Rare-earth and trace elements and hydrogen and oxygen isotopic compositions of Cretaceous kaolinitic sediments from the Lower Benue Trough,Nigeria:provenanceand paleoclimatic signif icance
Anthony T.Bolarinwa·Sunday O.Idakwo·David L.Bish
Abstract This study evaluated the Cretaceous(Campanian–Maastrichtian)kaolinitic sediments of the Ajali/Mamu and Enugu/Nkporo Formations from the Lower Benue Trough of Nigeria.A combined method of inductively coupled plasma–mass spectrometry and isotope ratio mass spectrometry was used to investigate trace and rareearth element geochemistry and hydrogen and oxygen isotopic compositions.These data were then used to infer the sediments’provenance and paleoclimatic conditions during their deposition.The sediments contained low concentrations of most trace elements,with the exceptions of Zr(651–1352 ppm),Ba(56–157 ppm),V(38–90 ppm),and Sr(15.1–59.6 ppm).Averagevaluesof Co and Niwere 1.5 and 0.7 ppm,respectively.Traceand rareearth element values were lower than corresponding values for upper continental crust and Post-Archean Australian Shale,with the exception of Zr.The samples showed only slight light rare-earth enrichment and nearly f lat heavy rare-earth depletion patterns,with negative Eu and Tm anomalies,typical of felsic sources.Geochemical parameters such as La/Sc,Th/Sc,and Th/Co ratios support that the kaolinitic sediments were derived from a felsic rock source,likely deposited in an oxic environment.18O values ranged from+15.4 to+21.2‰for the investigated samples,consistent with a residual material derived from chemical weathering of felsic rock and redeposited in a sedimentary basin(typical values of+19 to+21.2‰).While in the basin,the sediments experienced extended interactions with meteoric water enriched inδD andδ16O.However,the variation inδD andδ16O values for the investigated samples is attributed to the high temperature of formation(54–91°C).TheδD andδ18O values suggest that the sediments,although obtained from different localities within the Lower Benue Trough,formed under similar hot,tropical climatic conditions.
Keywords Rare-earth and trace elements·Oxygen/hydrogen isotopic composition·Kaolinitic sediments·Lower Benue Trough·Nigeria
1 Introduction
The chemical composition of weathered rocks depends largely on the parent materials,the rate of weathering,and well-established concepts on element mobility during weathering(Singh et al.2005).The relative distribution of immobile elements—such as La and Th(felsic rocks);and Sc,Cr,and Co(basic rocks)—has been used to infer relative contributions,from sources within or outside the depocenter,of kaolinitic sediments from different environments(Wronkiewicz and Condie 1990).Larger light rare earth element(LREE)/heavy rare earth element(HREE)ratios and negative Eu anomalies are generally found in deposits originating from felsic rocks,whereas maf ic rocksproducedepositsthat have lower LREE/HREE ratios and small Eu anomalies(Cullers 1995).
The hydrogen and oxygen isotopic compositions of kaolinite are determined primarily by temperature and by the isotopic composition of the water present during crystallization(Savin and Epstein 1970;Lawrence and Taylor 1971,1972).BecauseδD and O values of meteoric water vary systematically with latitude and altitude(Craig 1961;Dansgaard 1964;Yurtsever and Gat 1981),the stable isotope composition of kaolinite formed during weathering is normally considered to ref lect the location,and hence the mean temperature,of the landscape surface(Lawrence and Taylor 1971,1972).For example,lower kaoliniteδD and O values are characteristic of cooler regions,typically located at higher latitudes and/or altitudes.For oxygen,post-depositional isotopic exchange between kaolinite and water is virtually non-existent(O’Neil and Kharaka 1976).Thus,thisclimatic signature is typically preserved.Measured hydrogen(H2/H1)and oxygen(18O/16O)isotope ratios for kaolinites in weathering prof iles can be used to estimate the isotope fractionation factor(Savin and Epstein 1970a).Indeed,a‘‘supergene/hypogene’’line has been established to distinguish between clay minerals formed under normal Earth surface conditions and those formed under higher burial temperatures(Sheppard et al.1969).Thus,hydrogen(2H/1H)and oxygen(18O/16O)isotope studies can be a powerful tool in understanding the genesis,temperature of formation,and paleoclimatic conditions under which a clay mineral formed(Sheppard and Gilg 1996;Savin and Hsieh 1998).
While several researchers(e.g.,Reyment 1965;Akande et al.1992;Zaborski1998;Idakwo,et al.2013;and Odoma et al.2015)have studied Lower Benue Trough stratigraphy,mineralization,and petroleum potential,there is a paucity of published work on trace elements,REEs,and O and H isotopic characteristics of the Cretaceous kaolinitic sediments from the area.The aim of this study was to determine the source of the sediments and the paleoclimatic conditionsunder which thesedimentsweredeposited using REEs and trace elements,aswell as Oand H isotopic compositions.Thisstudy used acombination of inductively coupled plasma–mass spectrometry and isotope ratio mass spectrometry method to evaluate kaolinitic sediments from the Lower Benue Trough to provide insight into the temperature of formation and the preserved climatic signature.
2 Geologic setting
The study area is near the Gulf of Guinea,south of the Chad Basin(6°00′00′′N to 8°20′00′′N and 6°50′00′′E to 8°00′00′′E)(Figs.1 and 2).We evaluated portions of the Ajali/Mamu and Enugu/Nkporo Formations,which are exposed at Aloji,Udene-Biomi,Agbenema,Ofejiji,Otukpa,Okpokwu,and Enugu,in the Lower Benue Trough(Fig.2).
Sedimentation in the Lower Benue Trough began with the Albian Asu River Group of marineorigin,it constitutes the shales,limestones,and sandstone lenses within the Abakaliki Formation of Abakaliki area and the Limestones from Mfamosing in the Calabar Flank(Petters 1982;Figs.2 and 3).This group preceded the deposition of the Ezeaku Formation(Ezeaku Shales of Turonian age)and Makurdi Formations.These shales of Ezeaku are thick,f laggy,calcareous and non-calcareous with Limestones(sandy or shelly)and calcareous sandstones.Exposures of these shales are observed in the southern part of Otukpa with varying thickness from~305 m in the northern part to~610 m southward.The Ezeaku Formation is overlain by the marine Cenomanian–Turonian Nkalagu Formation,composed of black shales,limestones,and siltstones.Deformation during the Mid-Santonian within the Benue Trough resulted in the displacement of major depositional axis towards the west,thus,leading to the formation of the Anambra Basin.Anambra Basin represents the post-deformational sedimentation in the Lower Benue Trough.The process of sedimentation in the Anambra Basin started with the marine and paralic shales of the Enugu and Nkporo Formations during the Campanian–Maastrichtian,this marks a depositional cycle represented by the brackish marsh and fossiliferous pro-delta facies of the Late Campanian–Early Maastrichtian(Fig.2).The Ajali and Owelli Formations’f luvio-deltaic sandstones lie conformably on the Mamu Formation and its lateral equivalents are observed in most places.The Nkporo cycle represents an overall regression for an epoch during which the coalbearing Mamu Formation and the Ajali Sandstone accumulated while the onset of another transgression was marked during Paleocene with the Nsukka Formation and the Imo.Finally,within the Lower Benue Trough,there was returned regressive conditions marked by the Eocene Nanka sands,thus,suggesting more predominance f loodtidal currents than weak ebb-reverse currents(Zaborski 1998;Obaje 2009).
The kaolinitic clay,a unit in the Mama and Ajali Formations,has an exposed,weathered surface at Aloji,along the Ayingba-Itobe road.The mine pit reveals massive dirty white clay about 9.90 m thick,brown and gray clays about 0.75 m thick,brown clay about 1.55 m thick,and conglomeritic sandstone about 0.50 m thick(Fig.4a)The exposed kaolinitic clay within the Enugu/Nkporo Formations at Enugu is massive,and has a thickness of about 61 m,including a 20-m thick dirty white layer at the bottom,a grayish layer of 1 m,a mixed brown and dirty white layer of 38 m,and a lateritic capping of 2 m(Fig.4b).
The kaolinitic Cretaceous(Campania-Maastrichtian)sediments of the Ajali/Mamu and Enugu/Nkporo Formations from the Lower Benue Trough of Nigeria were selected for study because of the abundance of sediment within these formations and the comparative lack of published data on its hydrogen(2H/1H)and oxygen(18O/16O)isotopic compositions and trace element and REE compositions.
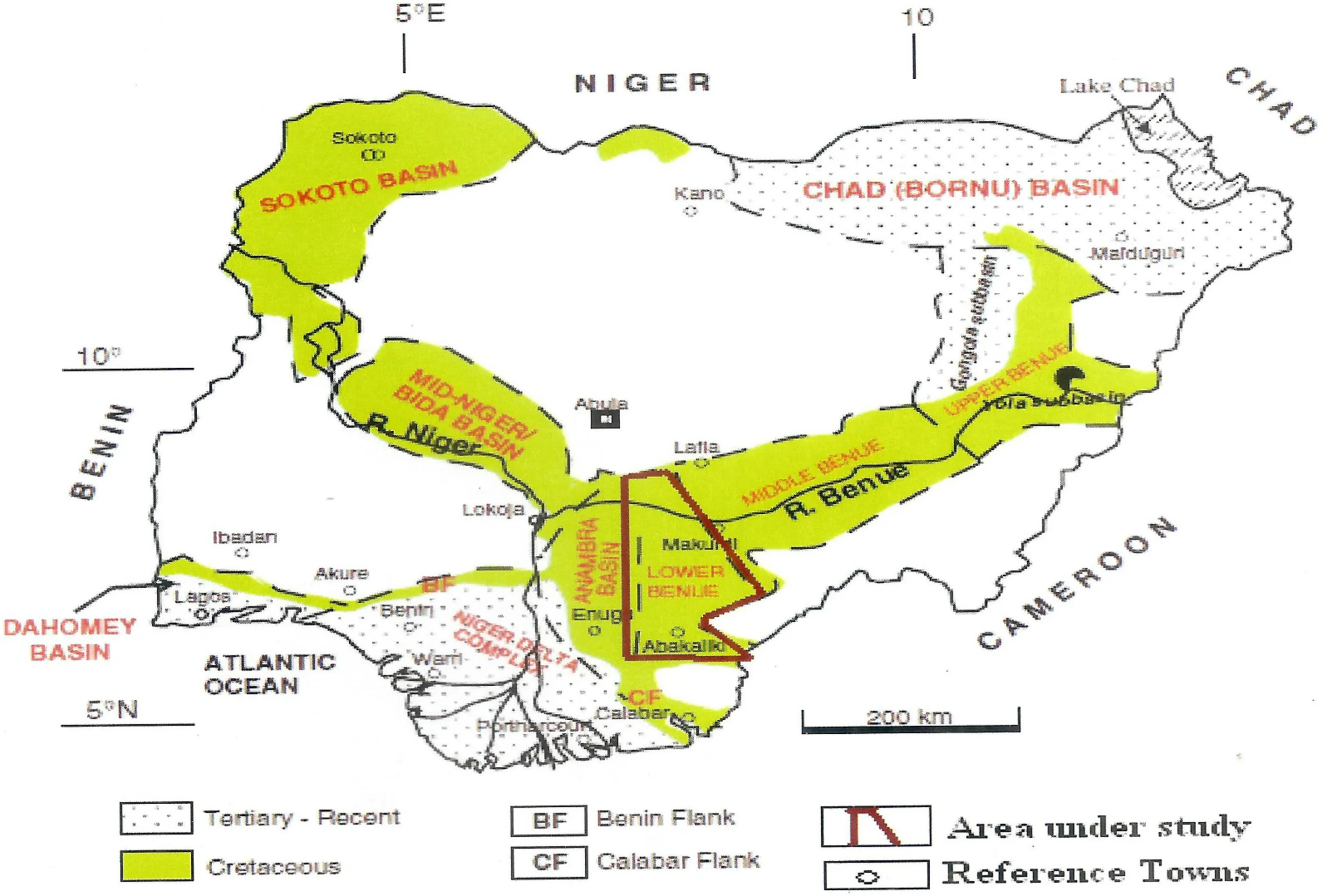
Fig.1 Map of Nigeria showing the position of the Lower Benue Trough(Modif ied after Obaje 2009)
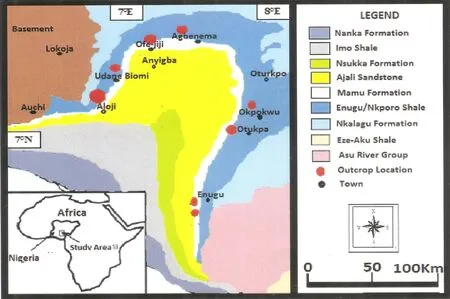
Fig.2 Geologic map of the Lower Benue Trough showing sample locations
3 Methods

Fig.3 Idealized N–Sstratigraphic cross-section across the Benue Trough and the relationship to the Niger Delta and the Chad Basin(vertical scale exaggerated;erosion and uplift not considered).(After Obaje 2009)
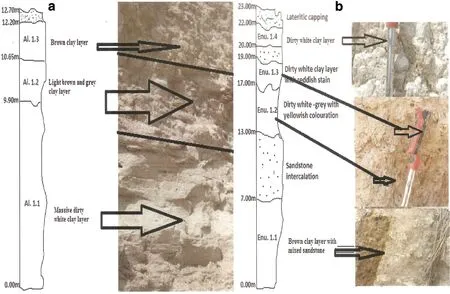
Fig.4 Lithostratigraphy of kaolinitic-rich deposits a at Aloji along the Ayingba-Itobe road,Kogi State(N 07°24′45′′and E006°56′17′′)and b along the Abakaliki-Onitsha road,within Iva Valley Enugu State(N06°27′54.6′′and E007°27′17.1′’)
Thirty-four sediment sampleswere collected from different vertical sections of the exposures in Aloji,Udene-Biomi,Agbenema,Ofejiji,Otukpa,Okpokwu,and Enugu in the Lower Benue Trough(Fig.2).Efforts were made to avoid weathered horizons (Fig.4).Samples for chemical analyses were selected based on variety and location within the Lower Benue Trough for a representative distribution.The samples were pulverized and packaged for trace element and REE analyses at the ACME Analytical Laboratories Ltd,Canada,using an inductively coupled plasma–mass spectrometer(Perkin-Elmer Elan 6000).Trace element and REE analyses used powdered and pressed pellets prepared from 3 to 5 g samples that were digested by weighing 0.2 g aliquots in a graphite crucible mixed with 1.5 g lithium metaborate/tetraborate(LiBO2/LiB4O7)f lux.The crucibles were placed in an oven and heated at 980°C for 30 min.The cooled bead was dissolved in 5%HNO3(ACS grade nitric acid diluted in demineralized water).Calibration standards and reagent blanks were added to sample sequences.A selection of 34 elements(Ba,Co,Cs,Ga,Hf,Nb,Rb,Sn,Sr,Ta,Th,U,V,Y,Zr,La,Ce Pr,Nd,Sm,Eu,Gd,Lu,etc.)was determined for the clay samples.Hydrogen and oxygen isotopic analyseswere carried out at the Department of Geological Sciences,Indiana University,Bloomington,Indiana,U.S.A.Extraction of H for isotopic analysis followed the principle of Bigeleisen et al.(1952).Samples(from 0.741 to 2.767 mg)were degassed at 50°C in a vacuum to remove adsorbed moisture;H2O was extracted from the clays by heating the samples in a Mo crucible with an induction furnace to 1404°C,and H2O was converted to H2gas by reduction over hot U.Yield ranges of 100±2%of the theoretical amounts were determined monometrically.Hydrogen isotopic composition was measured on a Delta Plus XP isotope ratio mass spectrometer(IRMS)with a TCEA inlet spectrometer calibrated using Vienna Standard Mean Ocean Water(VSMOW).
Oxygen for isotopic analysis was liberated quantitatively from dried and thoroughly out-gassed samples(2.9–4.2 mg)that were dried at 200°C for 12 h before reaction with bromine pentaf luoride at 550°C,following Clayton and Mayeda(1963).Oxygen was subsequently converted to CO2by reaction with hot graphite at a temperature of about 550–600°C.Theδ18O value of this gas was measured using a Thermo Scientif ic MAT 253 dual inlet mass-spectrometer calibrated using VSMOW.The precision of the isotope measurements was estimated at±0.2‰for O and±2‰for H.
4 Results
4.1 Trace elements
The sediment sampleswere depleted in trace elementswith the exception of Zr.The Zr range(651–1352 ppm)of the samples was higher than the Zr content of Post-Archean Australian Shale(PAAS)(210.00 ppm),upper continental crust(UCC)(190.00 ppm),and North American Shale Composite (NASC) (210.00 ppm).In contrast,Ba(56–157 ppm)was lower than PAAS(650.00 ppm),UCC(550.00 ppm),and NASC(636.00 ppm);V(38–90 ppm)was lower than PAAS(150 ppm),UCC(60 ppm),and NASC(130 ppm);and Sr(15.1–59.6 ppm)(Table 1)was lower than PAAS(200.00 ppm),UCC(143.00 ppm),and NASC(350.00 ppm).Average values of Co and Ni were 1.5 and 0.7 ppm,respectively.These concentrations are much lower than the corresponding values of 10 and 20 ppm,respectively,for Co and Nicontents of UCC.The average value of 53.1 ppm obtained for V is similar to an average of 60 ppm obtained by Taylor and McLennan(1985)for UCC.This indicates that although vanadium is considered to be associated with organic matter,it is hosted in the samplesby detrital silicate minerals(Stow and Atkin 1987).All values for PAAS and UCC in this paragraph from Taylor and McLennan(1985);for NASC,from Gromet et al.(1984).
Other trace elements were generally lower than average crustal values.Zircon was signif icantly enriched in all samples,with values up to 1352 ppm.The Sr and Ba contents showed a general depletion in comparison with UCC values (Table 1, Fig.5), averaging 27 and 89.41 ppm,respectively.Strontium and barium mostly reside in plagioclase and K-feldspar,respectively(Puchelt 1972).A depletion of Ba could be due to recrystallization of clays and progressive destruction of feldspars,probably due to preferential loss during weathering and erosion associated with hydration energy(Cullers et al.1988;Liu et al.2013).
The average Th value of 14.5 ppm of the samples was slightly higher than the corresponding UCC value of 10.7 ppm(Fig.5).
The correlation coeff icients of some of the trace elementsrelevant to thedetermination of their associationsare presented in Table 2.The results show positive correlation between Ba and Co,Ba and Ni,and Ba and Sr.Positive correlations also exist between Ni and Sr,Ni and Co,and Th and La,suggesting that all these trace elements are associated with clay minerals.The prominent negative correlation between Zr and Co,aswell asthat of Zr and Ni,combined with higher Zr concentration,probably ref lects the concentration of heavy minerals during recycling and sorting(Table 2).
The La/Sc(5.1–8.4),La/Th(2.1–3.9),Th/Sc(0.6–2.8),and Th/Co(10.4–20.6)values suggest a mixed source for the clay samples(Table 3)and when compared to ratios derived from felsic rocks,maf ic rocks,UCC and PAAS,indicate that such ratios are within the range of felsic source rocks(Table 4).Th/U(3.2–6.4),Cu/Zn(0.3–12.6),and Cu+Mo/Zn(0.4–12.7)suggest an oxidizing depositional condition of the sediments within the Lower Benue Trough(Table 4).However,two samples(Al.1.1 and Al.1.2)returned high ratios,probably due to the high concentration of copper in these two samples(Table 4)as a result of copper’s association with organic matter.The UCC-normalized trace elements(Fig.5)show elevated Hf and Zr,but low Sr and Rb values,consistent with felsic crustal rocks.
4.2 Rare earth elements
The REE compositions of the kaolinitic sediments presented in Table 5 show that Ce was>100 ppm in many of the samples.La,Nd,and Y were>25 ppm,whereas Pr,Sc,Sm,Dy,and Gd values were between 4.0 and 10 ppm.Other REEs,notably Eu,Tb,Ho,Tm,and Lu were<2.0 ppm.These REEvalues are generally lower than the values obtained by Okunlola and Idowu(2012)in the adjacent Bida Basin at the northwestern side of the Lower Benue Trough(Fig.1).The investigated clay samples had fractionated REE patterns with(La/Yb)cnvarying from 5.79 to 14.50 and(Gd/Yb)cnvarying from 0.14 to 2.05(Table 5).These characteristics indicate that the original source area was felsic and the negative Eu anomaly is regarded as evidence for a differentiated source,similar to granite(Taylor and McLennan 1985,1985;McLennan 1989;McLennan et al.1993)(Table 5).The REE data are comparable to PAAS values (Table 6). The Eu concentrations of the sediments were very low(Table 5),and the chondrite-normalized REE plot(Fig.6)shows that the sediments were LREE-enriched,with a near-f lat HREE pattern with very low to negative Eu and Tm anomalies.The two samples that showed high Ho composition suggest contamination,possibly from a maf ic source.Overall,the REE compositions were consistent with a felsic source rock.

Table 1 Trace element composition(ppm)of kaoliniticsediments from Lower Benue Trough,Nigeria

Fig.5 UCC normalized trace elements plot of the kaolinitic sediments of the Lower BenueTrough,Nigeria
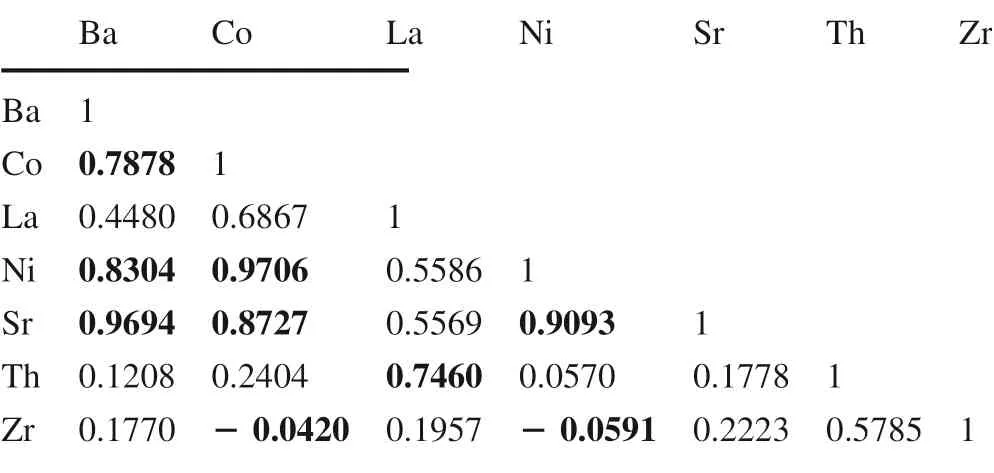
Table 2 Correlation coeff icients of trace elements in the kaolinitic sediments of Lower Benue Trough,Nigeria
4.3 Hydrogen and oxygen isotopes
Hydrogen and oxygen isotopic compositions of the sediments are reported in the conventional delta(δ)in parts per thousand(‰)relative to VSMOW(Gof iantini 1984).The δD valuesof theclay fell between-50.80 and-64.40‰,and theδO18values between 15.40 and 21.20‰(Table 7).The oxygen and hydrogen fractionation factors betweenkaolinite and water were calculated using Eqs.1 and 2(Savin and Epstein 1970),and we used theδ18O=-5‰andδD=-30‰values of Friedman(1964)for local ground/meteoric water in equilibrium with kaolinite as an alternative way to calculate the fractionation factors for both oxygen and hydrogen isotopic compositions of the investigated clay deposits.The values obtained here are in good agreement with those of Savin and Epstein(1970),suggesting that the kaolinite in these samples was in oxygen/hydrogen isotopic equilibrium with local ground water.

Table 3 The calculated trace element ratios for the kaolinitic sedimentsof Lower Benue Trough,Nigeria

Table 4 Range of elemental ratios in this study compared to ratios derived from felsic rocks,maf ic rocks,upper continental crust and Post-Archean Australia shale

?
Based on revisions of existing empirical data,Sheppard and Gilg(1996)proposed the following oxygen andhydrogen isotope fractionation equations between clay mineral(kaolinite)and water:
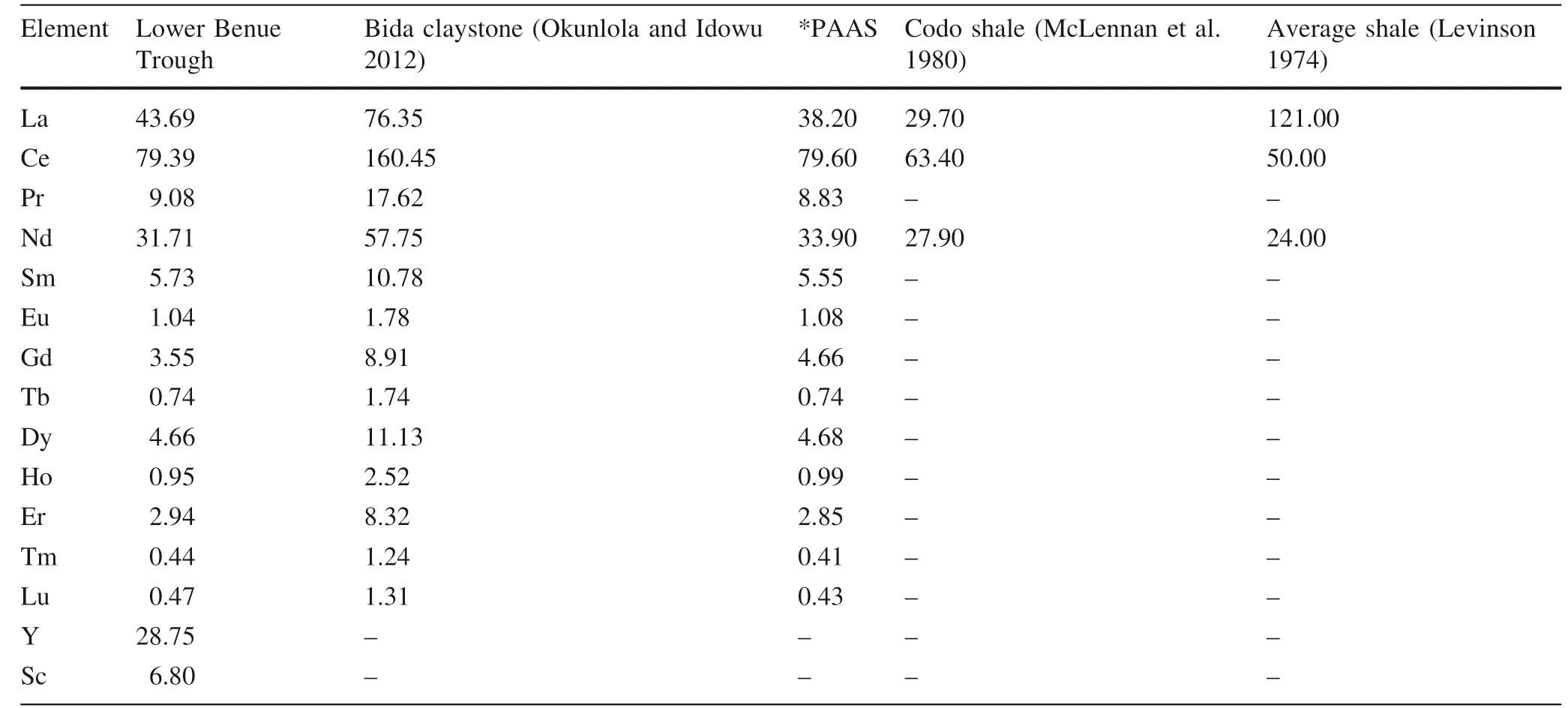
Table 6 Average rare-earth elements of Lower Benue Trough clay compared to world averages

Fig.6 Chondrite-normalized REE plot for the kaolinitic sediments of the Lower Benue Trough,Nigeria


Table 7 Hydrogen and oxygen isotopic compositions of kaolinitic sediments of Lower Benue Trough,Nigeria with calculated temperature of formation

where18αandDαare the oxygen and hydrogen isotopic fractionation factors between kaolinite and water,respectively,and T is in Kelvin.The equation of oxygen isotope fractionation was employed to calculate the temperature of formation for these kaolinitic deposits(Table 7).The results show that temperatures of 54–91°C were attained during the weathering and deposition of the kaolinitic sediments within the superf icial level of the Earth.TheδD andδ18O values are plotted in Fig.7,with some scatter between the kaolinitic line of Savin and Epstein(1970),as modif ied by Sheppard and Gilg(1996),and the supergene/hypogene line(S/H)of Sheppard et al.(1969).
5 Discussion
The geochemical signatures of clastic sedimentshave often been used to ascertain provenance and tectonic setting(Taylor and Mclennan 1985;Cullers et al.1987;Cullers 1995;Armstrong-Altrin et al.2004).In particular,trace element and REE contents are particularly powerful indicators of origin and process.For example,Th and La,which are consistent with a more felsic source,and Sc and Cr,consistent with a maf ic source,have been exploited to distinguish between felsic and maf ic provenance by many authors(McLennan et al.1980;McLennan 1989;Wronkiewicz and Condie 1990;McLennan and Taylor 1991).In addition,La/Sc,Th/Sc,and Th/Co ratios generally return signif icantly and radically different values in felsic and basic rocks(Wronkiewicz and Condie 1990;Cox et al.1995;Cullers 1995).Some of the Th/Sc values for the Benue Trough sediments were greater than the UCC average value of 0.79,suggesting that the sediments may not have originated from the same source but rather from mixed sources(Tables 3 and 4).
Depletion of transition metals in the Benue Trough samples may be attributed to the abundance of felsic components in the source(Liu et al.2013).The Th/Co,Th/Sc,and La/Sc ratios for clay samples from this study were compared with those of felsic and basic rock-derived sediments,UCC and PAAS values,and the Bida claystone(Tables 3 and 4),revealing that the chemistry of the Benue Trough rocks is consistent with a felsic source.In addition to these ratios,the higher LREE/HREE ratios and low Eu anomalies(Table 5)corroborate the felsic source-rock characteristics of the kaolinitic sediments.Chondrite-normalized REE distributions(Fig.6)show that the deposits are LREE-enriched,with an almost f lat HREE pattern,and very low to negative Eu and Tm anomalies.
Hallberg(1976)used Cu/Zn and(Cu+Mo)/Zn ratios as redox determinants.He stated that high Cu/Zn and(Cu+Mo)/Zn ratios indicate reducing depositional conditions,while low Cu/Zn and(Cu+Mo)/Zn ratios suggest oxidizing conditions.In this study,almost all Cu/Zn ratios for the sediments are within the range of 0.2–3.6,except for Al.1.1(12.6)and Al.1.2(6.8)(Table 3),consistent with oxidizing depositional conditions.The U/Th ratio may also be used as a redox indicator,with a higher ratio found in organic-rich mudstones(Jonesand Manning 1994).A U/Th ratio<1.25 suggests oxic depositional conditions,whereas values>1.25 suggest suboxic to anoxic conditions(Nath et al.1997).The samplesanalyzed in thisstudy have consistently very low U/Th ratios of 0.2–0.3,suggesting deposition of thesedimentsin an oxic environment.
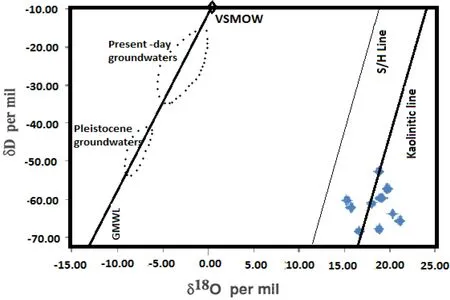
Fig.7 TheδD andδ18O plot of the kaolinitic sediments of the Lower Benue Trough on the Savin and Epstein(1970)diagram as modif ied by Sheppard and Gilg(1996).The concentration of the data around the kaolinitic and the supergene/hypogene(S/H)lines of Sheppard et al.(1969)indicates formation or equilibration of kaolinite with meteoric waters
Jones and Manning(1994)also suggested that Ni/Co ratios<5 indicate oxic environments,whereas ratios>5 indicate suboxic and anoxic environments.All the analyzed samples in this study had Ni/Co ratios of<1(Table 3),further conf irming that the Lower Benue Trough kaolinitic sediments were deposited under oxic chemical conditions.
The clay mineral in these investigated deposits,kaolinite,can form in a wide range of environments near the Earth’s surface during weathering, diagenesis, or hydrothermal processes(Murray and Janssen 1984;Savin and Lee 1988).In order to know the possible conditions of kaolinite formation,it is important to use other tools to ref ine information on genesis and potential paleoclimate.According to Savin and Lee(1988),the isotopic composition of clay minerals(e.g.,kaolinite)may ref lect geologic conditions during their formation,provided that the mineralsdid not undergo changesin isotopic composition after deposition.For example,the O isotope composition of kaolinitesof sedimentary origin usually varies from+19 to+23‰,and kaolinite from residual depositshas18Ovalues between+15 and+19‰(Murray and Janssen 1984).In the present study,the18O of kaolinite in the Benue Trough measured+15.4 to+21.2‰.This range is consistent with a residual material derived from chemical weathering of felsic rock,which was subsequently transported by water over a very short distance(colluvial deposit)(+15.4 to+19‰),or a relatively long distance(alluvial deposit)and deposited in a sedimentary basin(+19 to+21.2‰),where the sediments experienced longer interaction with meteoric water enriched inδD andδ18O,resulting in variedδD and δ16O values due to the generally high temperature of formation(54–91°C)(Table 7).The values recorded in Table 7 are characteristic of kaoliniteformed under hot and humid climatic conditions(Hassanipak and Eslinger 1985;Mizota and Longstaffe 1996).From the above-mentioned data,the paleoclimate was probably much hotter.Kaolinitesformed during weathering at high latitudesor altitudes,or at a large distance from the coast,where meteoric water is more depleted inδD andδ18O,generally have relatively low temperature of formation(Hassanipak and Eslinger 1985;Mizota and Longstaffe 1996).TheδD andδ18O values of the kaolinitic sediments of the Benue Trough measured in the current study suggest that the Cretaceous clay deposits formed under climatic conditionsmuch hotter than the present day,yielding the calculated temperature shown in Table 7.Thecurrent isotopic datawerecompared with published data(Table 8),which clearly illustrate the contrast between highδ18O clay minerals formed at equatorial latitudes,and the higher latitudes around Australia with lowerδD andδ18O values.
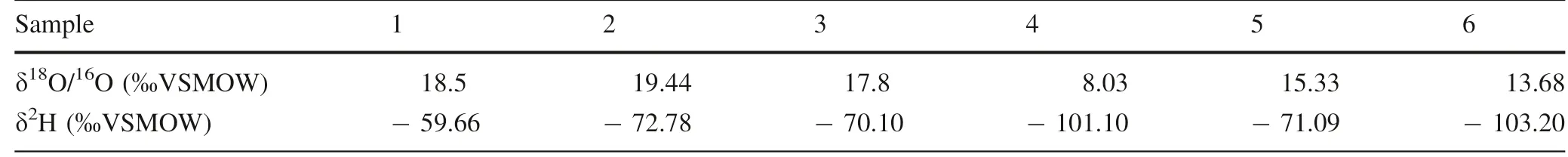
Table 8 Averagehydrogen and oxygen isotopic compositionsof kaolinitic sedimentsof Lower Benue Trough,Nigeriacompared to other world averages
A few samples in theδD andδ18O diagram(Fig.7)fell between the kaolinitic line of Savin and Epstein(1970)as modif ied by Sheppard and Gilg(1996)and the supergene/hypogene line(S/H)of Sheppard et al.(1969),suggesting formation or equilibration of kaolinite with meteoric waters at surf icial temperatures.The distribution of the data does not support a hydrothermal origin(S/H line of Sheppard et al.1969)because hydrothermal deposits are characterized by much lowerδ18O values(Sheppard et al.1969;Gazis et al.1987).The slight deviations of some samples from the kaolinitic line suggest post-depositional modif ication of the isotopic composition of these clay deposits,perhaps due to the interaction between earlier-formed kaolinite and downward percolating meteoric water(Longstaffe and Ayalon 1990).The similarity in theδD and δ18O values of the Cretaceous deposits suggests that though the clay samples were taken from different localities within the Benue Trough,they formed under almost thesameclimatic conditions(Table 7).The small variation inδD values observed in the investigated clay deposits(Fig.7)suggests that theδD values either do not ref lect differences in pore-f luid composition,or that they were subsequently affected by processes that minimized any initial differences between the colluvial and alluvial deposits.The variation in values could be related to considerable amounts of quartz,which might cause a signif icant effect of non-clay minerals on the isotopic composition of the clay deposits.However,the isotopic composition of the water in contact with the clay mineral during formation,the clay-water fractionation factors,the extent to which clay-water isotopic equilibrium was established,and the temperature at which fractionation occurred cannot be ruled out as contributing factors(Savin and Epstein 1970).TheδD values for some samples(Fig.7)suggest that the clay mineral is in hydrogen isotopic equilibrium with the present formation water,as supported by theδ18O values.Such a re-equilibration of hydrogen isotopes appears to have a signif icant effect at temperatures<100°C(Table 7).At higher temperatures(~200°C),H+-exchange,rather than OH-replacement,would be the controlling mechanism (Longstaffe and Ayalon 1990).
6 Conclusions
Geochemical data,such as La/Sc,Th/Sc,and Th/Co ratios,show that the Cretaceouskaolinitic sediments(Ajali/Mamu and Enugu/Nkporo Formations)were derived from felsic source rocks rather than maf ic source rocks.This conclusion is also broadly supported by the REE data.The hydrogen and oxygen isotopic compositions of the investigated clay deposits are very similar,suggesting their formation under similar hot and humid climatic conditions.The geochemical data suggest the deposition of the kaolinitic sediments under hot and humid climatic conditions closer to the equator than the present location.The minor scatter of the stable isotope data between the kaolinitic and supergene/hypogene lines suggests equilibration of kaolinite with meteoric waters at surf icial temperatures rather than hydrothermal origin.
The data from trace elements,REEs,and O2and H2isotopeson the kaolinitic-rich sediment were very useful as they provide information on the origin,source,and depositional process,including the altitude of deposition and ancient climatic conditions.
AcknowledgementsThe authors appreciate the assistance of Matthew Lilley,Benjamin Underwood,and Peter Sauer of the Department of Geological Sciences,Indiana University,Bloomington,Indiana,USA for their assistance with the oxygen extraction and with oxygen and hydrogen isotope analyses.
- Acta Geochimica的其它文章
- Correction to:The enhanced element enrichment in the supercritical states of granite-pegmatite systems
- Hydrogeochemical evaluation and statistical analysis of groundwater of Sylhet,north-eastern Bangladesh
- Effects of mineral-organic fertilizer on the biomass of green Chinese cabbage and potential carbon sequestration ability in karst areas of Southwest China
- Trace element partitioning between amphibole and hydrous silicate glasses at 0.6-2.6 GPa
- Geochemistry of subsurface Late Quaternary ironstones in Rajshahi and Bogra Districts,Bangladesh:implications for genetic and depositional conditions
- Mercury speciation,bioavailability and risk assessment on soil-rice systems from a watershed impacted by abandoned Hg mine-waste tailings

