CDC42在慢性结肠炎小鼠发病中的作用及可能机制
董乐妹 夏芳芳 吴芳
[摘要] 目的 探讨CDC42在慢性结肠炎小鼠结肠中的变化及与淋巴细胞因子水平的关系。 方法 36 只C57BL/6小鼠随机分为对照组、模型组和美沙拉嗪干预组 ,每组 12 只。应用TNBS乙醇灌肠诱导慢性结肠炎模型,美沙拉嗪组于诱导模型后开始每日予美沙拉嗪(5-ASA)灌胃。造模开始后14 d处死小鼠, 检测结肠组织 IFN-γ、IL-17A、IL-4和TGF-β的mRNA表达水平以及结肠组织CDC42、P38 蛋白表达水平。 结果 模型组结肠组织 CDC42,P38表达较对照组明显升高(P<0.05),美沙拉嗪干预组较模型组表达降低(P<0.05)。模型组IFN-γ、IL-17A的mRNA表达水平较对照组明显增高(P<0.05);美沙拉嗪组较模型组表达水平下降(P<0.05)。模型组的IL-4和TGF-β表达水平较对照组升高,但差异无统计学意义(P>0.05),美沙拉嗪组较模型组表达水平增高(P<0.05)。 结论 CDC42可能通过P38调节淋巴细胞因子的水平参与小鼠慢性结肠炎的发病。
[关键词] 慢性结肠炎;CDC42;免疫;Th1/Th2
[中图分类号] R735.3 [文献标识码] A [文章编号] 1673-9701(2019)14-0037-04
[Abstract] Objective To study the changes of CDC42 in the colon of mice with chronic colitis and its relationship with lymphocyte factor levels. Methods 36 C57BL/6 mice were randomLy divided into control group, model group and mesalazine intervention group, with 12 rats in each group. TNBS ethanol enema was used to induce chronic colitis model, and gavage administration of mesalamine(5-ASA) was performed daily after induction of the model in the mesalazine group. Mice were sacrificed at 14 days after the start of modeling. The mRNA expression levels of IFN-γ, IL-17A, IL-4 and TGF-β in colon tissues, and CDC42 and P38 protein expression levels in colon tissues were detected. Results The expression of CDC42 and P38 in the colon tissue of the model group was significantly higher than that in the control group(P<0.05), and the expression in the mesalazine intervention group was lower than that in the model group(P<0.05). The mRNA expression levels of IFN-γ and IL-17A in the model group were significantly higher than those in the control group(P<0.05). The expression level of IFN-γ and IL-17A in mesalazine group was lower than that in the model group(P<0.05). The expression levels of IL-4 and TGF-β in the model group were higher than those in the control group, but there was no statistical significance(P>0.05). The expression level of IL-4 and TGF-βin mesalazine group was higher than that in the model group(P<0.05). Conclusion CDC42 may participate in the pathogenesis of chronic colitis in mice through P38, which regulates the level of lymphocyte factors.
[Key words] Chronic colitis; CDC42; Immunity; Th1/Th2
炎癥性肠病(inflammatory bowel disease,IBD)包括溃疡性结肠炎和克罗恩病[1]。近年国内的IBD发病率呈逐年上升趋势。IBD病因和发病机制至今尚未完全明确。目前认为免疫因素在该病发病中作用较为肯定,其中CD4+T细胞发挥了重要作用。Th1/Th2失衡一直被认为是IBD的重要因素之一[2]。Treg细胞和Th17细胞的发现成为Th1/Th2失衡学说的重要补充[2]。Thl细胞以分泌IFN-γ和IL-2为主,Th2细胞以IL-4、IL-5、IL-10为主。Th17细胞以分泌IL-17A和IL-22为主[3],Treg细胞以分泌IL-10和TGF-β、IL-23p19为主[2]。CDC42是Rho GTP酶蛋白家族中的一员,属于细胞内信号转导因子[4]。是MAPK通路的上游信号分子[4],有研究发现,CDC42与淋巴细胞的分化和成熟有关。本研究拟在动物水平研究慢性结肠炎发病中CDC42的可能作用及其与淋巴细胞因子的关系。
1 材料与方法
1.1 实验动物、试剂与药品
C57BL/6小鼠[常州卡文斯实验动物有限公司,许可证号SCXK(苏)2016-0010],美沙拉嗪(5-ASA)颗粒(爱的发制药公司,500 mg/袋,批号H20040727),RT(逆转录)试剂盒(Promega,批号0000123564),TRIZOL(Invitrogen,上海,规格:100 mL,批号:15596-018),RT-PCR实时荧光定量试剂盒(Promega,批号0000157591),2,4,6-三硝基苯磺酸(TNBS,美国Sigma公司,批号DX78935),苯巴比妥钠注射液(0.1 mg/1 mL,上海新亚药业公司,国药准字H31020501)等。
1.2 实验分组及实验时间
健康雄性C57BL/6小鼠36只(8~10周龄,体重16~22 g),随机分为正常对照组(n=12),模型组(n=12)和美沙拉嗪组(n=12)。本实验于2016年12月~2017年12月期间进行。
1.3 TNBS慢性结肠炎模型制备及标本采集
小鼠灌肠前禁食10 h,术前予苯巴比妥钠(10 mg/kg体重)腹腔注射麻醉,予3.5F导管连接1 mL注射器行灌肠。TNBS造模组和美沙拉嗪组首次予以2.25 mg TNBS和50%乙醇(共150 μL)混合液灌肠[5],7 d后予3.375 mg TNBS和50%乙醇(共150 μL)混合液重复灌肠。对照组仅用等体积的0.9%氯化钠溶液灌肠。灌肠后携鼠尾倒立不少于30 min。美沙拉嗪干预组于造模第2天开始每日予5-ASA(50 mg/kg体重)灌胃,对照组和TNBS造模组予等量蒸馏水灌胃。每天记录小鼠饮食、饮水、体重、粪便、活动情况。造模开始后第14天处死小鼠,留取血标本、结肠组织,测定血和结肠组织相关细胞因子水平。
1.4 观察指标
1.4.1 结肠HE染色 取结肠组织固定,包埋,切片,行HE染色,光镜下观察。
1.4.2 结肠相关细胞因子mRNA的real-time PCR测定 分离留取远端结肠组织,按说明书Trizol提取组织总RNA,测定RNA浓度并稀释至同一浓度。逆转录得cDNA。PCR步骤如下:95°C变性2 min,95°C 15 s、60°C 30 s、72°C 30 s扩增40个循环。取GAPDH为内参,ΔΔCt分析得各指标的相对表达丰度。
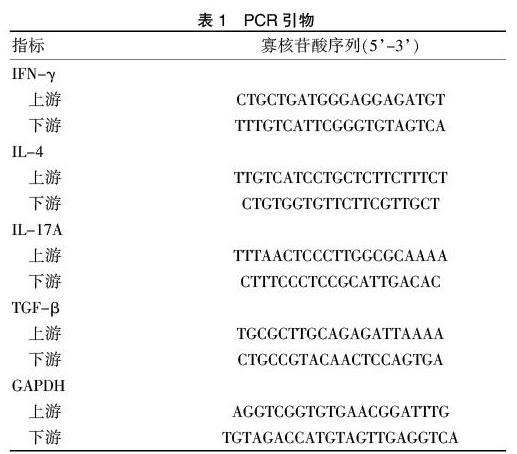
1.4.3 结肠组织CDC42和P38蛋白的western blot测定 用RIPA和蛋白酶抑制剂从小鼠结肠组织标本提取蛋白,BCA试剂盒测定蛋白浓度,所有样本稀释至同一浓度,10% SDS-PAGE分离等量样本蛋白(50 μg),并转至PVDF膜,牛奶封闭,一抗孵育过夜,洗膜,二抗孵育,显色。PCR引物见表1。
1.5 统计学方法
实验采用SPSS20.0(SPSS for windows)进行统计,实验数据用均数±标准差(x±s)表示。两组间比较采用独立样本资料t检验。多组间比较采用单因素方差分析。P<0.05为差异有統计学意义。
2 结果
2.1 模型验证
TNBS组小鼠结肠肉眼可见结肠短缩、黏膜糜烂、溃疡等改变;光镜下见结肠黏膜坏死,黏膜下层大量炎细胞浸润等现象;美沙拉嗪组的结肠组织损伤较TNBS组轻;对照组结肠组织未见明显异常。
2.2 各组结肠组织IFN-γ、IL-17A、IL-4和TGF-β的mRNA表达
与对照组比较,模型组IFN-γ、IL-17A的mRNA表达水平明显增高(P<0.05);美沙拉嗪组较模型组表达水平下降(P<0.05)。模型组的IL-4和TGF-β表达水平较对照组升高,但差异无统计学意义(P>0.05),美沙拉嗪组较模型组表达水平增高(P<0.05)。见表2。
2.3 各组结肠组织CDC42和P38蛋白表达水平
与对照组比较,模型组CDC42和P38蛋白表达水平明显增高(P<0.05);美沙拉嗪组较模型组表达水平下降(P<0.05)。见表3。
3 讨论
目前认为,免疫因素参与炎症性肠病的发病和进展,其中CD4+T细胞发挥了重要作用。疾病早期,肠道受微生物抗原刺激产生的细胞因子使未分化T细胞分化为Th1、Th2和Th17亚群,并影响调节性T细胞(Treg)的功能[2-6]。Th1细胞以表达IFN-γ和IL-2为主,Th2细胞以表达IL-4、IL-5、IL-10为主,Th1和Th2亚群比例失调参与了炎症性肠病的发病。Th17细胞是一群介导炎症反应的重要细胞,以表达IL-17和IL-22为主[7],Treg细胞是一类具有免疫抑制作用的T细胞亚群,以表达IL-10、TGF-β和IL-23p19为主。Th1/Th2失衡,Treg/Th17转化失衡被认为是IBD发病的重要因素[8-10]。调整CD4+T细胞亚群之间的平衡,或许可以成为缓解肠道炎症的一个新的策略和手段。
在诸多影响Th细胞分化的因素中,信号转导是免疫细胞激活的关键步骤,对Thl和Th2的分化有非常重要的调节作用[11]。CDC42是Rho GTP酶蛋白家族中的一员,属于细胞内信号转导因子。CDC42一旦被激活会与多种效应分子结合,具有调节细胞极性、迁移、分化、存活等功能[12]。有研究发现,CDC42与淋巴细胞的分化和成熟有关[13]。
本研究采用TNBS灌肠制备小鼠慢性结肠炎的模型[14],并采用慢性结肠炎的治疗药物美沙拉嗪干预[15]。造模后小鼠结肠出现短缩、糜烂,证明造模成功,美沙拉嗪干预组的结肠炎症较造模组明显缓解。本研究同时测定了各组小鼠结肠黏膜组织的Th1、Th2、Th17和Treg细胞的代表细胞因子IFN-γ、IL-4、IL-17和TGF-β水平。结果显示,TNBS造模后促炎细胞因子IFN-γ、IL-17在模型组明显增高,美沙拉嗪降低促炎因子的水平。而相对起抗炎作用的IL-4、TGF-β在模型组虽较对照组表达有所上升,但差异无统计学意义;美沙拉嗪治疗后IL-4、TGF-β水平较模型组增高。此外,模型组结肠组织的CDC42水平明显高于对照组,美沙拉嗪干预组CDC42水平下降,且与Th1和Th17优势促炎细胞因子的变化趋势相同。至此,本文得出:CDC42可能参与慢性结肠炎的发病。
作為Rho家族的GTP结合蛋白,CDC42与MAPK信号通路存在一定的联系[16],CDC42是MAPK通路的上游信号分子[17],而MAPK下游信号通路P38、ERK、JNK与炎症反应存在关系[18]。CDC42与MLK1结合后激活P38,导致MAPK-NF-κB通路活性改变[19,20],发生多级级联反应,最终导致广泛的炎症因子表达改变。本实验同时测定了结肠组织P38蛋白的表达水平,结果显示其变化趋势与结肠CDC42相同,与文献相符。
因此本文得出结论:在小鼠TNBS慢性结肠炎模型中,CDC42可能通过MAPK途径调节炎症因子水平参与结肠炎的发病。本实验由于条件限制,仅开展动物水平研究。在接下来的研究中,为进一步证实CDC42在慢性结肠炎发病中的作用,需待分离结肠黏膜内淋巴细胞行进一步研究。
[参考文献]
[1] Marquez L,Shen C,Cleynen I,et a1.Effects of haptoglobin polymorphisms and deficiency on susceptibility to inflammatory bowel disease and on severityofmurine colitis[J].Gut,2012,6l(4):528-534.
[2] Fuss I J,Neurath M,Boirivant M,et a1.Disparate CD4+lamina propria(LP) lymphokine secretion profiles in inflammatory bowel disease.CrohnS disease LP cells manifest increased secretion of IFN-γ,whereas ulcerative colitis LP cells manifest increased secretion of IL-5[J].J Immunol,1996,157(3):1261-1270.
[3] Raza A,Yousaf W,Giannella R,et a1. Th17 cells:Interactions with predisposing factors in the immunopathogenesis of inflammatory bowel disease[J]. Expert Rev Clin Immunol,2012,8(2):161-168.
[4] Zhang Y,Zhou ZH,Bugge TH,et al.Urokinase-type plasminogen activator stimulation of monocyte matrix metalloproteinase-1 production is mediated by plasmin-dependent signaling through annexin A2 and inhibited by inactive plasmin[J].J Immunol,2007,179(5):3297-3304.
[5] Terai T,Osawa S,Tani S,et al. Induction of murine TNBS colitis is strictly controlled by a modified method using continuous inhalation anesthesia with sevoflurane[J].Dig Dis Sci,2014,59(7):1415-1427.
[6] Cua DJ,Sherlock J,Chen Y,et al.Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain[J]. Nature,2003,421(6924):744-748.
[7] Kalim KW,Yang JQ,Li Y,et al.Reciprocal Regulation of Glycolysis-Driven Th17 Pathogenicity and Regulatory T Cell Stability by Cdc42[J].J Immunol,2018,200(7):2313-2326.
[8] Hundorfean G,Neurath MF,Mudter J.Functional relevance of T helper 17(Th 17) cells and the IL-1 7 cytokine family in inflammatory bowel disease[J].Inflamm Bowel Dis,2012,18(1):180-186.
[9] Padoni I,Iliev ID,Rossi G,et a1.Dendritic cells produce TSLP that limits the differentiation of Thl7 cells,fosters Treg development,and protects against colitis[J].Mucosal Immunol,2012,5(2):184-193.
[10] Xu J,Yang Y,Qiu G,et a1.Stat4 is critical for the balance between Thl7 cells and regulatory T cells in colitis[J].J Immunol,2011,186(11):6597-6606.
[11] Yates A,Callard R,Stark J.Combining cytokine signalling with T-bet and GATA·3 regulation in Thl and Th2 differentiation:A model for cellular decision-making[J].J Theor Biol,2004,231(2):181-196.
[12] Galan JE.Interaction of Salmonella with host cells through the centisome 63 type III secretion system[J]. Curr Opin Microbiol,1999,2(1):46-50.
[13] Procyk KJ,Rippo MR,Testi R,et al. Distinct mechanisms target stress and extracellular signal-activated kinase1 and Jun N-terminal kinase during infection of macrophages with Salmonella[J].J Immunol,1999,163(9):4924-4930.
[14] 楊舒,王新月,景姗,等.不同浓度三硝基苯磺酸和乙醇诱导的大鼠克罗恩病模型[J].中西医结合学报,2011(11):1242-1247.
[15] Shah B,Mayer L.Current status of monoclonal antibody therapy for the treatment of inflammatory bowel disease[J].Expert Rev Clin Immunol,2010,6(4):607-620.
[16] Hanna R,Hubbeling I,Takaya J,et al.NR4A1 (Nur77) deletion polarizes macrophages toward an inflammatory phenotype and increases atherosclerosis[J]. Circ Res,2012, 110(3): 416-427.
[17] Chrétien A,Dierick JF,Delaive E,et al.Role of TGF-beta1-independent changes in protein neosynthesis, p38alphaMAPK,and cdc42 in hydrogen peroxide-induced senescence-like morphogenesis[J].Free Radic Biol Med,2008,44(9):1732-1751.
[18] Lavin Y,Lavin Y,Mortha A,et al.Regulation of macrophage development and function in peripheral tissues[J].Nat Rev Immunol,2015,15(12):731-744.
[19] Qi M,Elion EA. MAP kinase pathways[J]. J Cell Sci,2005, 118(Pt 16):3569-3572.
[20] Spann NJ,Garmire LX,McDonald JG.Regulated accumulation of desmosterol integrates macrophage lipid meta-bolism and inflammatory responses[J].Cell,2012,151(1):138-152.
(收稿日期:2018-10-18)

