Advanced imaging in surveillance of Barrett’s esophagus: Is the juice worth the squeeze?
Sara A Cerrone, Arvind J Trindade
Abstract Esophageal cancer is on the rise. The known precursor lesion is Barrett’s esophagus (BE). Patients with dysplasia are at higher risk of developing esophageal cancer. Currently the gold standard for surveillance endoscopy involves taking targeted biopsies of abnormal areas as well as random biopsies every 1-2 cm of the length of the Barrett’s. Unfortunately studies have shown that this surveillance can miss dysplasia and cancer. Advanced imaging technologies have been developed that may help detect dysplasia in BE. This opinion review discusses advanced imaging in BE surveillance endoscopy and its utility in clinical practice.
Key words: Barrett’s esophagus; Advanced imaging; Chromoendoscopy; Endomicroscopy
INTRODUCTION
Barrett’s esophagus (BE) is the development of specialized intestinal metaplasia in the esophagus. The exact incidence of BE is not known, but it is estimated to be from 0.2%-2% per year[1]. It is a premalignant lesion for adenocarcinoma of the esophagus.Though the rates of esophageal squamous cell carcinoma and distal gastric cancers are declining, the incidence of esophageal adenocarcinoma is rising more than any other malignancy[2]. Reports have quoted an average annual increase of up to 17%[2]. This increase may be due to environmental and population factors, but also due to insufficient detection of Barrett’s and insufficient surveillance protocols for patients with known BE[3,4]. The Seattle protocol guides the surveillance procedure of many endoscopists, which consists of 4-quadrant biopsies at intervals of every 1-2 cm and separate samples of areas of mucosal irregularity, may miss a significant number of areas of low-or high-grade dysplasia[5]. In a large multicenter study, 53% of patients who underwent consecutive surveillance endoscopies documenting non-dysplastic tissue or intestinal metaplasia without dysplasia, developed high grade dysplasia and/or cancer within a mean of 3 years[1].
Unfortunately, the mortality for esophageal adenocarcinomas is very high, with a mean 5-year survival rate of less than 20% for advanced disease. Many patients are diagnosed at presentation with advanced disease, and there is a need to find better means to identify patients at earlier stages. BE confers a 30-40 fold increased risk for esophageal adenocarcinoma, but it is unclear if such focus on surveillance in BE patients has improved outcomes[2]. Patients with surveillance-detected BE have higher rates of survival at two years compared to patients that are diagnosed outside of a surveillance program (73.3% vs 12.5%, P = 0.02), yet few patients (3.9%) are diagnosed with BE before their cancer diagnosis[2]. In other studies, there was no association between surveillance in BE and decreased risk of death from esophageal adenocarcinoma (OR = 0.99; 95%CI: 0.36-2.75) and the detection of advanced disease was equivalent in surveillance and non-surveillance groups[4]. Therefore, current surveillance strategies may be ineffective in improving patient outcomes.
Endoscopic surveillance of known BE may be improved through advanced imaging. Advanced imaging technologies allow visualization of abnormalities that may not be seen on routine endoscopic evaluation. These are also termed as red flag technologies as they point attention to abnormal areas that can be consistent with dysplasia or early cancer.
Preservation and Incorporation of Valuable Endoscopic Innovations (PIVI) was developed in 2011 by the American Society of Gastrointestinal Endoscopy (ASGE) to recognize important clinical questions and develop diagnostic and/or therapeutic thresholds for endoscopic technologies related to these clinical questions[6]. In 2016,performance thresholds were established to evaluate real-time imaging-assisted modalities used for endoscopic targeted biopsies in the endoscopic surveillance of non-dysplastic BE which included chromoendoscopy (using acetic acid and methylene blue), electronic chromoendoscopy [using narrow-band imaging (NBI)], and both probe and endoscopic based confocal laser endomicroscopy (CLE). Volumetric laser endomicroscopy (VLE) was not evaluated in the PIVI initiative given the recent release on the market at the time and thus lack of studies. These per-formance thresholds included: (1) Sensitivity of ≥ 90% and a negative predictive value of ≥ 98%for detecting high grade dysplasia (HGD) or early adenocarcinoma (EAC) compared to standard protocol, and (2) Imaging technology with high (80%) specificity to allow reduction in the number of biopsies compared to random biopsies. Acetic acid chromoendoscopy, narrow band imaging and endoscopic CLE met the thresholds set by the ASGE PIVI, and thus the ASGE Technology Committee endorsed using these modalities to guide surveillance in patients with previously non-dysplastic BE.
Given the number of tools on the market, the main question to ask is, is it worth performing any of these technologies? Ultimately it comes down to how cumbersome is it to perform the technology compared to the yield of additional cases of dysplasia being detected that changes management. Cost to the patient and health care system is also a factor that can contribute to usage of a technology. We describe the technologies from these viewpoints in this mini-review.
DYE CHROMOENDOSCOPY
Dye chromoendoscopy uses chemical agents to highlight mucosal changes of dysplasia to allow for improved detection of abnormalities[3]. Such dye agents include methylene blue, indigo carmine and acetic acid. Methylene blue is absorbed by nondysplastic intestinal-type epithelium and can also be used to detect Barrett’s mucosa.In several studies comparing rates of detection of intestinal metaplasia and dysplasia between methylene blue and 4-quadrant biopsies, the rates for methylene blue were similar than 4-quadrant biopsies, but the number of biopsies used to detect those changes were lower with methylene blue[7]. Other studies have found four-quadrant biopsies detect significantly more dysplasia than methylene blue[8]. In addition, there is concern that methylene blue may damage DNA in Barrett’s epithelium potentially leading to errors in diagnosis[9]. These solutions are difficult to handle and use endoscopically and therefore their use is not standard. Indigo carmine has also been shown to be effective in detecting mucosal patterns, but when compared to highresolution white light endoscopy, indigo carmine showed no significant difference in detecting early neoplasia[10,11]. Given its cumbersome use and lack of incremental yield vs high definition white light endoscopy, its use is also not standard.
Acetic acid can be used to enhance different mucosal pit patterns in columnar epithelium. Certain pit patterns have shown high sensitivity and specificity for intestinal metaplasia. In a meta-analyses, acetic acid chromoendoscopy has shown a sensitivity of 96.6%, negative predictive value of 98.3% and a specificity of 84.5% in detecting dysplasia or esophageal adenocarcinoma[6].
Acetic acid is a safe, rapid, and inexpensive. It can highlight ridged and villous patterns that are associated with mucosal abnormalities associated with dysplasia.Therefore, its use is high yield in surveillance in BE[12].
ELECTRONIC CHROMOENDOSCOPY
Electronic chromoendoscopy techniques involve the use of NBI (Olympus America,Center Valley, PA, United States) and I-Scan (Pentax Medical, Montvale, NJ, United States). Blue light imaging and linked color imaging (Fujifilm, Tokyo, Japan) are newer electronic chromoendosocpy platforms. They will not be discussed here due to limited data in Barrett’s esophagus. NBI highlights vascular patterns on the mucosal surface by using spectral narrow-band optical fibers[13]. Figure 1 shows a patient with Barrett’s and high-grade dysplasia with NBI imaging. I-Scan uses a post-processing technology to highlight contrast between squamous and columnar epithelia[3].Patterns detected by NBI have been shown to predict histology. In one study, a ridge/villous pattern predicted the presence of intestinal metaplasia with a sensitivity of 93.5% and a specificity of 86.7%, and an irregular/distorted pattern predicted highgrade dysplasia with a sensitivity of 100% and sensitivity of 98.7%[14]. In a prospective,blinded, tandem endoscopy study of 65 patients comparing NBI-targeted biopsies to random biopsies via the Seattle protocol with high definition white light, NBI was able to identify more patients with dysplasia (57% vs 43%, P < 0.001) and also found high grades of dysplasia (18% higher grade vs 0%, P < 0.001)[15]. In another study comparing results in patient who were first screened for Barrett’s with high definition white light and then NBI-targeted biopsies at a 6-wk interval, there was no significant difference in detection of intestinal metaplasia and dysplasia (P = 0.15), but NBI required a significantly fewer number of biopsies to make a diagnosis (3.6 vs 7.6, P <0.001)[16]. A study comparing computed virtual chromoendoscopy to conventional chromoendoscopy with acetic acid, a sensitivity of 83% and 92% for high grade intraepithelial neoplasia was found, respectively, with no significant difference between the two methods (P = 0.617)[17].
Overall, electronic chromoendoscopy shows high sensitivity for the detection of high-grade dysplasia in patients with BE and provides a means to more efficiently biopsy patients. Its ability to detect low grade dysplasia is comparable to that of highresolution white light endoscopy. We recommend using electronic chromoendoscopy given its ease of use (turning on a switch on the endoscope processor), low cost (already incorporated into the scope technology), and that it meets PIVI thresholds.
CONFOCAL LASER ENDOMICROSCOPY (CLE)
A CLE examination of the gut mucosa is performed using endoscopically delivered laser light. This light is reflected through a pinhole onto sensors that relay the signals to a computer, which provides a cross-sectional microscopic image of the mucosa[18].CLE is almost analogous to looking real time at a microscope during the endoscopy exam. There is limited data on the learning curve for use of CLE in Barrett’s, but it appears favorable[19]. This allows for detailed analysis of the intestinal mucosa and in vivo histology during ongoing endoscopy[20]. CLE has shown high accuracy rates (85%-94%) for the detection of high-grade dysplasia and a sensitivity of 80% in the identification of advanced neoplasia with good interobserver agreement[20,21]. In an exvivo study, CLE was shown to have positive and negative predictive values for highgrade dysplasia/early cancer of 44% and 83%, respectively[22]. When combined with high-definition white light endoscopy, probe-based CLE was able to detect all cases of high-grade dysplasia/early cancer in this study, but this was not statistically significant compared to these imaging methods alone[23].

Figure 1 A patient with Barrett’s and high-grade dyspalsia with narrow band imaging. A: A segment of Barrett’s esophagus on high definition white lightendoscopy (HDWLE); and B: narrow band imaging (NBI) from a patient with prior long segment disease post two sessions of endoscopic resection, 4 sessions of radiofrequency ablation, and one session of cryotherapy. The HDWLE did not show any features concerning for dysplasia. The NBI shows an area of disrupted vessels (upper yellow arrow, lower yellow arrow) concerning for dysplasia.
Currently endoscope-based CLE (e-CLE) is not available for use in the United States, but the probe-based CLE (pCLE) version (Cellvizio, Mauna Kea Technologies,MA, United States) is. The pCLE version images a small area at a time and thus has a narrow field of view. Thus, it is cumbersome to use in long segments of Barrett’s where advanced imaging is more of a need vs short segments. In our practice we find CLE to be helpful when wanting to examine a specific area if considering a biopsy vs endoscopic resection, however its routine use for surveillance is limited given its narrow field of view; especially since it does not meet PIVI thresholds. It should be noted that CLE also requires intravenous fluorescein which has been reported to be safe in GI procedures[23].
VOLUMETRIC LASER ENDOMICROSCOPY (VLE)
VLE (NvisionVLE, Ninepoint Medical, Bedford, MA, USA) is the latest advanced imaging technology in Barrett’s. It is second-generation optical coherence tomography using infrared light to produce real-time, high-resolution, cross-sectional microstructure imaging of tissue[24]. VLE can scan a 6-cm length of the esophagus in approximately 90 s, providing surface and subsurface wide-field cross-sectional imaging with an axial resolution of 7 μm, and to a depth of 3 mm[25]. Ex-vivo studies comparing VLE features to endoscopic resection specimens have demonstrated sensitivities of 86%-90% and specificities of 88%-93% for the detection of dysplasia in BE[26]. The benefits to VLE is that an entire segment of Barrett’s can be imaged in a short period of time, abnormalities can be laser marked for targeting, the interobserver variability among experts is limited[27], and the learning curve for image interpretation appears favorable[28]. In a large retrospective study comparing the dysplasia yield in BE’s patients undergoing Seattle protocol biopsies, VLE without laser markings, and VLE with laser markings (VLEL), both VLE with and without laser marking had statistically significant differences in dysplasia yield compared to Seattle protocol, (14% vs 1%, P = 0.001) and (11% vs 1%, P = 0.003), respectively[25]. VLE appears to be a safe form of advanced imaging. In a case series on 52 patients, the safety and feasibility of the NVision VLE system was assessed. Of the 52 patients undergoing VLE, only 2 minor adverse events were reported which includes mucosal lacerations that did not require therapy or intervention[29]. VLE does not appear to significantly increase endoscopic risk to patient but can lead longer procedure times,estimated 22 ± 6 min standard deviation, which can be of anesthetic concern[30].
The downside to VLE is that a large amount of information is presented that may be overwhelming or time consuming to interpret. Artificial intelligence (AI) technology has been developed that has recently been released and is under study in a prospective fashion[31]. This may help physicians process the large amount of information and images that are presented at one time. The AI technology is termed intelligent real-time image segmentation (IRIS) and highlights three VLE features associated with dysplasia . The three features of dysplasia include hyper-reflective surface, hypo-reflective structures, and a lack of layering. A hyper-reflective surface indicates a high surface signal (appears darker) relative to the subsurface. The image feature is represented as a pink color bar at the tissue surface. The lack of layering image feature is represented by an orange color bar at the exterior edge of the VLE image space. The hypo-reflective structure (usually glandular structures) is represented by a blue image overlay on top of the structure. IRIS displays an en face image of the scanned esophagus. There is also a luminal en face view that reconstructs the Barrett’s segment in regard to the three features. These allow for easier identification of overlap between the three colors and is high yield for areas that could be dysplastic. Figures 2-4 show VLE images with IRIS in patients with Barrett’s esophagus and high-grade dysplasia. Figure 5 shows the endoscopy view of the targeted laser marks (upper yellow arrow, lower yellow arrow) placed using volumetric laser endomicroscopy.
Prospective in-vivo studies are needed looking at the sensitivity and specificity of VLE in dysplasia detection for BE. A prospective multi-center study examining this has been completed and we are awaiting results[32]. We suspect VLE is the most sensitive tool for the detection of dysplasia in BE. For reference, acetic acid chromoendoscopy, narrow band imaging and endoscopic CLE have been shown to have high sensitivity of close to 90% in various studies. The VLE scoring system is evolving as quickly as the technology is being developed. The traditional current VLE scoring system (OCT-SI) generates a dysplasia score after the combination of 2 independent criteria (surface to subsurface signal intensity and glandular archi-tecture). The novel VLE diagnostic algorithm (VLE-DA) where a segment of BE is first characterized as having complete or partial effacement, then further categorized by subsurface intensity and number of atypical glands respectively. In a head to head comparison for the detection of dysplasia, pCLE, OCT-SI and VLE-DA were evaluated. The sensitivity for pCLE was 76% (95%CI: 59-88), for OCT-SI was 70% (95%CI: 52-84) and for VLE-DA was 86% (95%CI: 69-96)[33]. Finally cost utility studies are needed comparing the benefit of finding dysplasia and preventing cancer and its associated costs.
CONCLUSION
Advanced imaging in BE can be useful in management of these patients if it helps increase yield of dysplasia detection or help change management in a procedure. The decision to perform these procedures ultimately depends on if the benefit outweighs the cost and added time performing the procedure. In our practice the added benefits of narrow band imaging and volumetric laser endomicroscopy outweighs the costs and added time and thus we have incorporated this into our Barrett’s surveillance routine.
Future research may dictate which advanced imaging techniques become incorporated in the gastrointestinal society guidelines, but for now if the sensitivity,specificity, and cost of an exam is acceptable locally for a center/endoscopic imaging expert[34], then the advanced imaging tool is generally acceptable and thus worth the squeeze!

Figure 2 Volumetric laser endomicroscopy with artifical intelligence from the same patient as in Figure 1 with an en-face view showing an area of overlap(yellow arrow) between three features of dysplasia (orange is lack of layering, blue is glandular structures, and pink is a hyper-reflective surface).

Figure 3 Volumetric laser endomicroscopy from the same patient showing cross-sectional view of the area of overlap (yellow arrow 5.73” 5.41”) between three features of dysplasia (orange is lack of layering, blue is glandular structures, and pink is a hyper-reflective surface).

Figure 4 Volumetric laser endomicroscopy with artificial intelligence showing an up close snap shot of the abnormal area of overlap between three features of dysplasia (orange is lack of layering, blue is glandular structures, and pink is a hyper-reflective surface).
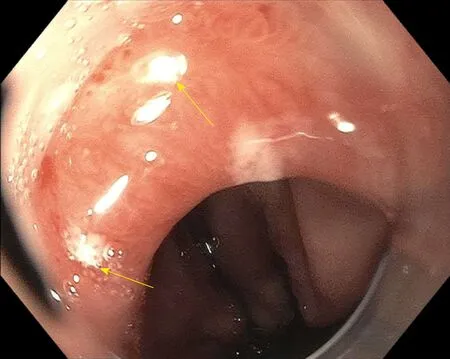
Figure 5 Endoscopy view of the targeted laser marks (upper yellow arrow, lower yellow arrow) placed using volumetric laser endomicroscopy. This corresponds to the same area highlighted by narrow band imaging. The pathology showed high-grade dysplasia.
 World Journal of Gastroenterology2019年25期
World Journal of Gastroenterology2019年25期
- World Journal of Gastroenterology的其它文章
- Fate plasticity in the intestine: The devil is in the detail
- Revisiting the liver’s role in transplant alloimmunity
- Hepatocellular carcinoma: Therapeutic advances in signaling,epigenetic and immune targets
- Hepatocellular carcinoma: Mechanisms of progression and immunotherapy
- Epidemiology of hepatitis E in South-East Europe in the "One Health" concept
- Infections with Helicobacter pylori and challenges encountered in Africa
