The enhanced element enrichment in the supercritical states of granite-pegmatite systems
Rainer Thomas·Paul Davidson·Karen Appel
Abstract In this paper,we show that supercritical f luids have a greater signif icance in the generation of pegmatites,and for ore-forming processes related to granites than is usually assumed.We show that the supercritical melt or f luid is a silicate phase in which volatiles;principally H2O are completely miscible in all proportions at magmatic temperatures and pressures.This phase evolves from felsic melts and changes into hydrothermal f luids,and its unique properties are particularly important in sequestering and concentrating low abundance elements,such as metals.In our past research,we have focused on processes observed at upper crustal levels,however extensive work by us and other researchershavedemonstrated that supercritical melt/f luids should be abundant in melting zones at deep-crustal levels too.We propose that these f luids may provide a connecting link between lower and upper crustal magmas,and a highly eff icient transport mechanism for usually melt incompatibleelements.In thispaper,we explorethe unique featuresof thisf luid which allow the partitioning of various elements and compounds,potentially up to extreme levels,and may explain various features both of mineralization and the magmas that produced them.
Keywords Granites·Pegmatites·Supercritical state·Extreme element enrichment
1Introduction
During the study of melt inclusions in minerals of granites and pegmatites using high-pressure homogenization experiments(cold-seal pressure and hydrothermal rapidquench experiments)we found two different inclusion types,type-A and type-B melt inclusions in pegmatites,as well as evolved granites(Table 1),which represent conjugatemeltsresulting from melt–melt immiscibility along a pseudobinary solvus boundary.In an extensive series of published studies(see Thomas and Davidson 2016a)we have demonstrated that the two conjugate melt fractions evolve to more peraluminous and less water-rich composition(type-A melts)and to more peralkaline and very water-rich compositions(type-B melts).In the case of granites,in addition to the predominant inclusions representing the main crystallization sequence,we also found these extremely water rich melt inclusions,which cannot be assigned to the classic crystallization processes.Further studieshaveshown that these inclusion types,although rare in granites,are present in many granites and are dominant in pegmatites.Based on the Raman spectroscopic determination of water a careful analysis of such inclusion in pegmatites has led us to the conclusion that these inclusions represent pseudo-binary solvi(Fig.1).In Thomas and Davidson(2016a,b)we demonstrated that by normalising the peak temperatures and water concentration of the two-phase f ield of all the studied solvus systems can be brought together in a good approximation(the theorem of corresponding states is valid here).Note that the water concentration in the water-rich melt is a proxy for the critical density(Fig.4 in Thomas and Davidson 2016a).

Table 1 Experimental base:solvusdatafor pegmatites and granites,obtained from melt inclusionsin quartz(and topaz)using therapid quench cold-seal pressure vessel technique
As consequence of the demonstration that some melt inclusions form a pseudo-secondary solvus,it follows that at temperaturesand pressuresabovetheir critical valuesthe melt or f luid issupercritical,and that although transient and fugitive,this state is not a rare case.It is particularly important to note that in the vicinity of the critical point,namely for normalised temperatures in the range of 0.90–1.2 (730°C±20°C) small changes in these parameters causes enormous changes in the normalised density and,in consequence,in all the properties and behavioursof thissubstance,acommon behaviour noted in many supercritical systems(Bolmatov et al.2013;Pioro and Mokry 2011).It should be noted that such inclusions are found in granites and pegmatites at upper crustal levels,where they survive at temperaturesand pressuresconsistent with those found in felsic magma chambers generally,provided there are signif icant concentrations of volatiles and f luxing components,such as alkali carbonates and bicarbonates,water,etc.However,the origin of such melt/f luids may lie elsewhere.We propose a mechanism similar to Candela’s‘‘bubble plume’’(Candela 1997)but which can operate from lower crustal melting zones up to emplacement,and is at least as eff icient in sequestering metals and volatiles.
The extreme and unusual enrichment of some elements found in MI from a number of pegmatites(Thomas and Davidson 2016a)is further proof that supercritical conditionsarereal phenomenon during crystallization of granites and pegmatites.Furthermore,in the case of miarolitic pegmatitesin the Ko¨nigshain granite,wecan show that due to variation in pressure and cooling the supercritical state can repeatedly appear sequentially,i.e.that new aliquots may be created whenever conditions permit,and existing aliquots may evolve as temperature and pressure decrease,i.e.a conjugate melt fraction exsolved at one temperature may re-intersect the 2-phase boundary again at a lower temperature.
2 General properties of the near critical volatilerich melts
Figure 2 gives the results of water determinations on water-rich melt inclusions in quartz and topaz from a number of granites and pegmatites(listed in Table 1).The pseudo-binary solvus curves for granites and pegmatites are shown using normalized temperature and water concentration(seealso Fig.4 in Thomasand Davidson 2016a).The second curve(empty dots,light grey curve and some points nearby)are F-rich melt inclusions in topaz representing topaz-albite-granites.With these exceptions,almost all data points sit closely on the solvus curve using normalized coordinates.The f itting parameters for waterrich melt inclusionsin granites,topaz-albitegranite F3,and for the pegmatites are summarized in Table 2.Apart from the granite F3,in normalized coordinates the pegmatites show astonishing very good f it.Thus for the pegmatites listed in Table 2,the temperature and the water concentration in the melts are the predominant process-determining parameters. Variations in the composition(excluding water)are of secondary signif icance(F excepted).In the case of the water-rich melt inclusions in granites the f itting can be improved signif icantly(see Table 2)by excluding the anomalous data that was inf luenced by higher f luorine concentrations,i.e.the F-rich topaz granites.However,from thedata in Table 2 it isclear that although the water-rich granite and pegmatite melt groups show some differences despite the good f it,there are also signif icant similarities regarding temperature and water concentration as well as the strong element enrichment in the near-critical range.

Fig.1 Melt inclusions in granite quartz(Kirchberg,Saxony).Showing the solvus curve for this granite(top left)and the position of three different melt inclusions(a,b,c)on this curve.The melt inclusions contain a water-rich glass[about 15,31,and 38%(g/g)H2O,respectively]and a water-rich sub-phase(bubble)
Thus the dimensionless thermodynamic coordinates used,taken together with a substance’s compressibility factor,provide the basis for the simplest form of the theorem of corresponding states,which very well describes the behavior of physical quantities near the critical point.Given the complicated processes generating such inclusions,and the differences in major element concentrations,the good f it for water-rich granitic and pegmatitic melt inclusionssuggeststhat asimplefundamental processmust lie behind their origins.
Further analyses of melt inclusions,especially those from thecritical region around thesolvuscrest,haveshown that this region is characterized by some extraordinary physico-chemical properties (Thomas and Davidson 2016a).According to Pioro and Mokry(2011)all thermodynamic properties undergo signif icant or dramatic changes within the critical and near-critical regions.

Fig.2 The pseudo-binary solvus curves for granites and pegmatites using normalized temperature and water concentration.Values have been normalized to the critical temperature(TC)for each system at the point where homogenization by critical behavior begins(Ehrenfriedersdorf=718°C, Zinnwald=705°C, Malkhan=721°C,Ko¨nigshain=750°C,and Tanco=762°C)so that the data from different systems can be shown on the same plot.TC is the critical temperature;H2O-crit is the water concentration at the critical point.Note:each point represent the mean of measurements on up to 100 melt inclusions.The source granites and pegmatites are listed in Table 1,top plot isthedatafor normal granites(solid dots)and F-rich melt inclusions in topaz representing topaz-albite-granites(empty dots)

Table 2 Fitting parameter for the solvus curves in normalized coordinates
· Extremely high diffusion rates,low dynamic viscosity and extremely low surface tension values.
· High density,comparable to ordinary liquids.
· High thermal motion and diffusivity,approaching that of gases.
· Dramatic change in speciation in a direction of highly volatile compounds(e.g.,formation of alkali carbonate complexes).
· High f luctuations of thermodynamic quantities(dynamic viscosity,volume expansivity,specif ic heat,thermal conductivity,Prandtl number)of substances in this region.At the critical point the density and the dynamic viscosity undergo an almost vertical drop,whereas the kinematic viscosity and the specif ic enthalpy undergo a sharp increase.The volume expansivity,specif ic heat,thermal conductivity and Prandtl number have peaks near the critical and pseudocritical points.The pseudocritical point(characterized with Ppcand Tpc)being a point at a pressure above the critical pressure and at a temperature(Tpc>Tcr)corresponding to the maximum value of the specif ic heat at this particular pressure.The magnitudes of these peaks decrease very quickly with an increase in pressure(see Pioro and Mokry 2011).
· Extraordinarily strong enrichment of some common and rare elements and compounds.
· A small decrease in pressure causes a large increase in the density of the supercritical phase in direction of an ordinary melt.
· Many other physical properties also show large gradients with pressure near the critical point(solvent strength,relative permittivity,etc.).
The f luid-like viscosity,the melt-like wetting and the extraordinary element carrying capacity make the supercritical f luids an ideal agent for chemical transport(see Ni et al.2017)in pegmatites and granites,and not only in subduction zones.Calculations based on the measured compositions of type-B melt inclusions(Aude´tat and Keppler 2004;Thomas et al.2012)show that such melt/f luids have viscosities on the order of 10-3Pa s,similar to pure water.Such f luids would have extremely high viscosity contrastswith typical felsic magmas(on theorder of 105Pa s at 800°C),thus these supercritical melt/f luids would have very high mobility when traversing a crystal mush,or even a still f luid magma,behaving in a similar manner to melt-or bubble-plumes.
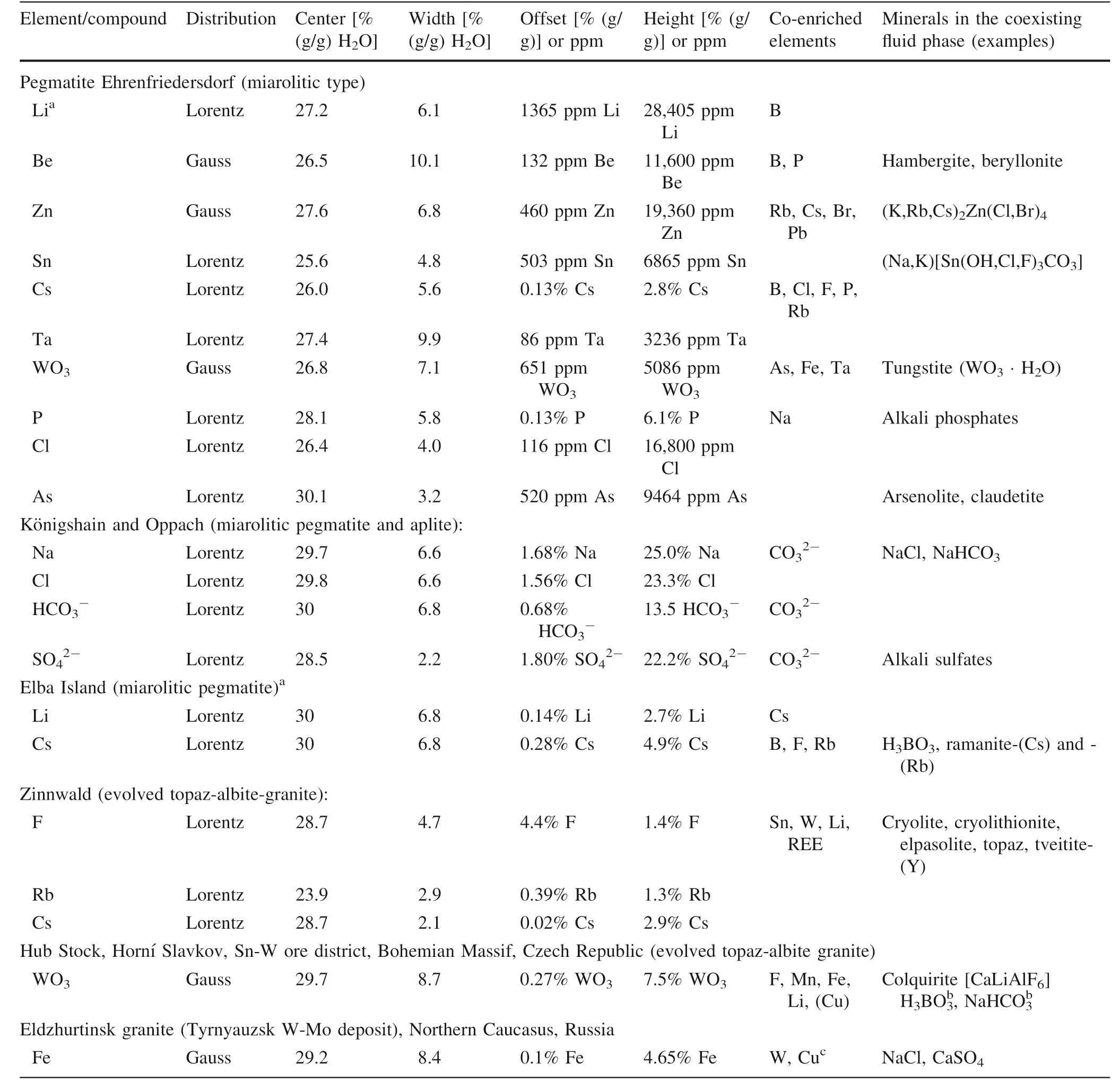
Table 3 The f itting parameters for element distributions in the range of the critical point of pseudo-binary melt-water systems
2.1 Extreme enrichment of some elements
We can show(Table 3,Fig.3a,b)that some elements(Li,Na,Rb,Cs,Be,B,C,F,Cl,P,S,As,Zn,Fe,Sn,Ta,W)are enriched in MItrapped in the critical region of pegmatites,as well as some granites,to extreme values which cannot be explained simply with experimentally obtained partition coeff icients.To a good approximation the distribution of these elements and compounds can be described by Gaussian and Lorentzian functions.The ratio between the offset and the highest values of the element distribution curves represents the partitioning between pegmatite and granite melts and the supercritical f luids,respectively.In Fig.4 are given the element partitioning between granitic rock and some studied pegmatites and its supercritical f luids.We observe a weak,but general increase of the partition coeff icients with the atomic number,and a signif icant difference between miarolitic pegmatites and supercritical f luids of two orders of magnitude,relative to the granite reference.
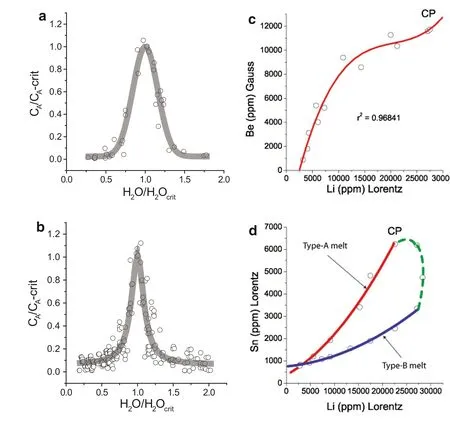
Fig.3 Plot of theelementsor compoundsversus H2Oconcentration given in Table 3,leveled as(CA)using normalized coordinates[CA/CA-crit versus H2O/H2O-crit]show Gaussian(a)or Lorentz(b)distribution curves.The Gaussian distribution is based from 38 data points(each is the mean of 2–10 measurements)and the Lorentzian distribution is based from 216 data points(the same number of measurements).Similarly the Ehrenfriedersdorf pegmatite:(c)showing strong correlation of Be vs.Liin thenear critical range,and(d)thecorrelation of Sn vs.Li(simplif ied).CPis the critical point of the solvus
Thus,the given elements are strongly enriched in the supercritical state.However,the strong enrichment of some elementsin the supercritical state isone sideof the process.The other side is the f luid-like viscosity,the melt-like wetting,and the element carrying capacity,which can be changed dramatically by variations in pressure,temperatureor thematrix composition.Asaresult of such changes,a supercritical f luid may separate into immiscible f luids and/or melts,or lose its supercritical identity by reacting with the rock matrix(Ni et al.2017;Manning 2006).After our homogenization studies,the quartz and feldspar of the graphic granite zones often contains near-critical melt inclusions,demonstrating that the supercritical state is often present at the early beginning of the pegmatitecrystallization.

Fig.4 Element partition coeff icients between granitic rocks(Ro¨sler and Lange1975)and thestudied pegmatites(generally miarolitic)and its supercritical f luids.The following elements are plotted as atomic numbers for Li,Be,C,Na,P,S,Cl,Zn,As,Rb,Sn,Cs,Ta,and W against the partition coeff icients.Note that the scale is logarithmic,indicating just how great the differences are
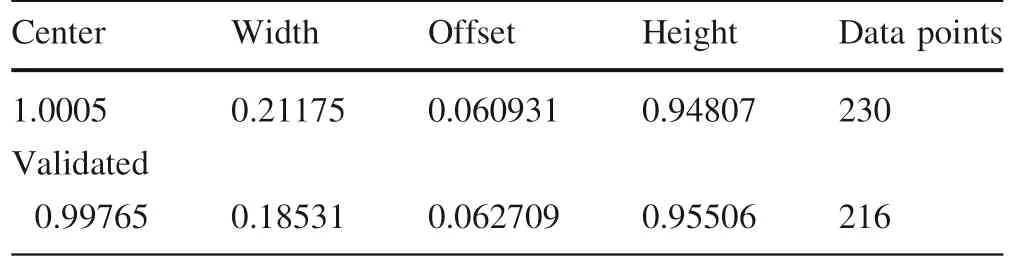
Table 4 Fitting parameters for the reduced Lorentz distribution of the concentration of elements and compounds(CA)in dependence of the reduced water concentration(H2O/H2Ocrit)
Furthermore,the extreme enrichment of elements in the near-critical range is a very robust proof for the supercritical state.The normalized f itting parameters for all the elements and compounds in question are given in Table 4.According to Hollas(1996)the Gaussian form is produced mainly by thetemperatureand in thecaseof the Lorentzian prof iles according to Peach(1981)by the temperature and density(pressure).In this context it is very important to note that all elements and compounds forming such distributions are strongly correlated with each other in a given deposit(Fig.3c,d).From Fig.3d we see the origin of a very strong scattering of the elements around the critical point(CP)depending on the concurrent trapping of the different melt inclusion types(type-A versus type-B melt)and related f luid inclusions(Type-C).
We suggest that the extraordinary properties of these supercritical melts,extremely high diffusion rates linked with the extreme low viscosity,favor the selective partitioning of some elements into these supercritical melt/f luids.It is an observed phenomenon that in supercritical f luids small variation in pressure and temperature result in large variations in properties,given normal variations within a pluton,such as crack-seal events in the carapace,that conditions may brief ly favor sequestration of specif ic elements,providing enhanced partitioning from the surrounding melt,resulting in signif icantly differing element concentration in immiscible patches created at different times.In this context we note here that melt and f luid inclusions trapped in quartz of a single growth zone with extreme enrichment of one element may adjoin a growth zone with inclusions showing completely different elements concentrations.In the case of the Ehrenfriedersdorf chamber pegmatite,zinc-rich inclusions are may be found only some microns apart from tungsten-rich ones(see Borisova et al.2012)—inclusion#019 contain 75,300 ppm Zn and 1.8 ppm W and inclusion#020 40,300 ppm Zn and 4620 ppm W.In addition to such extreme cases,there are often very NaCl-rich f luid inclusions,with or without KCldaughter in adjoining inclusions.In this context it is worth noting that MIdecorating growth planes may result from a sudden pressure drop,such as a crack-seal event in the granitecarapace,which induce sudden pressure quenching.Similar extreme heterogeneity was reported by Kamenetsky et al.(2002)although with a different explanation.
However,an analysis of the inclusion diversity in pegmatite quartz clearly shows that a vapor phase can also coexist under most conditions,implying the existence of three phase equilibrium:melt 1+melt 2+vapor.Due to pressure variations,the composition of the vapor phase can vary over broad limits depending on the position of the vapor curve relative to the solvus curve(Vogel 1959).The most interesting case should be the one where the vapor curve cuts the critical point.At this point we should have themost extreme conditionsfor avolatile-rich melt system.That issue was previously discussed in Thomas and Davidson(2012)and for which we can use Vogel’s(1959)explanation,namely that the coexisting vapor curve cut exactly the critical point of the solvuscurve.Thiscombines a number of factors[discussed in Thomas and Davidson(2012)],including thepropertiesof supercritical f luids,and the known solubility of metals in magmatic vapors(e.g.,Candela 1997),all of which point towards the sometimes extreme enrichments that have been noted.
Recently,in Thomasand Davidson(2016b)in astudy of melt inclusions in quartz crystals from miarolitic pegmatites in the Ko¨nigshain granite(E-Germany)demonstrated that some major elements such as Na,Cl,and C(as bicarbonate ion)can also be co-enriched to extremely high values,and as a rule,that the concentration around the critical point obey a Lorentz distribution.The extreme enrichment of NaCl in the near-critical region provides a mechanism for the formation of halite-rich f luid inclusions related to magmatic-hydrothermal systems.That is true also for f luid inclusions very rich in some rare elements beside halite.Gaussian distribution was only found in the case of Be and Zn in the Ehrenfriedersdorf pegmatite and W in the topaz-albite granite from Hub Stock,Hornı´Slakov-Kra´sno Sn-W ore district,Bohemian Massif,Czech Republic,as well as for Fe in the Eldshurtinsk granite(Caucasus).In the Ehrenfriedersdorf pegmatite Be is more or less simultaneous correlated to two different ligands:P and B.

◀Fig.5 Water-rich melt inclusions after rehomogenization under pressure in smoky quartz from a miarolitic cavity from the Hilbersdorf quarry in the Ko¨nigshain granite.(a)The daughter halite crystals show that the f luid phase is oversaturated in NaCl(at room temperature)demonstrating the extreme enrichment of NaCl at nearcritical conditions.G—silicate glass,NaCl—halite,Fl—f luid phase,V—vapor bubble.(b)The melt inclusion in the upper f igure contains daughter crystals of halite and nahcolite in the f luid sub-phase.The inclusions in the lower f igure are halite and nahcolite-rich f luid inclusion,probably generated by phase separation at sub-critical conditions.All inclusions shown in 4a and 4b are in close proximity.V=vapor phase(composition undetermined),Fl=aqueous f luid phase
Typical inclusions are shown in Fig.5.It is important to note that the three different inclusions were found in adjoining growth zones,or even the same growth zone,of quartz from a single miarolitic cavity in which melt inclusions dominate,evidence that the contents of the inclusions were coexisting immiscible phases.This demonstratesclearly thehigh variability of phasesinsideof a single miarole.It is also important here that the coexistence of the very different primary melt inclusion types were found in the central part of quartz prism,thus demonstrating that the process involved have to be magmatic.
The numerical data,calculated from melt inclusion data and Fig.5b in Thomasand Davidson(2016a)arepresented in Table 3 under point‘‘Ko¨nigshain.’’
The distribution of Na against the water concentration over the critical region is shown in Fig.6.Open circles in this f igure represent measured data,the closed circles are calculated.Note that Na represents the bulk concentration derived from NaCl and NaHCO3.This example shows a simple way of the formation of daughter crystal-rich f luid inclusions by oversaturation of the supercritical f luid(see Kamenetsky et al.2002).In some cases the f luid phase of near-critical melt inclusions also contains very high concentrations of alkali sulfates,borates and chlorides,such as the rare alkali-rich zinc tetrachloride/bromide with the simplif ied formula K2ZnCl4(see ESM Figs.1 and 2).
If we look at the f itting parameter the highest enrichment of all elements and compounds in question are correlated to the critical point of the solvus curves.The small variation in the water concentration around the critical point primarily results from analytical problems due to the water being present as liquid,vapor,and dissolved in silicate glass in the same inclusion,and also to small but varying degrees of diffusive water loss.
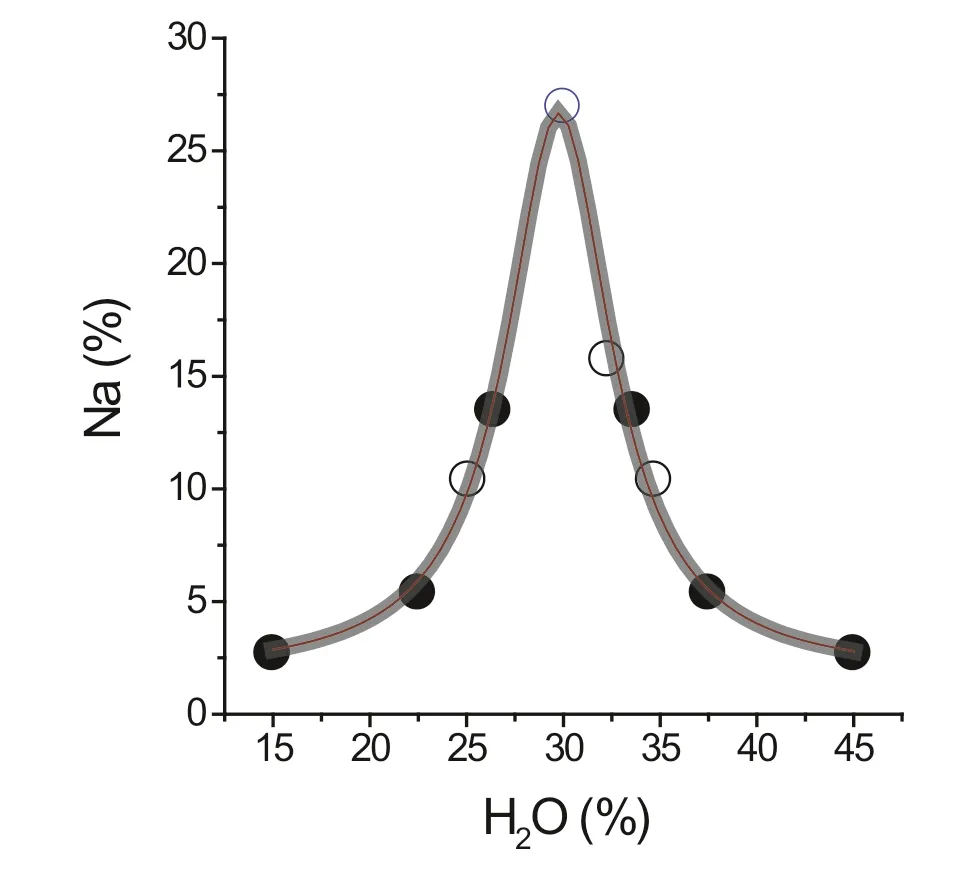
Fig.6 Plot of the Na distribution in melt inclusions in smoky quartz from the Hilbersdorf quarry in the Ko¨nigshain granite using normalized coordinates[H2O/H2O-crit versus CNa/CNa-crit]
One might expect that f inding MIwith compositions on thecritical point of thesolvuscurve might be astatistically rare event,however it is not,we have often observed and analyzed such inclusions,typically over a narrow range centering on the solvus crest.The intersection of the oneand two-phase f luid f ields is rather broad,for that reason we tend to use the term‘‘near-critical’’for melts to cover inclusions trapped across the top of the solvus crest.This observation implies that at or near the critical point,the diffusiveinf lux of thenamed elementsand compoundsinto the water-rich critical melt stabilize this melt against cooling,and increases the stability down to temperatures lower than the nominal solvus crest(Fig.2).In the case of the Ko¨nigshain-granite-pegmatite system,the lowest temperature is around 650°C,where the trapping of NaCl–NaHCO3–H2O±CO2f luids occurs.These inclusions are generated by the exsolution of new bicarbonate-rich supercritical melts after cooling below the minimum supercritical temperature.The relationship is indicated by the high nahcolite concentration of the melt and NaCl-rich f luid inclusions,as well as by the small amount of silicate material in the primary f luid inclusions.Note:nahcolite is stable only at low temperatures.
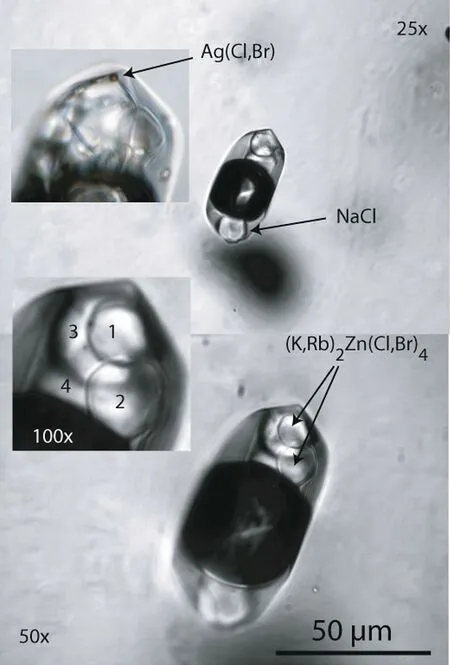
Fig.7 A typical high-temperature f luid inclusion with numerous daughter crystals,most are soluble at room temperature.Given their close relationship to a growth zone containing melt inclusions,and because such inclusions also contain signif icant boric acid concentrations,it seemsreasonablethat thisinclusion type isrelated directly to the pegmatite melt system.According to microscopic,Ramanspectroscopic,Synchrotron radiation induced X-ray f luorescence studies(Rickers et al.2004,2006)and by femtosecond LA-ICPQMS microanalysis(Borisova et al.2012)the following elements could beunambiguousdetected in relative high concentrations:B,Na,K,Rb,Cs,Sr,Cl,Br,I,Mn,Fe,Zn,Mo,Ag,Sn,Sb,and Pb,mostly as simple and complex halogenides(see ESM Fig.1:element mapping).Zinc forms complexes of the type(K,Rb,Cs)2Zn(Cl,Br)4,Ag is present as chloro-,brom-and iodargyrite,tin as Na[SnCl3]and Pb probably as cotunnite[PbCl2].Mn and Fe could not clear assigned to specif ic mineral phases
2.2 We call this process‘‘Enhanced Supercritical Craig-Effect’’
The strong and selective enrichment of some solutes between the two liquid phases,where one phase(generally the less polar phase)is percolating through a more stationary melt system(generally the more polar phase)is similar to the countercurrent distribution described by the process of partition chromatography according to Craig’s model(e.g.,Michal 1973),wherein thiscasethenumber of extraction steps trends to inf inity,similar to the industrial process of moving-bed chromatography.With such a process the 104–109fold enrichment of some specif ic elements in supercritical f luids(e.g.,Thomas and Davidson 2016a)can be explained.
Additionally,thegeneration of unusual f luid phaseshigh in borates,chlorides,sulfates,bicarbonates,phosphates,and others can be explained by the continuous operation of this process.One example is the extreme enrichment of Zn as(K,Rb,Cs)2Zn(Cl,Br)4-complex and other elements in f luid inclusions genetically connected with the Ehrenfriedersdorf pegmatite system(see Fig.6a,b).For the formation of this Zn-complex a simultaneous enrichment of Rb,Cs,and Br in addition to Zn isnecessary.According to data presented by Borisova et al.(2012)this enrichment is not proportional.
Another example is the extreme enrichment of La(Nd)in an alkalicarbonate-rich supercritical f luid in connection with the formation of gem-quality tsavorite crystals containg lanthanite-(Nd)[(Nd,La)2(CO3)3·8H2O](see Thomas et al.2018).Here is the supercritical f luid phase also connected to a volatile-and alkali-rich silicate melt,which is in this case not a pegmatite system sensu stricto.
The formation of inclusions such as shown in Fig.7a,b demonstrate that unusual element enrichments can happen by such a process,and unusual element combinations are possible and have a rational explanation.Furthermore,the element enrichment can vary within wide limits.However,given the extremely fugitive nature of these f luids,this extreme enrichment processes is often completely hidden,and can only be seen by the‘‘conservation’’asmelt or f luid inclusions.
That such enhanced enrichment effects are not only connected to pegmatites can be shown by Re-enrichment by a factor of 109under fumarolic conditions which is necessary for the formation of macroscopic rheniite crystals[ReS2]in nature(see Fig.4 in Korzhinsky et al.1994).Another example is the extreme mobility of Be in boronrich f luid systems indicated by the formation of bromellite[BeO](Thomas and Davidson 2010).
2.3 Conditions under which supercritical melt patches can be generated
Our studieson water-rich melt inclusionsin pegmatitesand granites have shown that the solvus crest of almost all of thesystemswestudied fall into awater concentration range of about 30%,at typical granite system temperatures of about 700°C or higher.Depending of the primary water concentration of the particular melt patches the supercritical state can be reached very rapidly by the simple crystallization of nominally water-free minerals.For example,if the starting concentration of water is 8%(not a rare case—see Thomas and Davidson 2012)a 76.5%degree of crystallization leads to the critical conditions(30%H2O),provided the residual volatiles cannot diffuse out rapidly,with a transition into the supercritical state,which enable a separate geochemical evolution path.The intensity of the local patch-formation and its conf luence and movement generate different melt/f luid systems with local different extension.It may be that the formation of rare element minerals(e.g.,zircon),distributed inside the granite,are the result of such micro-patch formation.
Furthermore,the existence of water-rich melt inclusions in granites and pegmatites forming similar pseudobinary solvuses(see Fig.2),especially if we use normalized conditions,and the presence of critical and near critical inclusions in most pegmatites,point to the speculation that the parental pegmatite melt is probably supercritical on pegmatite emplacement.This implies that it became supercritical while moving up through the magma and crystal mush of the granites.Thus moving-bed chromatography or the Craig-effect operated both before and after pegmatite emplacement.Likewise,such supercritical melt/f luids not trapped as pegmatites or in MI must disassociate into highly concentrated hydrothermal f luids and haplogranitic residual melts.Another indirect proof of the existence of supercritical phases that is the formation of‘‘strange’’melt inclusions with unusual high concentration of rare elements.As an example,in quartz of the Ehrenfriedersdorf pegmatite we have found often extreme Rb and Cs-rich melt inclusions(some analyses are given in the ESM Table 1,and as an example,a plot of Cs2O versus B2O3are given in the ESM Fig.3)with up to 7%Cs2O in addition to high B2O3concentrations.Theformation of this type of melt inclusions can be traced back to a phase separation process as a result of the sequential transition into,and out of,the supercritical state according to the scheme
Extremely enriched silicate melt←supercritical state→highly evolved fluid.
The enrichment of elements in the supercritical state is one side of the process and the growth of minerals containing these elements(for example by enhanced Ostwald ripening)is the other side.
Pressure quenching due to repeated crack-seal events in the granite carapace may provide the necessary cyclic conditions.In an analogous way the extreme Pand B-rich melt inclusions can so be generated.
2.4 Why are the water rich melt inclusions so hard to f ind?
Water is present in such inclusions as molecular water as an inclusion sub-phase(e.g.,Fig.1a–c)with a relatively large volume fraction[~50%(vol/vol)].During homogenization on a heating stage,or in a horizontal furnace under ambient pressure,the pressure inside the inclusion increases very rapidly,and destroys most such inclusions via leaking through microcracks,or by partial or total decrepitation.Eventually avirtually dry glassremains,thus the strong correlation of Be,as an example,with other lithophile elements is lost(see to this the discussion of London and Evensen(2002)regarding the f irst results of Webster et al.(1997)on melt inclusion in quartz of the Ehrenfriedersdorf pegmatite).Only by homogenization using high pressure devices can the inclusion keep the primary volume ratios.Furthermore,the mystery of the large scatter and the‘‘non-correlation’’of typical elements in pegmatites can now be explained,and it can be shown that between these elements there are strong correlations.Given the instability of such volatile-rich melts at low temperatures,very rapid quenching is also necessary to preserve the inclusion content as homogeneous metastable glass.Another,very important point is the level of expectations:water-rich melts should not appear in the standard experiments dealing with granite-water systems,and it is easy to ignore any outliers as decrepitated or otherwise unrepresentative.The observation of the‘‘heavy phase’’of many Russian and other experimenter at highpressure experimentsmaybe aindirect hint to the formation of the supercritical state in the hydrothermal systems(see also the discussion to this by Peretyazhko et al.2004 and references therein,Smirnov 2015).
Another very important reason why such inclusions describing primary processes are rare or hard to f ind,is the fact that due to recrystallization processes,in quartz often initiated by theα-βtransition,the primary inclusion populations are partially or totally eliminated.
3 Discussion
3.1 Possible signif icance of supercritical f luids and melts
For some time there has been disagreement in economic geology as to whether or not orthomagmatic ore bodies represent the product of metal-rich magmas,or simply more eff icient methods of sequestering and transporting the necessary element from otherwise unexceptionally endowed magmas.Some studies on porphyry deposits,for example Dietrich et al.(2000)and Core et al.(2006)suggested on the basis of varying lines of evidence for the existence of highly enriched magma in the formation of a number of orebodies.For example,Carten et al.(1988)suggested Mo concentrations magma in the apex of the seriatestock in the Henderson Porphyry Mo deposit ashigh as 13,000 ppm.In contrast,others(e.g.,Aude´tat and Li 2017)provided evidence for metal endowments in the stocks below a number of economic and sub-economic porphyry bodies that were only marginally enriched,2–25 ppm Mo in a number of stocks associated with Climax-type porphyry Mo deposits.
To resolvesuch apparent conf lict Mutschler et al.(1981)and more specif ically Shinohara et al.(1995)and Cloos(2001)proposed that extremely largevolumes of low-grade magma convected up into the stocks that underlie many porphyry deposits,losing their metals and volatiles by degassing at the top of the stock,then the depleted magma sinking back down under gravity into the main magma chamber in a continuous cycle.In this paper we propose a possiblealternativesolution,in that both thehigh-and lowenriched magma models are simply looking at two ends of a continuous spectrum,and that it is the intermediate stage that allows at least the initial sequestration needed to raise metal concentrations from ppm levels in magmas to wt%concentrations in orebodies.
3.2 Problems with existing models
Smith(1948)demonstrated that granite magma with initial 2%water under high pressure conditions would form an aqueous solution immiscible with the residual silicate solution after 96%crystallization.In analogy to this scenario Aude´tat and Pettke(2003)used the Rayleigh fractionation curves of Cs to calculate the degree of melt crystallization on the basis of a given Cs concentration in the residual melt.The starting point was a constant H2Osolubility in the melt of 4.5%.Recently Aude´tat and Li(2017)used the same argument to postulate that melt inclusions that they measured with 6000 ppm Mo are not representative of a f luid that formed the porphyry Mo mineralization(Aude´tat and Li 2017,p.447).
Most researcher dealing with granite and rhyolite-related ore deposits regard the crystallization of the corresponding magmasmoreor lessasan undisturbed continued process.For example,Aude´tat and Pettke(2003)and Aude´tat and Li(2017)use the Rayleigh fractionation curves of Cs to estimate the degree of crystallization without allowing for other effects.Other authors accept melt inclusions only as relevant if they ref lect the experimental results on synthetic granite-water systems going back to the work of Goranson(1931)and the interpretation by Smith(1948).However,careful observations on melt inclusions in granite and pegmatite systems using highpressure homogenization experiments over some tens of years has shown that this is only a f irst approximation,invalid for evolved and even moreprimitive magmasin the f inal stages of crystallization.We suggest that more complex melt evolution pathways need to be considered.
3.3 Melt evolution
In the case of Ko¨nigshain granite,we have observed that H2O-rich melt inclusions with ten or more%water are not rare,often connected to fast moving‘‘melt injections’’from a greater depth.If such injections accumulate near the granite roof a separate evolution in miarolitic cavities is inevitable,and is documented in Thomas and Davidson(2016b).Moreover,such local melt accumulations are possible at multiple points in the evolution by crystallization.Given partial crystallization of approximately anhydrous phases,the formation of supercritical melt patches with about 30%water is practically inevitable,provided that diffusion and mixing isnot excessive,and wef ind such indication’s nearly in each intrusive and extrusive granitic system we have studied.Furthermore,the appearance of these inclusion types is not only restricted to pegmatite.In a study of the topaz-albite-granite from Zinnwald(Thomas et al.2005)such inclusions were seen and described.Using the Ehrenfriedersdorf pegmatite as an analogy,a pseudobinary solvus for this granite system was constructed(Thomas et al.2005).In these occurrences we found two different inclusion types,type-A and type-B melt inclusions in pegmatites,as well as evolved granites(Table 1),which represent conjugate melts resulting from melt–melt immiscibility along a pseudobinary solvus boundary.Such a system is the simplest representation of real volatile-rich silicate melt-f luid systems in nature.This pseudo-binary solvus curve with a critical point at the solvus crest is the principle result of this extensive series of published studies(see Thomas and Davidson 2016a).Furthermore,we have demonstrated that the two conjugate melt fractions evolve to more peraluminous and less water-rich composition(type-A melts)and to more peralkaline and very water-rich compositions(type-B melts).
An important point,however,is that above the solvus crest a single-phase melt exists in which volatiles and silicate melt are completely miscible in any proportion(Thomas et al.2000).Various studies have observed such f luids and they have been given various names,silicothermal f luids(Wilkinson et al.1996),supercritical f luids(Bolmatov et al.2013;Gorbaty and Bondarenko 1998),aqueous aluminosilicate polymers(Manning 2004;Mysen 2014;Smirnov 2015).Since these f luids exist as a singlephase f luid above the critical two-phase boundary we prefer the term supercritical melt/f luids to highlight their main features.These are that two almost immisciblephases at lower temperatures and pressures are miscible in any proportion abovea critical point,that the single-phase f luid hasmelt-likeand aqueousf luid-likeproperties,and that the new phase has properties unlike either silicate melts or aqueous f luids.This phase has been shown to be stable at conditions consistent with those in felsic magma chambers,>700°C and 1–10 kbar(Sowerby and Keppler 2002;Thomas et al.2000),and that solubilities of otherwise incompatible elements can reach extremely high levels(Bureau et al.2007).Such a phase has also been produced and observed in HDAC experiments(Kawamoto et al.2014;Schmidt et al.2014).Weproposethat thisphasemay prove to be the intermediary between felsic magmas and the orebodies and hydrothermal f luids that form them,and that the properties and behavior of these f luids are critical in the process of sequestering the otherwise rare elements from huge volumes of magma.
3.4 Supercritical melt/f luids in the upper crustal level
The existenceof melt inclusions with compositions consistent with supercritical melt/f luidsin granitesand pegmatites at upper crustal levels,along with experimental evidence(Veksler et al.2002)demonstrates that such melt/f luids can existunder these PTX conditions,provided thatthey havethe necessary compositions.However,this still leaves open the question of their origins,given that their compositions are notably different from thebulk compositionsof thegranites and pegmatites that enclose them.Manning(1994,2004),Bureauand Keppler(1999)and Aude´tat and Keppler(2004)demonstrated that aluminosilicate melt and aqueous f luids are completely miscible in all proportions at lower crustal pressures,coining the term ‘‘Aqueous aluminosilicate polymers’’(Manning 2004).Moreover,that such f luids would be extremely mobile,and readily sequester high concentrations of otherwise incompatible elements from rocksor meltscarrying only low concentrations.In addition to metals such f luids would have high partitioning coeff icients for elements such as F,B,and P,as well as alkali carbonates and bicarbonates,all of which are considered f luxing components,sincesingly or in combination they can signif icantly reduce the solidus of a silicate melt(Sowerby and Keppler 2002;Thomas et al.2000).The enrichment of these f luxing components is the prerequisite for the generation of PTX conditions in which the supercritical state can develop its extraordinary and selective ability of extreme element enrichment,and their focusing into highly mobile,effectively immiscible rapidly moving incompatible element rich plumes.
Once generated,such f luids would have the capacity to sequester more metals and f luxing elements,thus further decreasing their solidus and enhancing their ability to survive ascent into regions of lower temperature and pressure.Moreover,this capacity should actually increase with the distance the melt/f luids migrate.Whatever their origin,their composition determines their ultimate fate,unless trapped in MI,at some point they must cool below their solidus and disassociate into a haplogranitic melt(≈eutectic feldspar,quartz,mica granite)essentially indistinguishable from any other granite,plus a volume of very concentrated aqueous solution,an ideal candidate for a hydrothermal f luid.Furthermore such‘‘end-member’’high-temperature aqueous f luids can completely overprint pegmatite characteristics in situ,and leave behind an apparent‘‘hydrothermally’’formed pegmatite or,in the extreme cases,only hydrothermalites.As an example the pycnite rock from the Sn-W deposit at Altenberg in the East Erzgebirge/Germany was produced by overprinting of a simple feldspar-quartz stockscheider pegmatite by extremely F-rich high-temperature metasomatic f luids(Thomas and Davidson 2013).Another extreme example is the Landsverk 1 pegmatite in the Evje-Iveland/Norwegian,where overprinting by hydrothermal solutionsgenerated by the same pegmatite has nearly completely erased the primary signatures of the pegmatite(see Mu¨ller et al.2017).Only remnants of primary melt inclusions can be found,thus allowing the reconstruction of the magmatic end stage of this pegmatite.
We would suggest that the intensive re-crystallization and overprinting of many pegmatites by late high-temperature f luids,often loaded with alkali carbonates,sulfates and/or boric acid,generate much of the controversies surrounding pegmatites.It isnot necessary that external f luids away perform the transformation in pegmatites.The crystallization of predominant nominally water-free minerals causes the mass of molecular water to increase steadily during the pegmatite evolution from high to low temperatures,resulting in the pegmatites‘‘stewing in their own juice’’complicating unraveling the processes involved.
Asan aside,we havediscussed theprocessesof extreme enrichment in thecontext of granite/pegmatitesystems,but it should be noted that the TPX conditions for the critical state can fall within peak metamorphism conditions in some rocks of unusual compositions.Primary melt inclusions have been noted in crystals previously assumed to have been formed during metamorphism,from metamorphic f luids,for example in green vanadium grossular from Tanzania,which imply crystallization from an exotic partial melt(Thomaset al.2018).Near critical melt inclusions with 27.7%H2O and up to 8.4%(g/g)MgCO3[corresponding to about 6.9%(g/g)BeCO3]havealso been found in‘‘metamorphic’’emeraldsfrom the Habachtal in Austria,suggesting that similar extreme enrichment processes are not restricted to purely magmatic or pegmatitic systems.
4 Conclusions
From our data and results of others the formation and existence of supercritical f luids during different crystallization stages of granitic and pegmatitic melts isapparent.Furthermore,supercritical f luids have extreme properties:very high diffusivity,very low viscosities and surface tensions,among others.Given thesepropertiessupercritical f luids provide an excellent mechanism for sequestering metals and volatiles and have a great impact to the texture of inf luenced rocks.
At temperatures and pressures consistent with felsic magma chambers a phase effectively immiscible with under-saturated felsic melts can exist,in which aluminosilicatemelt and volatiles,principally water,are miscible in any proportion.This phase can only exist above a twoliquid phase boundary,the crest of which appears to be around 730°C±20°C,with about 30 wt%H2O.Below this the phase splits into conjugate pairs which evolve down temperature towards H2O saturated melts(≤8 wt%H2O)and hydrothermal f luids(≥92 wt%H2O).This phase,which we term supercritical melt/f luid,but which appears consistent with other names,such as silicothermal f luid,has extremely high partitioning coeff icients for a range of melt incompatible elements and compounds,principally metals and volatiles.These supercritical melt/f luids being effectively immiscible with under-saturated felsic magmas,and having very high viscosity,density and surface tension contrastswith those magmasare capable of segregating into f luid pockets(patches,f luid globules or droplets)and migrating through that magma by processes such asbuoyancy or f ilter pressing.Given thehigh mobility and partitioning coeff icients such f luid pockets a process similar to moving bed supercritical f luid chromatography may allow such melt/f luids to sequester the very high concentrations of melt incompatible elements and compounds found in MIin pegmatites.Given the variability in composition of this melt/f luid,and the different styles of interaction with host magmas or rocks,a diverse range of pegmatites may form,depending on local conditions.Such melt/f luids would also form an excellent intermediate phase between felsic melts and hydrothermal f luids,permitting the sequestering of high metal and volatile concentrations and delivering them to pegmatites,or feeding porphyry or other types of ore deposits,depending on the characteristics of a given magma chamber.
In contrast to supercritical f luids formed at very high pressures,e.g.,at subduction zones(Ni et al.2017)the extreme water-rich melt inclusions in granites and pegmatites represent the most direct and reliable and robust record of natural samples of supercritical f luids.
AcknowledgementsWe would like to take this opportunity to dedicate this paper to Dr.James Webster,with our thanks and gratitude for his unf lagging assistance,collaboration and encouragement over the years.Over a long period,Dr.Webster has worked with us unravelling the mysteries of melt–melt immiscibility,in addition to the enormous body of work he has achieved in other areas of geology and geochemistry.We thank Prof.Huaiwei Ni for valuable suggestions and the hint to some important references.
Compliance with ethical standards
Conf lict of interestThe authors declare that they have no conf lict of interest.
- Acta Geochimica的其它文章
- Correction to:The enhanced element enrichment in the supercritical states of granite-pegmatite systems
- Hydrogeochemical evaluation and statistical analysis of groundwater of Sylhet,north-eastern Bangladesh
- Effects of mineral-organic fertilizer on the biomass of green Chinese cabbage and potential carbon sequestration ability in karst areas of Southwest China
- Trace element partitioning between amphibole and hydrous silicate glasses at 0.6-2.6 GPa
- Geochemistry of subsurface Late Quaternary ironstones in Rajshahi and Bogra Districts,Bangladesh:implications for genetic and depositional conditions
- Mercury speciation,bioavailability and risk assessment on soil-rice systems from a watershed impacted by abandoned Hg mine-waste tailings

