Effect of hydro-alcoholic extract of Olea europaea on apoptosis-related genes and oxidative stress in a rat model of torsion/detorsion-induced ovarian damage
Majid Shokoohi, Malihe Soltani, Seyed-Hosein Abtahi-Eivary, Vahid Niazi, Mohammad Javad Rafeei Poor,Hooman Ravaei, Ramin Salimnejad, Maryam Moghimian✉, Hamed Shoorei
1Student in Nursing, Student Research Committee, Gonabad University of Medical Sciences, Gonabad, Iran
2Women’s Reproductive Health Research Center, Tabriz University of Medical Sciences, Tabriz, Iran
3Department of Basic Sciences, Faculty of Medicine, Gonabad University of Medical Sciences, Gonabad, Iran
4Department of Tissue Engineering and Applied Cell Sciences, School of Advanced Technologies in Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran
5Department of Biology, Hakim Sabzevari University, Razavi Khorasan, Sabzevar, Iran
6Physiology Research Center, Faculty of Medicine, Iran University of Medical Sciences, Tehran, Iran
7Research Laboratory for Embryology and Stem Cells, Department of Anatomical Sciences and Pathology, School of Medicine, Ardabil University of Medical Sciences, Ardabil, Iran
8Department of Anatomical Sciences, Faculty of Medicine, Birjand University of Medical Sciences, Birjand, Iran
Keywords:Ischemia/reperfusion Oxidative stress Olea europaea Ovarian torsion Apoptotic gene expression
ABSTRACT Objective: To evaluate the impact of Olea (O.) europaea extract on markers of oxidative stress and apoptosis of ovarian tissues in a rat model of torsion/detorsion-induced ovarian damage.Methods: A total of 28 Wistar female rats were randomly assigned into 4 groups, with 7 rats in each group. The sham group received a 2.5 cm longitudinal incision in the midline part of the abdomen which was then sutured with 5-0 nylon thread; the torsion/detorsion group underwent torsion induction for 3 h followed by reperfusion for 10 days; the torsion/detorsion+O. europaea group received 300 mg/kg hydro-alcoholic extract of O. europaea 30 min before detorsion, followed by reperfusion for 10 days; and the O. europaea group only received 300 mg/kg hydro-alcoholic extract of O. europaea for 10 days. After the treatment period, blood samples were taken; the levels of estrogen, glutathione peroxidase, superoxide dismutase, and malondialdehyde were assayed.The histological changes, as well as the rate of apoptosis in ovarian tissues, were also carried out by histomorphometric analysis at day 10 post-procedure.Results: Histological comparisons demonstrated a significant detrimental change in the torsion/detorsion group as compared with other groups. The number of pre-antral and antral follicles and corpus luteum was significantly decreased in the torsion/detorsion group compared with the sham group, while treatment with O. europaea could enhance their numbers (P<0.05). The index of apoptosis and the number of atretic body in the ovarian tissue were significantly higher in the torsion/detorsion group compared with the sham group (P<0.05). The concentrations of glutathione peroxidase, estrogen, and superoxide dismutase as well as the mRNA expression of Bcl-2 were considerably diminished in the torsion/detorsion group while they were elevated in the torsion/detorsion+O. europaea group (P<0.05) compared with the torsion/detorsion group. The serum malondialdehyde level and the mRNA expression of Bax were markedly increased during ischemia, while treatment with O. europaea significantly diminished the increased concentrations of malondialdehyde and Bax level in the torsion/detorsion+O. europaea group (P<0.05).Conclusions: O. europaea extract can reduce the degree of tissue damage induced by oxidative stress and apoptosis in the ovary following ovarian ischemia/reperfusion.
1. Introduction
Ovarian torsion, as one of the most prevalent gynecologic disorders, is defined when the adnexal vessels (such as ovarian and utero-ovarian ligaments) are twisted around their axis, leading to venous, arterial, or vascular occlusion[1]. This condition may result in necrosis, accompanied by gangrene, arterial incompetence, and hemorrhagic infarction, which is induced by venous and lymphatic blockage[2]. To date, no consensus exists on the minimum number of rotations required to create torsion, as well as the necessary time to induce necrosis after torsion induction[3]. Torsion is diagnosed via clinical manifestations and sonographic findings, but a definite diagnosis is only established in the case of surgery[1]. Oophorectomy of doubtful and/or the necrotic ovaries (exhibiting some degrees of vasodilation) after occlusion or torsion is the best surgical therapeutic option among clinical approaches. However, prior to the puberty period, the preservation of gonads has a more significant superiority over their destruction in the following period of life. Also,asynchronous contralateral torsion accounts for 2% to 5% of female reproductive diseases, so it is considered a clinical catastrophe.About one-fourth of pediatric ovarian torsion is characterized by abnormal ovaries; thus, the protection of gonads in children would be of great importance[2,3]. Considering the ovary plays a critical role in fertility and secretion of sexual hormones, the torsioned ovaries could cause detrimental effects on the reproductive system[3].
Torsion/detorsion (T/D)-induced ischemia/reperfusion (I/R) injury is a pathophysiologic event that causes histological damage to the female reproductive tract[4-6]. Torsion is associated with a marked reduction in perfusion of the ovary, followed by a lack of oxygen supply (ischemia) in a particular organ. Ovarian I/R is capable of initiating inflammatory cascades that could cause microcirculation disorders and induce damages to vascular endothelial cells that are mainly in charge of ovarian tissue injury. The overproduction of reactive oxygen species (ROS), namely hydroxyl radicals, hydrogen peroxide, and superoxide radicals is one of the I/R complications which are able to cause severe damages to reproductive tissues[4-9].The elevated levels of ROS result in DNA damage, endothelial destruction, and apoptosis of granulosa cells[10,11]. Hence, oxidative stress has deleterious effects on ovarian tissue, and the use of antioxidant substances could neutralize the harmful impacts of free radical agents on reproductive tissues. Natural products such as herbal extracts are considered bona fide alternative treatments for the alleviation of oxidative stress-induced ovarian tissue damage[12].
The leaves extracts of Olea (O.) europaea have been widely used in traditional medicine in European and Mediterranean countries such as Greece, Spain, Italy, France, Turkey, and Tunisia. The herb extract is usually applied in human foods, and it contains numerous bioactive compounds with antioxidant, antihypertensive, anti-atherogenic,anti-inflammatory, anti-diabetic, and anti-hypercholesterolemia properties. One of the essential bioactive compounds in O. europaea extract is secoiridoid oleuropein, which is a potent antioxidant with anti-inflammatory potentials, constituting 6%-9% of dry matter weight of the leaves. Other bioactive components found in O.europaea include secoiridoids, flavonoids, and triterpenes which have beneficial impacts on metabolism when used as a supplementary compound. The primary sources of polyphenols in olive are olive leaves and the industrial waste of olive oil, known as alperujo.Alperujo is an inexpensive source of natural antioxidants in which the concentrations of such compounds are up to 100 times higher than olive oil. Olive leaves possess the most potent antioxidant components compared with other parts of the plant[13,14].
Hence, concerning the antioxidant and anti-inflammatory potential of O. europaea, the current study aimed to assess the effect of this herb extract on the reduction of oxidative stress and tissue damages in the ovary of adult female rats induced by T/D.
2. Materials and methods
2.1. Plant collection process
In April 2018, the leaves of O. europaea were collected in the rural regions of Khorramabad, located in the western part of Iran (Lorestan province). The identification and characterization of gathered samples were performed by an expert botanist. The voucher specimens were deposited at Razi Herbal Medicine Research Center affiliated with Lorestan University of Medical Sciences (RH 1165)[15].
2.2. Extraction of hydroalcoholic mixture
For the preparation of the extraction from whole leaves of O.europaea, 500 g of O. europaea was air dried at room temperature. To continue the extraction procedures, the dried herb was grounded into a fine powder and dissolved in 2 L of 96% alcohol, then kept at 25 ℃for 48 h. Next, the mixture was frequently agitated, and the solution was filtered and then centrifuged at 3 000 rpm for 5 min. Finally, the resulting solution was transferred into an open-top container and then evaporated. 100 g of the semisolid extraction was achieved from whole leaves of O. europaea. The resultant extraction was dissolved in normal saline to gain appropriate concentrations of O.europaea extract[16]. It was shown that the main phenolic contents of the hydroalcoholic extract of the O. europaea leaves were oleuropein(356 mg/g), tyrosol (3.73 mg/g), hydroxytyrosol (4.89 mg/g)and caffeic acid (49.41 mg/g) when the components of the herb were analyzed by the high-performance liquid chromatography technique[17].
2.3. Study design
For this experimental study, 28 adult female Wistar rats weighing 200-250 g were purchased from the Razi Institute of Mashhad city, and they were maintained in standard conditions. During the experimental period, all of the animals had free access to food and tap water. The rats were randomly assigned into four groups, with 7 rats in each group: 1) the sham group received a 2.5-cm longitudinal incision in the midline part of the abdomen which was then sutured with 5-0 nylon thread; 2) the T/D group underwent ovarian torsion for 3 h while the animals received normal saline by oral gavage 30 min before detorsion and then daily received normal saline until the end of the treatment period (day 10); 3) the T/D+O. europaea group underwent ovarian torsion for 3 h and the animals were treated with 300 mg/kg hydro-alcoholic extract of O. europaea by oral gavage 30 min before detorsion[18]. And the animals daily received the O. europaea extract until the end of the treatment period (day 10)[2]; 4) the O. europaea group did not undergo operation and they only received 300 mg/kg hydro-alcoholic extract of O. europaea orally for 10 days. After day 10, the left ovary was resected for histopathological analyses.
2.4. Ethics
All experimental processes of the present research were conducted in accordance with the Guidelines of the Gonabad University of Medical Science, Gonabad, Razavi Khorasan Province (Iran)specified for the care and use of laboratory animals (ethical code:IR.GMU.REC.1394.10).
2.5. Surgical operations and sampling
When the experimental period was finished, the animals were anesthetized by intraperitoneal injection of ketamine (50 mg/kg) and xylazine (10 mg/kg). Animals were placed in a supine posture,and then a longitudinal incision was made in the midline of rats’abdomen, and the left horn of uterine, as well as adnexa, was exposed. After that, the left ovary of each animal was twisted 720 degrees around its axis in a counterclockwise direction. Next, the rotated ovary was fixed to the abdominal wall using 0.6 nylon sutures to avoid detorsion. The incision was sutured with 6/0 nylon suture. The rotated ovary was left in this situation for 3 h. Next, the O. europaea extract was administered by oral gavage 30 min before the release of torsion. After 3 h, the torsioned ovary was returned to the normal condition to complete the detorsion process. Next, the reperfusion procedure was accomplished, and the ovary was left for seven days in this status. At the end of the reperfusion period, all of the animals were anesthetized by ketamine (50 mg/kg), and xylazine(10 mg/kg) and then the blood samples were taken from the heart of each animal to evaluate oxidative stress indices and sex hormone levels. Also, ovarian tissues were removed to assess the histological alteration as described previously[2]. Ovarian tissues were fixed in 10% formalin for 72 h, then dehydrated and paraffin embedded.The samples were sectioned at the thickness of 5 µm by a rotary microtome, and then tissue sections were stained with hematoxylineosin. Blood specimens collected from the animals were centrifuged at 4 000 rpm for 5 min. The isolated serum samples were collected and aliquoted into three microtubes (500 µL), and finally kept at -70 ℃until the analysis.
2.6. Histological analysis
The histological and histomorphometric studies of tissue sections obtained from each ovary of the animals were carried out. Tissue sections were evaluated spirally in clockwise directions from the cortex to the medulla region. In each tissue section, the number of atretic and yellow bodies, as well as the frequency of preantral, antral, and corpus luteum cells were counted under a light microscope at ×100 magnification (Carl Zeiss, Germany).
2.7. Apoptotic cell detection
The rate of apoptosis in follicles was evaluated by the terminaldeoxynucleoitidyl transferase mediated nick end labeling (TUNEL)assay using the In Situ Cell Death Detection Kit, POD TUNEL assay (Boehringer Mannheim, Germany) according to the recommendations provided by the manufacturer. All procedures were implemented in accordance with the protocols provided by the commercial kit as follows: 1) ovarian sectioned tissues were deparaffinized and rehydrated in descending gradient of ethanol; 2)samples were incubated with 20 mg/mL proteinase K for 20 min in humid room temperature; 3) endogenous peroxidase activity was blocked by the incubation with 3% hydrogen peroxide in methanol for 10 min; 4) ovary sections were incubated with the TUNEL solution containing deoxy-nucleotide mixture and terminal deoxynucleotidyl transferase enzyme at 4 ℃ overnight; 5) tissue specimens were then incubated with the anti-fluorescein antibodyperoxidase solution at room temperature for 30 min; 6) finally, tissue sections were treated with diaminobenzidine for 15 min. All steps mentioned earlier were conducted separately by rinsing the samples in phosphate-buffered saline after each stage. Finally, tissue sections were stained with hematoxylin for 1 min. Then, tissue samples were dehydrated, cleared, and mounted with Entellan (Merck,Darmstadt, Germany). Apoptotic cells appeared in dark brown and were homogeneous[19]. For calculation the apoptotic index in each type of follicles, the number of TUNEL-positive cells was counted,then divided into the total number of granulosa cells and expressed as the percentage. Next, the mean apoptotic index of each group was calculated and analyzed by the ImageJ software.
2.8. RNA isolation, cDNA synthesis, and real-time polymerase chain reaction (RT-PCR)
The left ovaries of all animals in each group were collected in three replicates on day 10 of the treatment period to analyze the expression of apoptosis-related genes. In each group, the total RNA content was extracted utilizing the TRIzol reagent (Invitrogen, CA, USA) based on the manufacturer’s instructions. Then, the RNA concentration was determined by using the spectrophotometry method, and it was adjusted to a concentration of 500 ng/mL. Using oligo dT, total isolated RNA was reverse-transcribed into cDNA by the Moloney murine leukemia virus reverse transcriptase. The primer sequences of each gene were listed in the below:Forward primer (Bax): GGCGAATTGGAGATGAACTG;Reverse primer (Bax): TTCTTCCAGATGGTGAGCGA;Forward primer (Bcl-2): CTTTGCAGAGATGTCCAGTCAG;Reverse primer (Bcl-2): GAACTCAAAGAAGGCCACAATC;
Jovial18 George clearly enjoyed helping19 others while he spread cheer and told jokes -- the same jokes, over and over again, all day long, one patient at a time. We all enjoyed his presence that Christmas day.
Forward primer [glyceraldehyde-3-phosphate dehydrogenase(GAPDH)]: ATGGAGAAGGCTGGGGCTCACCT;
Reverse primer (GAPDH): AGCCCTTCCACGATGCCAAAGTTGT.The GAPDH gene was applied as an internal control[20].
2.9. RT-PCR
RT-PCR was performed on the Applied Biosystems (UK, Lot No. 1201416) and the relative gene expression was conducted with SYBR green-based RT-PCR. The thermal cycling conditions were as follows; initial denaturation at 95 ℃ for 10 min to inhibit the reverse transcriptase, followed by 40 cycles of 15-second denaturation at 95 ℃, annealing for 30 s at 58 ℃, 30-second elongation at 72 ℃, and the extension step of 95 ℃ for 15 s, 60 ℃for 1 min, and 95 ℃ for 15 s. Next, the relative expression analysis of target genes mentioned above was carried out by the Pfaffl method (2-ΔΔCt, ΔΔCt =ΔCt sample-ΔCt control)[21].
2.10. Evaluation of serum biochemical parameters
2.10.1. Determination of malondialdehyde (MDA)
The level of MDA was determined by pouring 0.20 cm3of serum samples into microtubes containing 3.0 cm3of glacial acetic acid,to which 3.0 cm3of 1% thiobarbituric acid was added to 2% NaOH.The microtubes comprising the mixture solution earlier mentioned was placed in the boiling water for 15 min. The pink-colored product exhibited maximum absorbance at the wavelength of 532 nm when the cooling-down process was performed. Tetra-butyl-ammonium salt was employed for the preparation of a standard solution to obtain the calibration curve, as previously described[20].
2.10.2. Determination of activities of glutathione peroxidase(GPx) and superoxide dismutase (SOD)
The measurements of activities of GPx and SOD were conducted in serum samples of rats according to the protocols recommended by the commercial kits (Randox, UK). Briefly, GPx, by oxidizing glutathione, could reduce H2O2to H2O. Then, glutathione reductase catalyzed re-reduction of the oxidized form of glutathione. The GPx activity was measured in absorbance at 320 nm[22]. On the other hand, the reaction of superoxide radical and 2-(4-iodophenyl)-3-(4-nitrophenol)-5-phenyltetrazolium chloride forms red formazan,which was the base of measuring the activity of SOD at 505 nm[23].
The concentrations of serum estrogen hormone were determined by the enzyme-linked immuno sorbent assay method using the commercial kit (Demeditec Diagnostics, Kiel, Germany).
2.11. Statistical analysis
The statistical analysis was performed by the SPSS software version 20 (IBM, USA). All of the obtained values were expressed as mean and standard deviation of the mean (mean ± SD). The comparison of the values between the experimental group was determined by oneway analysis of variance followed by Tukey’s post hoc test. The level of statistical significance was set at P<0.5.
3. Results
3.1. Histological parameters of ovarian tissues
The histological analysis demonstrated a significant (P<0.05)decrease in the number of pre-antral follicles in the T/D group as compared with the sham group (Figure 1). Moreover, a significant(P<0.05) difference was found between the T/D group and other experimental groups when the frequency of the above cells was compared. Accordingly, a statistically significant reduction was observed in the number of antral follicles in the T/D group in comparison with the sham group (P<0.05). Also, the number of antral follicles were considerably increased in groups treated with the O. europaea extract (T/D+O. europaea group and O. europaea group), compared with T/D group (P both <0.05). The results indicated that the number of corpus luteum cells was considerably(P<0.05) lower in the T/D group when compared with the sham group. On the other hand, the percentage of corpus luteum cells was elevated in the T/D+O. europaea and O. europaea groups compared with the T/D group (P both <0.05). A significant increase was found in the frequency of atretic bodies in the T/D group when compared with the sham group (P<0.05). According to Table 1 and Figure 1, our findings indicated that the number of atretic bodies was markedly diminished in the T/D+O. europaea and O. europaea groups in comparison with the T/D group (P<0.05).
3.2. Apoptosis index
As depicted in Figure 2, the evaluation of the apoptosis index in ovarian tissues in the T/D group showed a significant increment in the percentage of apoptotic cells when compared with the sham group (P<0.05). The index of apoptosis in ovarian tissues of the groups treated with the O. europaea extract was significantly lower than the T/D group (P<0.05).
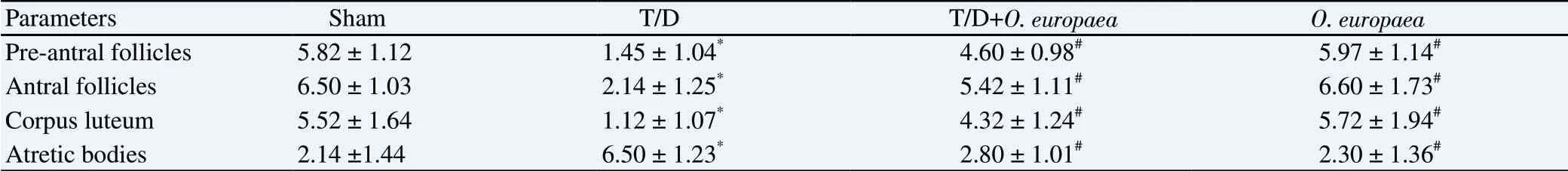
Table 1. Mean number of pre-antral follicles, antral, and corpus luteum along with atretic body in ovaries of female rats in all groups.
3.3. Analysis of gene expression
The expression ratio of Bcl-2 and Bax to GAPDH was illustrated in Figures 3 and 4. The expression ratio of Bax gene to the GAPDH gene was substantially (P<0.001) elevated in the T/D group compared with the sham group; however, the expression of Bax was diminished in both T/D+O. europaea group and O. europaea group comparing with the T/D group. Although the ratio expression of Bcl-2 to GAPDH was significantly (P<0.001) decreased in the T/D group in comparison with the sham group, it was dramatically increased in both T/D+O. europaea and O. europaea groups compared with the T/D group (P<0.001).
3.4. Serum parameters
The level of serum estrogen was significantly (P<0.05) reduced in the T/D group in comparison with the sham group. Moreover, as shown in Table 2, serum concentrations of estrogen were significantly(P<0.05) different between the T/D and other experimental groups.The activity of the SOD enzyme in serum samples of the T/D group was significantly lowered compared with the sham group (P<0.05).Also, as shown in Table 2, the serum activity of the SOD enzyme was elevated considerably (P<0.05) in the T/D+O. europaea and O.europaea groups when compared with the T/D group. The analysis of serum concentrations of the GPx enzyme indicated a significant decrease in the T/D group when compared with the sham group(P<0.05). According to Table 2, a marked difference was found between the T/D and other therapeutic groups regarding the levels of the GPx enzyme (P<0.05). The serum concentrations of MDA were statistically higher in the T/D group compared with that of the sham group (P<0.05). Conversely, as shown in Table 2, the serum levels of MDA were significantly diminished in the T/D+O. europaea group compared with the T/D group (P<0.05).
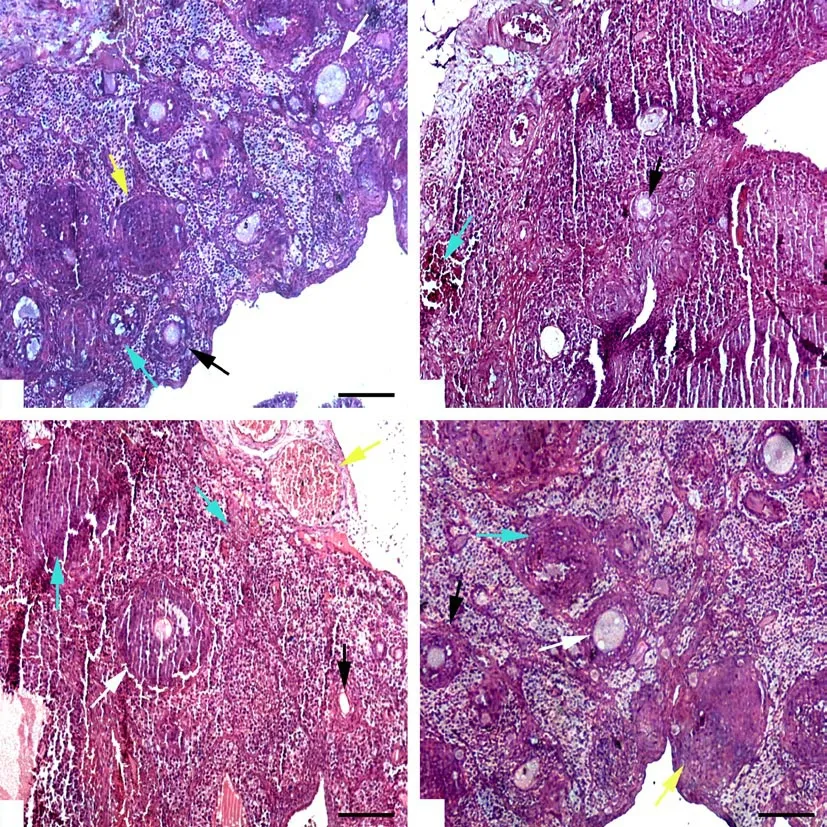
Figure 1. Histological analysis of sham group (A), T/D group (B), T/D+O. europaea group (C), and O. europaea group (D) 10 days post-surgery(hematoxylin-eosin staining). White and black arrows display the antral and pre-antral follicles, respectively. Green arrows show atretic bodies, and yellow arrows indicate corpus luteum cells. Scale bar: 100×. TD: torsion-detorsion.
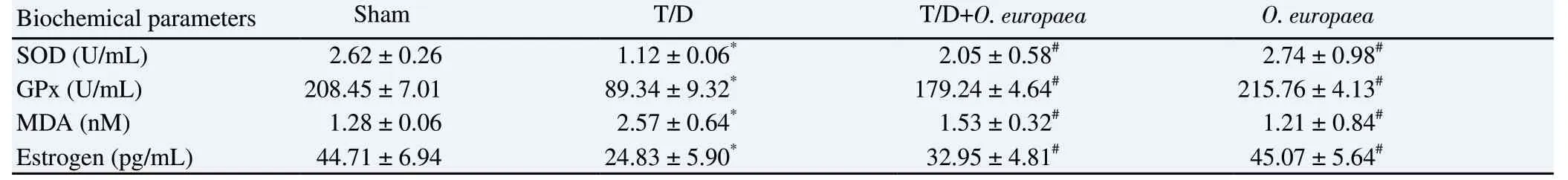
Table 2. Serum concentrations of superoxide dismutase, glutathione peroxidase, malondialdehyde and estrogen in different experimental groups.
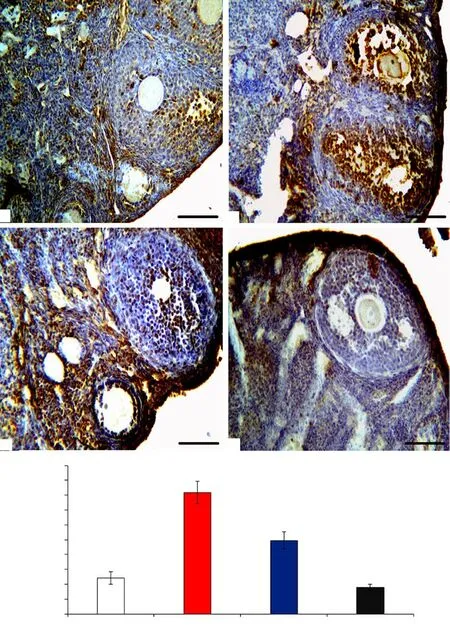
Figure 2. Apoptotic cells in ovarian tissue in the sham group (A), T/D group (B), T/D+Olea europaea group (C), and Olea europaea group (D) 10 days after surgery (hematoxylin-eosin staining; scale bar: 100×). All data are expressed as mean±SD. The asterisk (*) shows significant difference with sham group and the symbol (#) means a considerable difference with T/D group (P<0.05). TD: torsion-detorsion; OE: Olea europaea.
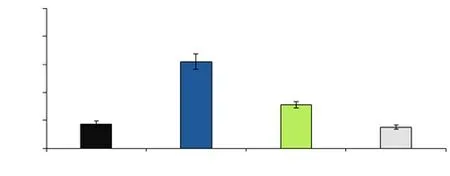
Figure 3. Comparison of expression ratio of Bax gene. All of the obtained values are expressed as mean ± SD. The asterisk (*) implies a significant difference compared with the sham group and the symbol (#) denotes a significant difference compared with the T/D group (P< 0.05). TD: torsiondetorsion; OE: Olea europaea.
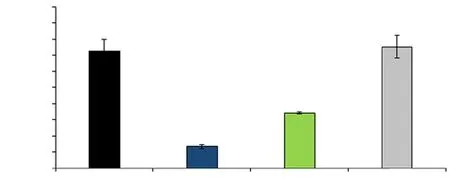
Figure 4. Comparison of expression ratio of Bcl-2 gene. All values are expressed as the mean ± SD. The asterisk (*) implies a significant difference versus the sham group and the symbol (#) denotes a significant difference versus the T/D group (P<0.05). TD: torsion-detorsion; OE: Olea europaea.
4. Discussion
In the human body, the level of ROS and antioxidant compounds are in equilibrium as the over-production of ROS results in oxidative stress. Studies have indicated that the reproductive system of women could be affected by oxidative stress during pre-puberty, puberty, or even menopause periods. Oxidative stress could be originated from the perturbation in the balance of pro-oxidant and antioxidant agents in which the human body would not be capable of eliminating the excessive amount of ROS from the body. The generation of ROS is regarded as a double-edged sword, implying that a particular amount of these radical and pro-oxidant chemicals is pre-requisite for the proper function of some biological phenomena such as the eradication of pathogens and so on; however, the elevated levels of ROS could cause some damages to vital macromolecules including DNA, protein, and lipids and they play a significant role in the development of some pathological events such as I/R-induced ovarian injury. It has been indicated that ROS play roles in some biological processes ranging from the maturity of the oocyte to fertilization, as well as the development of the embryo and gestation[2,24]. It has been shown that oxidative stress has a critical effect on the reduction of age-related fertility decline. Also, it has a significant impact on gestation, normal parturition, and the onset of preterm delivery[25]. Oxidative stress is the primary culprit of DNA damage in the ovulation process and ovarian epithelial cells while it could be prevented by the administration of antioxidant agents to individuals who are at risk of developing I/R-induced ovarian damage. Several lines of evidence have indicated that oxidative stress plays an essential role in the pathophysiology of infertility[24,25]. This fact was supported by similar reports demonstrating that oxidative stress contributes to the development of endometriosis, as well as tubal and peritoneal infertility[24,25]. It is thought that multiple mechanisms participate in I/R-induced tissue damage, including the increased production of ROS, elevation of proinflammatory mediators, and the initiation of pro-apoptotic factors in different tissues[26-29].
Antioxidant compounds have vital roles in the prevention of ROS overproduction and oxidative stress-induced infertility problems[7, 20,30-35]. Several reports have highlighted that ovarian I/R could be caused by the excessive generation of ROS, followed by the damages induced by oxidative stress[2,6]. Oxidative stress,induced by ovarian T/D may lead to the detrimental changes in ovarian tissues, along with hormonal alterations such as a decrease in the levels of GPx, SOD, MDA, and estrogen, as well as a reduction in the number of follicles in ovaries[2,36].
In the current research, ovarian torsion resulted in oxidative stress injuries, including histological damages and biochemical alterations.Such deleterious effects might be represented as a decrease in the number of follicles (pre-antral, and antral), as well as an increase in the number of atretic bodies and apoptosis of granulosa cells in follicles. Therefore, TD can stimulate the apoptosis process in ovarian tissue of rat, thereby leading to an increase in the production of ROS. The Bax (pro-apoptotic) and Bcl-2 (anti-apoptotic) proteins are two members of the Bcl-2 family, controlling the initiation of caspase activity[37-39]. Our findings revealed that the mRNA expression of Bax was significantly upregulated in ovarian tissue of the T/D group compared with other experimental groups. It has been reported that the overexpression of Bax finally leads to apoptosis[37, 38]. Contrariwise,the ratio of the Bcl-2 gene to the housekeeping gene expression, i.e.,GAPDH was significantly decreased in the TD group in comparison with other groups. Similar to our findings, Agarwal et al[24] showed that the production of ROS led to a decrease in the number of preantral, antral, and corpus luteum cells.
Moreover, Sapmaz-Metin et al[10] indicated that ovarian T/D causes apoptosis in follicular cells. Gencer et al[40] reported that ovarian T/D results in apoptosis and increases the activity of caspase-3, as well as the number of TUNEL-positive cells in the ovarian surface epithelium, follicular epithelial cells, and stromal cells. The results demonstrated that ovarian T/D caused some biochemical changes,such as the reduction in the levels of estrogen. In a study performed by Agarwal et al, they revealed that oxidative stress could lead to a reduction in the level of estrogen[24]. Moreover, our results highlighted that T/D-induced oxidative stress could reduce the activity of the SOD and GPx enzymes. Inversely, it also declined the concentrations of MDA levels in serum samples of female rats. These findings demonstrate a decline in the potency of the antioxidant defense system. In line with our results, Agarwal et al showed that ROS induction resulted in a reduction in levels of GPx and SOD enzymes[24]. Notably, our previous study indicated that the induction of ovarian torsion for three hours, followed by detorsion diminished the concentration of both SOD and GPx enzymes while the level of MDA was markedly increased[2]. The O. europaea extract has multiple active compounds such as secoiridoids, flavonoids,and triterpenes[13]. According to previous reports, the O. europaea extract has antioxidant, antihypertensive, anti-atherogenic, and antiinflammatory activities[13]. The O. europaea extract is an influential antioxidant agent as it contains considerable amounts of flavonoid and alperujo compounds which could neutralize ROS and prevent the formation of free radicals and lipid peroxidation[13].
In this context, we decided to use the O. europaea extract to decrease oxidative damage and apoptosis caused by ovarian I/R. Our results indicated that the O. europaea extract increased the percentage of follicles, while it reduced the frequency of atretic bodies, thereby preventing the overproduction of ROS and oxidative stress. Furthermore, the O. europaea extract protected ovarian tissue against apoptosis, and degenerative damage thought to be mediated by the antioxidant properties. It has been reported that the excessive generation of ROS results in apoptosis and subsequently increased expression of the Bax gene along with the decreased expression of the Bcl-2 gene[38]. The O. europaea extract diminished apoptosis index and down-regulated Bax expression and prevented apoptosis in ovarian tissue via the inhibition of the Bcl-2 activity. In line with our findings, Kaeidi et al[18] reported that treatment with the O. europaea extraction could protect testicular tissues against cell death and it is also capable of decreasing the expression of Bax and increasing the Bcl2 expression. We demonstrated that the O. europaea extraction elevated the level of serum estrogen in female rats induced by ovarian torsion. This might stem from the presence of alperujo and antioxidant compounds in the O. europaea extract, which protects ovarian tissue against oxidative damages. A study performed by Rodríguez-Gutiérrez et al[41] revealed that alperujo can increase the serum level of antioxidant enzymes and decrease oxidative stress.As similar to our results, it has been reported that the extraction of O. europaea possesses estrogenic components[42]. The presence of that antioxidant compounds in the O. europaea extract has enabled this herb to elevate the SOD and GPx enzymes and decrease the rate of lipid peroxidation as confirmed in a study conducted by Proietti et al[43]. Other reports also demonstrated that the extraction of O.europaea could decrease oxidative stress and increase the activity of antioxidant enzymes[13].
In conclusion, regarding the findings of the current investigation,the extraction of O. europaea increases the activity of antioxidant enzyme and serum levels of estrogen, and it protects against apoptosis of ovarian tissue. The herb extract is also capable of regulating the expression of the Bax and Bcl-2 genes and preventing T/D-induced oxidative stress in ovarian tissues.
Conflict of interest statement
The authors state no conflict of interest.
Foundation project
This study was supported by the Student Research Committee of Gonabad University of Medical Sciences (Grant No. 94/7).
 Asian Pacific Journal of Reproduction2019年4期
Asian Pacific Journal of Reproduction2019年4期
- Asian Pacific Journal of Reproduction的其它文章
- Cypermethrin triggers apoptosis, depletes granulosa cells, and induces endometrium thinning in female rats
- Influence of Glyphaea brevis twig extract on nucleus, tight junctions and expression of inhibin-β, stem cell factor, and androgen binding protein in TM4 Sertoli cells
- Effect of lyophilized aqueous leaf extract of Aquilaria subintegra on aphrodisiac properties in mice
- Soybean lecithin-based extender improves Damascus goat sperm cryopreservation and fertilizing potential following artificial insemination
- Pregnancy outcome of lactating dairy cows assigned for Presynch-Ovsynch synchronization program and inseminated either at detected standing heat or at scheduled fixed time
