorly investigated immune responses occurring in the protective mucus layer of the gut. It is, however, apparent that our understanding of IBD pathogenesis,including eosinophil participation in it, remains fragmentary and needs further thorough investigation. For this reason, it would be premature to speculate on possible therapeutic interventions targeting eosinophils in IBD.
Eosinophils in the pathogenesis of CRC
CRC is one of the most frequent oncological conditions with estimated global figures of 1801000 new CRC cases and 861700 deaths due to this disease in 2018[206]. Although there are many good reviews addressing various aspects of CRC pathogenesis[11-15],possible role of eosinophils in this process is usually overlooked despite the existence of reports deserving attention and briefly discussed below.
Eosinophil infiltration is often observed in malignancies, but for different tumours it was reported as either prognostically favourable or unfavourable[3,4,207-210].Nevertheless, the presence of eosinophils in CRC patients is strongly linked with a decreased disease risk, better prognosis and extended patient survival. Indeed,elevated blood eosinophil counts were associated with a decreased CRC development risk[211]as well as better prognosis[212-214]. Eosinophil infiltration of colorectal tumours is a common phenomenon, and higher numbers of infiltrating eosinophils detected both in the tumour tissue[215-217]and peritumourally[218-220]were repeatedly shown to be prognostically favourable. Despite these seemingly cogent findings, the quoted descriptive clinical studies could not provide any direct evidence of anti-cancer eosinophil action, and possible mechanisms of CRC growth inhibition by eosinophils remain poorly understood.
Tumour-associated inflammation is currently recognised among hallmarks of cancer[11]. Eosinophil accumulation accompanying inflammation-related cancer cell death and proliferation in CRC is one of its components that probably reflects Th2 immune responses enhanced at the expense of Th1 immunity[3]. Besides, eosinophils are likely to stimulate tissue remodelling and tumour-related angiogenesis[3,221]. The latter MBP-modulated effect may in theory promote tumour growth, however only non-cytotoxic MBP concentrations enhanced angiogenesis in vitro[221], and significant eosinophil infiltration in CRC is likely to produce high MBP concentrations. Ellyard et al[222]argued that some components of Th2-driven inflammation in cancer can be associated with anti-tumour activity of CD4+Th2 cells collaborating with tumourinfiltrating granulocytes, especially eosinophils that exert regulatory functions. In any case, it is now becoming clear that there are several mechanisms driving eosinophil attraction to tumours and defining their influence on malignant tissue. In particular, it was demonstrated in vitro that eosinophil chemotaxis could be induced by necrotic,but not viable cells of neoplastic intestinal epithelium[223]. Experiments in a xenograft mouse model indicated that eosinophil infiltration developed rapidly, involved mostly tumour necrotic areas or capsule regions and was not associated with the presence of CD4+T cells[224], thus suggesting that eosinophil chemotaxis depended on tumour-derived factors, such as damage-associated molecular pattern molecules(DAMPs) including the nuclear protein high mobility group box 1 (HMGB1)[225].Notably, the presence of cell-free cytotoxic MBP was confined to the necrotic areas of tumours[224]. There are also reports describing expression of eosinophil attractant ecalectin (variant of galectin-9) by human colorectal carcinoma cell lines[226]and of eotaxin 1 in tumours resected from CRC patients[227]. The latter phenomenon was recently investigated further in a mouse model by Hollande et al[228], who found that IL33 expressed by tumour cells induced eotaxin 1 production that led to eosinophil recruitment and degranulation-dependent suppression of tumour growth[228].Interestingly, eotaxin 1 concentration is negatively regulated by serine protease DPP4,and treatment with DPP4 inhibitor sitagliptin was shown to result in an increase in eotaxin 1 level[228]. Sitagliptin is a drug already approved by the US FDA for hyperglycaemia treatment, and it may potentially be re-purposed as a new antitumour agent promoting tumoricidal action of eosinophils[228,229]. Coming back to IL33,its role as an influential modulator of early stages of eosinophil development[22], was noted in the beginning of this review, but it is also a potent eosinophil activator stimulating their degranulation[230]. Moreover, it was reported that IL33-deficient mice had gut microbiota dysbiosis and were highly susceptible to both colitis and colitisassociated cancer[231], which could also be related to impaired eosinophil-driven responses. Hence, multiple parallel pathways are likely to be involved in generating eosinophil infiltration of colorectal tumours and direct killing of malignant cells, a phenomenon already proven experimentally. In vitro studies by French investigators assessing tumoricidal activity of eosinophils against Colo-205, a human colon carcinoma cell line, have shown that this effect was mediated by eosinophil-produced ECP, TNFα and proteolytic granzyme A[232]. The same group later reported that eosinophil attachment to Colo-205 cells depended on interaction between adhesion molecules LFA-1 and ICAM-1 that was upregulated by IL18[233]. Direct tumoricidal effect of tumour-infiltrating degranulating eosinophils was also observed in model experiments using genetically modified mice[234]. It is, however, obvious that the tumoricidal action of eosinophils in vivo may involve multiple interactions with other immune cells. Notably, there is evidence that eosinophils can promote either Th2 or Th1 immune responses, depending on varying cytokine profiles[86,235]. Carretero et al[236]have recently shown in experiments with xenograft-bearing mice that tumour-homing eosinophils secreted chemoattractants that guided CD8+effector T cells to tumours,eventually causing tumour rejection. Thus, it is apparent that different immune response scenarios can be involved in anti-CRC action of eosinophils.
Completing this final section of the review, the author is tempted to briefly mention already discussed ETosis as another possible, but hitherto poorly investigated factor in CRC. One interesting link here is provided by recently published results implicating inflammation-induced ETosis in extracellular matrix remodelling and awakening of dormant cancer cells[237]. As tumour-associated inflammation is a characteristic feature of CRC, and significantly increased extracellular trap formation in these tumours is now proven[238,239], it looks probable that previous reports of CRCassociated increase in the amount of DNA in stool[240,241]or on the surface of colorectal mucosa[190,242]at least partially reflected abundant ETosis occurring within mucus layers contacting tumour surface. In essence, cellular presence in the mucus overlaying colorectal tumour surface is quite similar to that depicted by Figure 3, the abundance of exfoliated malignant cells being the only major difference[190]. Although today there is no published evidence of eosinophil contribution in CRC-related ETosis, this evidence is very likely to emerge soon. In contrast to anti-cancer effects of eosinophils in fully developed tumours, which were discussed above, it is impossible to exclude carcinogenicity of eosinophil-derived DNA traps loaded with highly cytotoxic released granules damaging colonic mucosa. Such a carcinogenic action could be involved at early stages of CRC development, especially in IBD-associated context[243,244]. Conversely, eosinophil-driven ETosis may prevent further tumour expansion at later stages of advanced tumour growth.
The presented analysis of literature establishes a link between eosinophil presence and favourable CRC prognosis, but functional versatility of these multifaceted cells may comprise both anti-cancer and tumour-promoting features. Only several experimental studies addressing eosinophil roles in cancer could be highlighted,however it already becomes transparent that alternative mechanisms involving both direct effector action of eosinophils and complex cooperation with other immune cells, especially T lymphocytes, can be engaged in different circumstances. This fascinating area still poses numerous unanswered questions requiring further intense investigation.
CONCLUSION
Upon the presented analysis of the current literature it can be concluded that eosinophils are now regarded as multifunctional mobile cells routinely involved in controlling and regulating a range of biological pathways and responses in both health and disease. The versatility of eosinophils largely depends on the availability of numerous biologically active substances (cytotoxic cationic proteins, cytokines,growth factors, chemokines, enzymes) stored in their specific granules and ready for rapid release. The lamina propria of the human gut is one of the main destinations of eosinophils produced and matured in the bone marrow and transferred through the circulation. In the normal physiological conditions, the most important functions of gut-residing eosinophils appear to be their participation in the maintenance of the protective mucosal barrier and interactions with other immune cells, particularly in providing immunity to microbiota inhabiting the lumen of the gut. In health the latter regulatory role may be more important in the small intestine compared to the colon,mostly due to structural differences of mucus layers covering epithelial surfaces of these two segments of the intestine. Eosinophils are proven to be closely involved in the development of inflammation in IBD, when their uncontrolled cytotoxic effector functions cause damage to host tissues. However, their roles in CD and UC differ.While eosinophil impact in UC pathogenesis largely corresponds to Th2 immune response pattern, the involvement of these cells in CD pathogenesis is less clear.Eosinophils in IBD appear to be especially important in altering the structure and protective functions of the mucosal barrier and modulating massive neutrophil influx to the lamina propria followed by transepithelial migration to colorectal mucus. The author believes that accumulating evidence suggests that IBD-associated inflammatory process expands to the mucus overlaying the internal gut surface, and the presence of eosinophils in this mucus is well documented. It can be hypothesised that colorectal mucus presents a previously unexplored unique milieu for diseaserelated inadequate immune responses in the gut. These responses involving cytotoxic effects and ETosis exerted by both neutrophils and eosinophils on the both sides of the colonic epithelial barrier act as additional pathogenetic factors leading to colonic epithelium ulceration in IBD. However, further research is needed for testing the proposed hypothesis. Literature analysis also highlights an association between elevated eosinophil levels and better CRC prognosis. Mechanisms behind this link may involve both direct anti-tumour action of eosinophils and complex cooperation of eosinophils with T cells but remain to be elucidated. This challenging area still presents an important goal for future research.
It should be admitted that addressing all interesting topics related to eosinophil role in the gut was impossible for obvious reasons. A huge amount of literature covering gut immunity in health and disease exists, and certain selectivity was inevitable. Nonetheless, the author would like to specifically emphasise his opinion on the importance of investigating colorectal mucus in the context of major colorectal diseases including IBD and CRC.
 World Journal of Gastroenterology2019年27期
World Journal of Gastroenterology2019年27期
- World Journal of Gastroenterology的其它文章
- Stricter national standards are required for credentialing of endoscopic-retrograde-cholangiopan-creatography in the United States
- Colorectal peritoneal metastases: Optimal management review
- lmmune suppression in chronic hepatitis B infection associated liver disease: A review
- Device-assisted enteroscopy: A review of available techniques and upcoming new technologies
- ldentifying high-risk individuals for gastric cancer surveillance from western and eastern perspectives: Lessons to learn and possibility to develop an integrated approach for daily practice
- ls the treatment outcome of hepatocellular carcinoma inferior in elderly patients?
