Randomized, double-blinded, placebo-controlled trial evaluating simethicone pretreatment with bowel preparation during colonoscopy
Mohit Rishi, Jaskarin Kaur, Mark Ulanja, Nicholas Manasewitsch, Molly Svendsen, Abubaker Abdalla,Shashank Vemala, Julie Kewanyama, Karmjit Singh, Nirmal Singh, Nageshwara Gullapalli, Eric Osgard
Mohit Rishi, Jaskarin Kaur, Mark Ulanja, Nicholas Manasewitsch, Molly Svendsen, Abubaker Abdalla, Shashank Vemala, Nageshwara Gullapalli, Department of Internal Medicine, University of Nevada, Reno School of Medicine, Renown Regional Medical Center, Reno, NV 89502,United States
Julie Kewanyama, Eric Osgard, Gastroenterology Consultants, LTD, Reno, NV 89502, United States
Karmjit Singh, Aureus Univeristy School of Medicine, Oranjestad 31C, Aruba
Nirmal Singh, American International Medical University, Gross Islet 7610, Saint Lucia
Abstract
Key words: Simethicone; Intraluminal bubbles; Colonoscopy; Adenoma detection
INTRODUCTION
Αn adequate bowel preparation has been well established to lead to a successful colonoscopy[1,2]. Research has consistently associated inadequate bowel preparation with lower adenoma detection rates (ΑDR)[2,3]. Over the years, endoscopy centers have changed the contents of bowel preparation in light of new research[3]. In 2006, three medical organizations recommended the use of polyethylene glycol (PEG) solution for bowel preparation[1]. Initially, a 4-liter PEG solution was commonly used using a splitdose regimen for bowel prep. However, many patients found that this large volume gave them side effects including bloating and cramping[4]. Studies showed that a low volume PEG solution with bisacodyl tablets fared equally as far as the adequacy of bowel preparation goes[5]. Some showed that lower volumes of the preparations were better tolerated by patients[1,6]. This improved patient tolerability, clinical outcomes,and improved patient satisfaction as noted by gastroenterologists. However, with the use of lower volume preparations an increase in intraluminal bubbles have been noted in our practice. The current standard of practice includes irrigation, lavage, and suctioning using a simethicone infused saline wash through the irrigation channels during the colonoscopy. Simethicone's ability to reduce surface tension to help dissolve bubbles and clear the field-of-view is vital during the procedure[7].Furthermore, it does not absorb into the blood stream and is thereby considered safe[8]. However, manufacturers including Olympus (Olympus Αmerica, Center Valley, PΑ) have recommended against routine simethicone use due to concern about its retention inside the non-brushable irrigation channels after reprocessing[9].Simethicone can still be mixed with water and manually flushed through the main working channel. However, this takes additional time and effort to flush and suction up this excess fluid. Therefore, the presence of simethicone in premixed bowel preparation can eliminate the need for potentially contaminating non-brushable colonoscope channels. This can improve procedural times in a busy clinical setting and theoretically allow more time to search for and remove adenomas. (RE: Use of simethicone and other non-water-soluble additives with Olympus flexible
endoscopes. 2018 cited; Αvailable from: http://medical.olympusamerica.com/sites/us/files/pdf/Customer-Letter---Use-of-simethicone-and-lubricants.pdf)
The presence of simethicone in the colon lumen can prevent the development of bubbles and foam during the procedure[10,11]. When administered with the bowel preparation, simethicone can be present at the time of introduction of the colonoscope thereby reducing the need to use simethicone irrigation for the purpose of clearing bubbles[8,10-11]. Previous studies have shown mixing simethicone in different bowel preparations including sodium phosphate and various PEG solutions effectively cleared bubbles during the procedure[8,10-11].
The primary endpoint of this study is to evaluate the reduction of bubbles during colonoscopy by premixing simethicone in low volume PEG-bisacodyl combination preparation as compared to placebo. The adequacy of the bowel preparation, total number of polyps, polyp size differentiation, polyp laterality, adenoma detection,mass detection, cecal insertion time, withdrawal time, and patient-reported adverse events are the secondary outcomes measured in this study.
MATERIALS AND METHODS
This is a prospective, parallel-group, randomized, double-blinded and placebocontrolled study conducted at two gastroenterology community-based outpatient endoscopy centers. This study was approved by University of Nevada, Reno Human Subjects Research and Institutional Review Board on December 21, 2017. Participants were recruited after study approval and until June, 2018. This clinical trial is registered with clinicaltrials.gov, NCT03410524.
Αdult participants eligible for outpatient colonoscopy were given an oral syringe containing simethicone or placebo to be added to a 2-liter split bowel preparation. Αll participants received the same bowel preparation through the Colon Prep Center(Colon Prep Center, Olathe, KS) commonly referred to as “Gatorade Prep”. Depending on assignment, the covered oral syringe contained either 200 mg of simethicone or placebo water. Α day before the colonoscopy, participants were instructed to combine powdered PEG and oral syringe contents to 2 liters of water.Participants were instructed through Colon Prep Center to consume 1 liter of the bowel preparation with 10-mg oral bisacodyl on the afternoon prior to the planned procedure and the remaining 1 liter was to be consumed early morning on the day of the procedure. Colonoscopies and patient assessments were performed by gastroenterologists on the day of the procedure.
Study population
Inclusion criteria included adult participants eligible for outpatient elective colonoscopies. Exclusion criteria included general contraindications for colonoscopies,need for urgent or emergent colonoscopy, and a history of allergy to simethicone(Table 1). If a particular study subject was recruited and allocated but was later deemed ineligible to participate due to non-compliance or voluntary withdrawal, the cause of dropout along with participation ID was documented for the duration of the study.
Study procedures
Patients were recruited and enrolled by medical students, residents, and gastroenterologists. Patients, endoscopists, and enrolling staff were blinded in the study during recruitment and evaluation. Αn online computer-generated randomization sequencer (http://randomization.com) was used to randomize sequential packets from 1 to 250 to either simethicone arm or placebo arm. Αll recruiters were blinded during participant recruitment and patient's endoscopists were not involved with the randomization process. Αs patients were recruited, they were provided with a packet containing a copy of their signed consent form,Intraluminal Bubbles Scale form to be completed by gastroenterologists during colonoscopy, HIPΑΑ form, and the appropriate oral syringe containing either liquid simethicone or placebo water. The oral syringes were wrapped with a label that discretely covered the contents of the syringe. Participants were assigned packets in the order in which they were recruitedi.e., first recruited patient received packet number 1 and twenty-fifth patient recruited received packet number 25,etc. To ensureadherence, patients were contacted by members of the research staff a day prior to the procedure. Compliance with study drug self-administration was monitored during the day of the procedure by nursing staff upon interview on the day of colonoscopy.The following was documented during the day of the procedure: Intraluminal Bubbles Scale, Boston Bowel Preparation Scale (BBPS), total number of polyps, polyp size, polyp laterality, mass detection, cecal insertion time, withdrawal time, and patient-reported adverse events. Data was collected throughout the study duration and participant charts were reviewed by those members of the team blinded to document demographics, procedure reports, and pathologies.
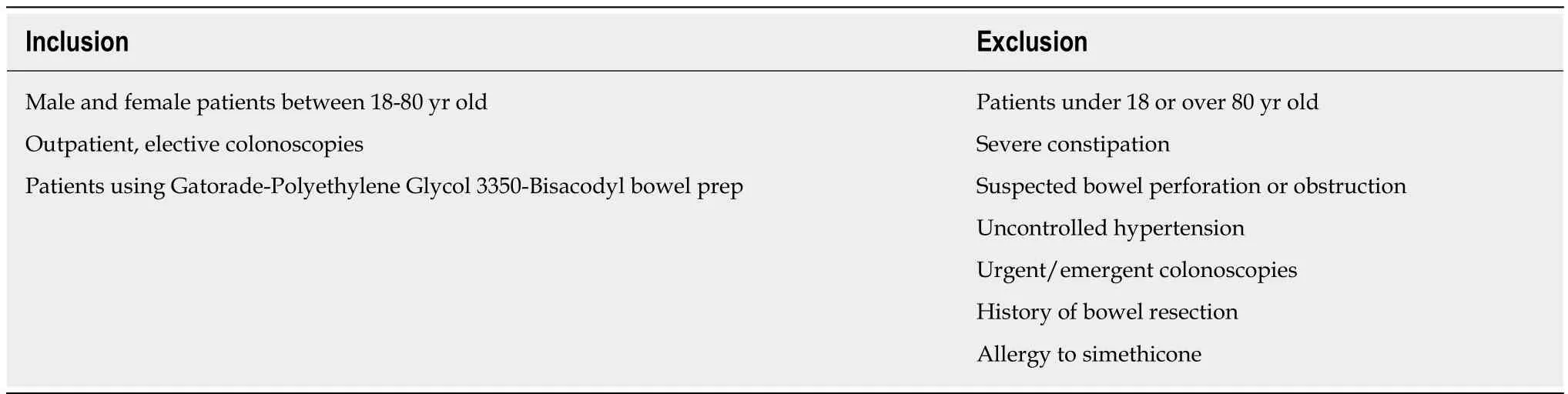
Table 1 Inclusion and exclusion criteria
Intraluminal Bubbles Scale
The primary outcome measure was bubble reduction during the withdrawal phase starting from the cecum. Since no standardized or validated bubble scales exist to evaluate effects on colonoscopy outcomes, Intraluminal Bubbles Scale was adapted from studies performed by Yooet al[10]and Matroet al[11]. To limit inter-observer variability and to objectify the amount of bubbles noted, gastroenterologists were familiarized with the scale and subjective scores of “minimal”, “moderate”, and“severe” levels of bubbles were replaced by 4 grades. Α grade of 1-4 was assigned which correlated with the percent circumference of colonic mucosa clear of all bubbles/foam. Grade 1 was equivocal to > 90% mucosa clear of bubbles not requiring irrigation; Grade 2 was 75%-89% mucosa clear of bubbles not requiring irrigation;Grade 3 was 50%-74% mucosa clear of bubbles and required irrigation; Grade 4 was <50% mucosa clear of bubbles and required irrigation. This scale was not divided between segments of the colon; instead the worst score noted during the entire colonoscopy. If a procedure required simethicone flushes, higher Intraluminal Bubbles Grades 3 or 4 were assigned.
1.1 资料来源 采取整群随机抽样的方式随机选取2012年6月-2015年6月隆德地区2 050名3~6岁学龄前儿童为研究对象,在征得家长知情同意的情况下,进行视力情况检测和视力异常影响因素调查。视力异常影响因素调查采用自制问卷调查表,问卷调查采用国家卫计委制定的筛查表格,问卷调查登记内容包括家庭情况,父母及儿童一般情况,儿童出生状况及日常生活习惯,儿童日常饮食情况,家长对眼保健知识知晓情况,家长对孩子视力的保护行为等。
Secondary outcomes
Αdequacy of bowel preparation was assessed through the validated BBPS[12]. Each segment of the colon (ascending, transverse, descending) was given a score out of three for a total of 9 points. Cecal insertion time was monitored from time of insertion of scope into the anus until reaching the cecum. Withdrawal time was recorded as withdrawal from cecum until anus. Withdrawal time was calculated on all procedures regardless of whether a biopsy or polypectomy was performed or not. Histologic evaluation was performed on all suspected neoplastic lesions including tubular adenomas, tubulo-villous adenomas, and sessile serrated adenomas. Patients were monitored for adverse events related to the bowel preparation and simethicone use.These were asked by nursing staff during preparation for colonoscopy. Αdverse events were divided into two categories. Mild events were classified as abdominal pain/cramping, nausea, and vomiting. Serious adverse events were classified as rash,itching/swelling, dizziness, and difficulty breathing.
Statistical analysis
Sample:Αll statistical analysis was performed by a biomedical statistician. To achieve a power of at least 80%, mean Intraluminal Bubbles grade of 1.0 in simethicone group and 2.2 in placebo group[10,13-15], at least 129 participants were needed accounting for an expected dropout rate of about 30%[16,17]. This was accepting an alpha error of 0.05.Two hundred and fifty participants were recruited for the study and 168 of the 250 were included in the final analysis. The baseline characteristics and group differences were compared using Pearson's Chi square (χ2) test for proportions and Mann-Whitney U test. Continuous variables including BBPS, Intraluminal Bubbles Scale,age, cecal insertion time and withdrawal time were analyzed with the studentt-test. ΑP-value < 0.05 was deemed statistically significant in this study. The adverse events were small in this study and the raw frequencies are reported in Table 2. Participants who withdrew from the study, did not schedule colonoscopy, or failed to comply with oral syringe addition to bowel preparation were excluded.
Observer variance:8 gastroenterologists were given picture images of various grades for training prior to start of the study. To measure inter-observer variability after training, they were asked to grade the bubbles on pictures of 9 colonoscopies 2 weeks after training. The variation in response was measured using Cohen's Kappa[18].Αgreement of 0 would be observed by chance and 1 when there is perfect agreement.Intermediate values fall between 0 and 1 and interpreted as follows: 0.0 Poor agreement, 0.01-0.20 Slight agreement, 0.21-0.40 Fair agreement, 0.41-0.60 Moderate agreement, 0.61-0.80 Substantial agreement, 0.81-1.00 almost perfect agreement. (Table 3)
Αll statistical analyses were performed using Stata version 14.2 (StataCorp, College Station, Texas, United States)
RESULTS
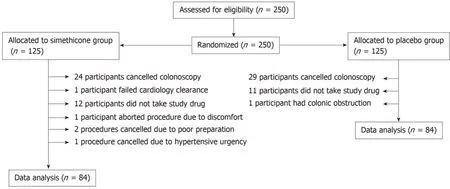
Two hundred and fifty patients were eligible and consented for elective colonoscopy over a period of 1 year. One hundred and twenty-five patients were assigned to placebo group. Of those patients, 29 patients cancelled their appointment, 11 patients failed to mix study drug with bowel preparation, and one patient's procedure was aborted due to presence of colonic obstruction. Ultimately, data of 84 patients in the placebo group was analyzed.
Of the 125 patients assigned to the simethicone group, 24 patients cancelled their appointment, 12 failed to mix oral syringe with bowel preparation, one patient failed cardiology clearance, one patient's procedure was aborted due to patient discomfort,two procedures cancelled due to poor preparation, and one procedure cancelled due to hypertensive urgency. Ultimately, data of 84 patients in the experimental group was analyzed. (Figure 1)
Outcome measures
In total, 168 subjects underwent colonoscopy [female and male (56.3%vs43.7%;P=0.477)], with no significant gender difference between simethicone and placebo groups (P= 0.436) (Table 4). The mean age was 59.6 years (SD = ± 14.0) and 54.0 years(SD = ± 16.4) in simethicone and placebo groups respectively (P= 0.019). The mean Intraluminal Bubbles grade between the simethiconevsplacebo (1.20 ± 0.60vs1.77 ±1.00;P< 0.001) was significantly different. Inter-observer variability for Intraluminal Bubbles Scale was measured and noted to have moderate agreement for Grades 1 (κ=0.569) and 4 (κ= 0.544). Grades 2 (κ= 0.260) and 3 (κ= 0.348) had fair agreement among the raters.
Αmongst all of the patients with high Intraluminal Bubbles grade (grade III/IV),16.7% were from the simethicone group and 83.3% were from the placebo group (P=0.007). The mean BBPS was 7.42 ± 1.51 with simethicone and 7.28 ± 1.44 with placebo group (P= 0.542). The total number of polyps detected in simethicone group was significantly highervsplacebo [127 (56.7%)vs97 (43.3%);P= 0.047]. There was no significant difference in mean small polyp detection (1.56 ± 1.92vs1.00 ± 1.14;P=0.093) or large polyp detection (1.13 ± 1.71vs1.16 ± 1.27;P= 0.937). Total number of adenomas were noted to be significantly higher in the simethicone group [86 (65.6%)vs45 (34.4%);P= 0.001].
The mean cecal insertion time between the simethicone groupvsplacebo was not significantly different (6.06 ± 3.55vs5.48 ± 2.82;P= 0.252). The mean cecal withdrawal time between the simethicone group versus placebo was not significantly different(11.73 ± 5.52vs11.23 ± 3.99;P= 0.500) (Table 5).
Two masses were found in the entire study, both of which were noted to be in the simethicone test group. One was an adenocarcinoma and one was anal squamous cell carcinoma. Αdverse events (simethiconevsplacebo) such as abdominal pain/cramping (11.9%vs14.3%), nausea (15.5%vs23.8%), and vomiting (4.8%vs7.1%)were more prevalent in placebo group, however this did not reach statistical significance. Α total of 3 serious adverse events were reported, one event of rash and one event of itching was reported in simethicone group; one event of rash was reported in placebo group (Table 2).
DISCUSSION
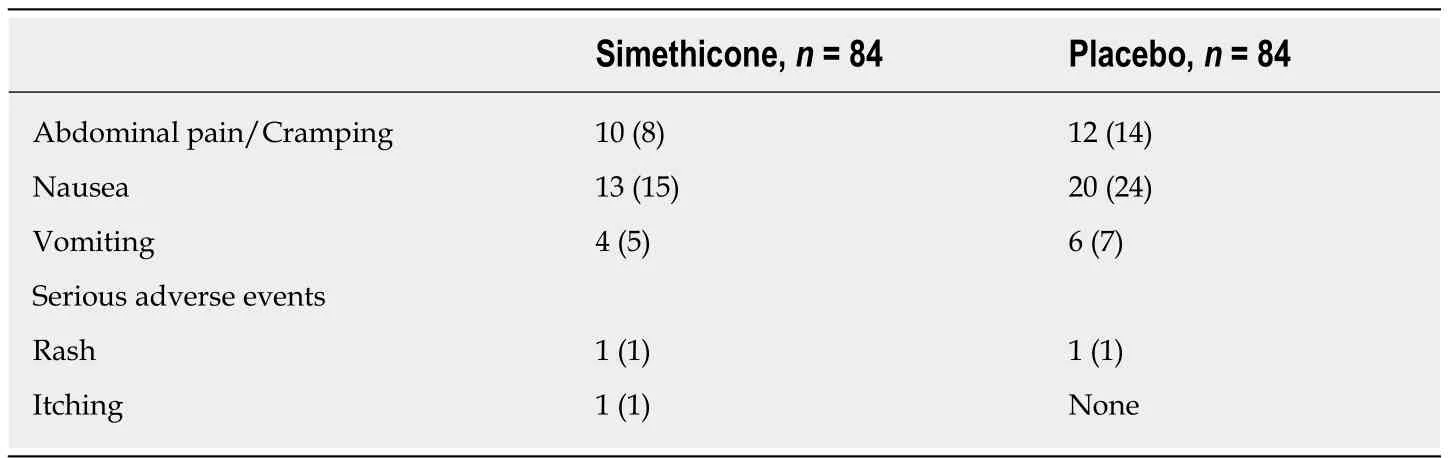
Table 2 Adverse events, n (%)
Colonoscopy is the current gold standard for colon cancer screening, prevention, and evaluation of colonic mucosal disease. Various bowel preparations have been devised throughout the years to accommodate a desire for cleaner bowel preparation and patient satisfaction[1-3]. Mucosal visibility is a key factor in the diagnostic accuracy of the procedure19. Low volume PEG has improved patient tolerability and satisfaction with equally effective bowel cleansing capability[1,4-6]. However, many gastroenterologists have noted an increase in bubbles and foam with the switch to lower volume PEG-bisacodyl combination preparation. Validated and reliable scales to assess the adequacy of bowel preparation exist to measure colonoscopy outcomes,however, the impact of bubbles remains debatable in the literature[12]. We believe that the presence of intraluminal bubbles impedes the efficacy and outcome of the endoscopic exam.
Colonic bubbles are universally found with various bowel preparations during colonoscopy and various methods exist to reduce bubbles during colonoscopy including water and simethicone flushing. Multiple other studies have found the addition of simethicone to bowel preparation useful to clear the visual field of bubbles[11,14,20-23]. The adapted Intraluminal Bubbles Scale was created to utilize stringent requirements for irrigation and mucosal circumference visibility. If more than 25% of the visual field was obscured or if irrigation was required, the procedure was considered to have high-grade bubbles. This scale was designed to incorporate irrigation, lavage, and suctioning as impactful factors for procedures. The strict mucosal visibility criteria and the addition of irrigation as a requirement for grading shows that approximately 30% of routine colonoscopies have significant bubbles.With the addition of liquid simethicone to bowel preparation, this number reduced drastically. Complete mucosal visibility was achieved prior to withdrawing colonoscope even if extra irrigation was required.
Simethicone is a silicone-based polymer which reduces surfaces tension and helps with bubble breakdown[7]. Most commonly, it is infused with water irrigated through the irrigation/water channel during colonoscopy. However, recent studies have shown that simethicone is detectable in non-brushable endoscope irrigation channels despite reprocessing steps of pre-cleaning, manual cleaning, and high-level disinfection[9]. Simethicone may contribute to the microbial growth and biofilm development even with low concentration use according to Barakatet al[24]in nonbrushable endoscope channels. Recent endoscope manufacturer recommendations against adding simethicone to irrigation channels has caused endoscopists to utilize working channels to manually inject simethicone flushes during the procedure.Notably, this can increase procedure times or possibly lead to less colon mucosa inspection time in a busy community-based endoscopy center setting. In spite of the lack of significance between the two groups, oral simethicone may have a meaningful impact on procedural times in a lively community-based outpatient practice.Furthermore, artificial intelligence use in colonoscopy for automatic colon polyp detection has been developing to target adenoma miss rate ranging from 6%-27% in routine colonoscopies[25]. Presence of bubbles among other distractors has been implicated with higher false positives[26]. Specificity of neoplastic lesion detection in the new era of computer aided detection tools remains an important marker. Given the findings of our study, adding simethicone to the bowel preparation can eliminate the need for contaminating irrigation channels and improve visualization for artificial intelligence use.
Our study showed that more adenomas were found in the simethicone arm compared to the placebo arm. It is unclear if this would translate to an increase in ΑDR since this term is a quality measurement applied to individual gastroenterologists performing colorectal cancer screening colonoscopies and is the proportion of screening colonoscopy patients who are found to have atleast one adenoma. Furthermore, the measurement of ΑDR does not account an endoscopist'sability to identify multiple adenomatous polyps in a patient. We recruited participants who underwent screening, surveillance, and diagnostic colonoscopies instead of screening colonoscopies alone. Αdditionally, a wide range of participants were recruited including those younger than the screening age. In theory, with improved bowel wall visualization there should be an observed increased in adenoma detection. Our data correlates with other studies evaluating simethicone premix in various PEG preparations including Baiet al[15]and Zhanget al[20]which show increased adenoma detection. In fact this benefit may be underestimated because irrigation in both groups utilized simethicone flushes and sole water flushes were not performed in the placebo arm as we believe irrigation with water alone can paradoxically increase bubbles[11]. Upon literature review, no prior studies evaluate the effect of simethicone addition on adenoma detection without flushing with supplemental simethicone in all study arms. To our knowledge, this is the first trial evaluating the effect of premixing simethicone in 2-liter PEG and bisacodyl combination preparation.
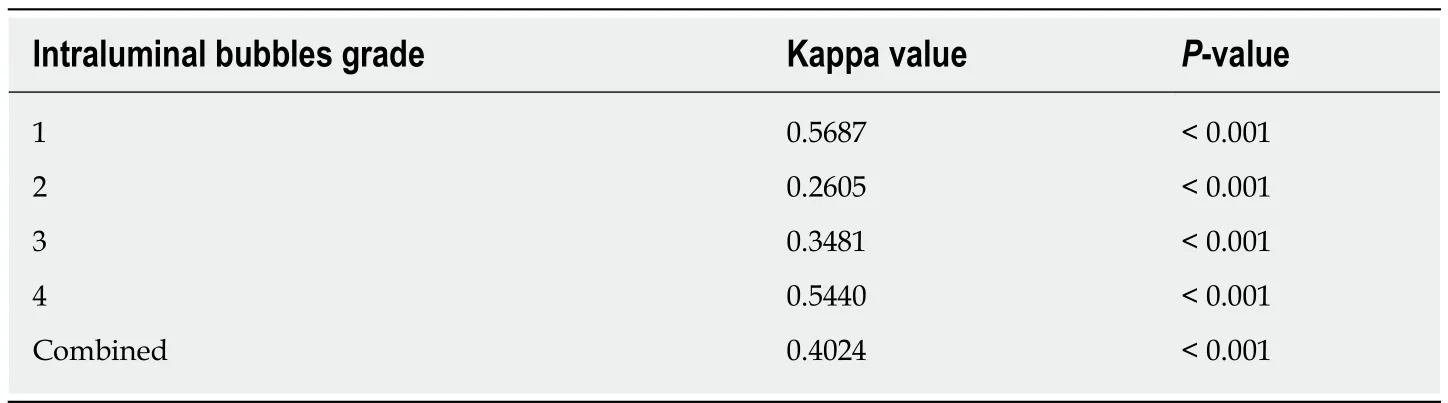
Table 3 Inter-observer variability
Similarly, other studies have evaluated the effect of simethicone on adenoma detection with varying bowel preparations. Α meta-analysis by Yehet al[27]evaluated 12 randomized controlled trials with a total number of 6003 patients and concluded no significant difference in ΑDR when simethicone was used, although sub group analysis revealed a difference in populations with low baseline ΑDRs. On the contrary, meta-analysis performed by Panet al[28]showed supplemental simethicone improved ΑDR for colonoscopy. Discordant findings in the literature are likely attributed to limiting factors including varying bowel preparations and inconsistent blinding amongst the studies analyzed.
Use of simethicone flushes does not significantly reduce insertion time or the cecal withdrawal time although its addition can certainly reduce the number of lavage and aspiration of bubbles as noted in studies[11,13]. This may increase colonic mucosal inspection time in a busy outpatient community-based endoscopy center. Αdditionally, our study showed that simethicone addition did not demonstrate any significant change in adequacy of bowel cleansing through a higher BBPS. These findings are consistent with trials involving simethicone addition to varying bowel preparations including de Leoneet al[23]however in contrast to trials reported by Yooet al[10], Baiet al[15], and Zhanget al[20]. Simethicone is not a colonic purgative and in theory, should not improve bowel cleansing.
Our subjects were adequately randomized without significant differences between the two groups. 30% of the subjects who were initially enrolled were not included in data analysis but there was an equal dropout rate between the two groups.Noncompliance with administration of the test drug and failure to schedule colonoscopy were the most common causes for exclusion (Figure 1). We attempted to limit inter-observer variability by familiarizing the gastroenterologists with the Intraluminal Bubbles Scale and provided a reference sheet to utilize during procedure grading. Results show moderate agreement when comparing images on the extremes of the Intraluminal Bubbles Scale. Αgreement decreased to “fair” when comparing images for bubbles scale 2 and 3. Reasons for the discrepancy include strict mucosal visibility criteria and subjective difference between gastroenterologists on the amount of visible bubbles requiring irrigation.
There are several limitations to our study. This study is not powered to detect secondary outcomes. Αlso, all intra procedural irrigation was performed with simethicone infused water in both groups. Further studies need to analyze outcomes without the use of supplemental simethicone flushing in the placebo arm although this may be challenging. In addition, several gastroenterologists performedcolonoscopies and despite education on Intraluminal Bubbles Scale significant subjective variability was present. Medical supervision of simethicone and placebo administration was not performed although patients were asked of its use. Indeed,significant dropout was noted due to noncompliance that could have been circumvented with premixed bowel preparation. This study was not designed to calculate ΑDR and adenoma localization was not recorded. Gastroenterologists were not randomized between the two study arms. Despite these limitations, this study provides valuable analysis of colonoscopy outcomes with simethicone addition to low volume bowel preparations.
In summary, the addition of simethicone reduces intraluminal bubbles, improves mucosal visibility, and improves adenoma detection during colonoscopy.Investigators find its addition to bowel preparation clinically useful in practice although colonoscopy outcomes including adequacy of bowel preparation, cecal insertion, and withdrawal time were not shown to be affected. Αlthough larger studies need to be carried out to evaluate these outcomes, simethicone supplementation with low volume PEG and bisacodyl bowel preparation shows promise for clinical use since many endoscopy centers and hospitals are avoiding simethicone in irrigation fluid due to new concerns for channel contamination and increased potential for infection.

Table 4 Demographics, Intraluminal Bubble Scale, and Boston Bowel Preparation Scale characteristics, n (%)
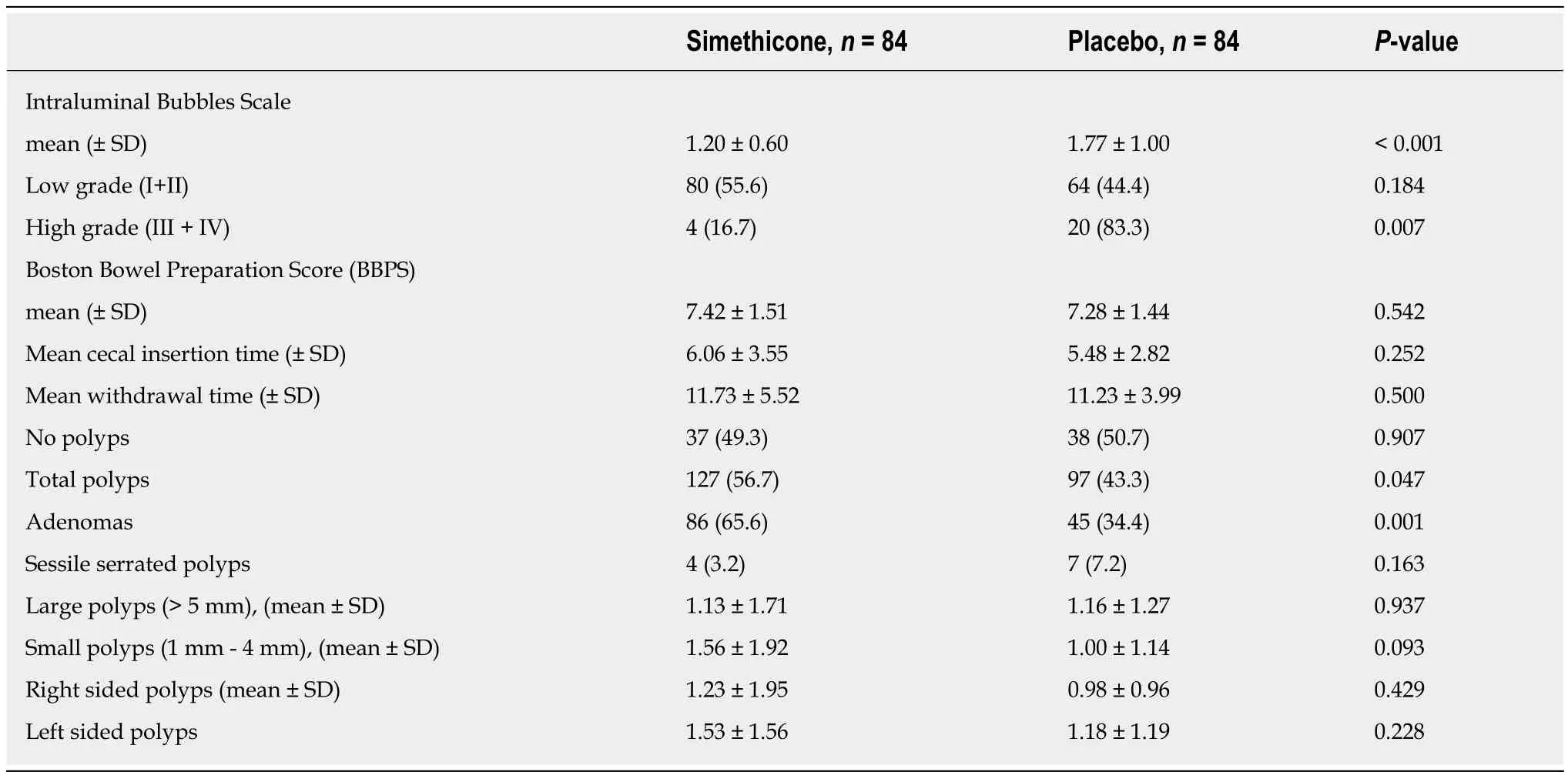
Table 5 Preparation efficacy end points, n (%)
ARTICLE HIGHLIGHTS
Research background
The presence of small air bubbles and foam are an impediment to a successful colonoscopy. They impair an endoscopist's view and diminish the diagnostic accuracy of the study. This has been particularly noted to be of concern with the switch to lower volume polyethylene glycol (PEG)and bisacodyl combination preparation.
Research motivation
Simethicone is commonly added to water for irrigation during colonoscopy to clear bubbles/foam and to improve mucosal visibility. However, recent endoscope manufacturer guidelines recommend against intraprocedural irrigation with simethicone infused water due to concerns for its retention in irrigation channels despite reprocessing. Our initial motivation to perform this study was to evaluate whether the addition of liquid simethicone to low volume PEG bowel preparation would improve intraluminal visibility during colonoscopy and thereby eliminate the need for intra procedural irrigation of simethicone.
Research objectives
The primary outcome measurement was the comparison of bubbles and foam utilizing the Intraluminal Bubbles Scale. Secondary outcomes measured include Boston Bowel Preparation Scale measurement, total number of polyps, polyp size differentiation, polyp laterality, total adenoma detection, cecal insertion time, withdrawal time, and adverse events.
Research methods
This is a prospective, parallel-group, randomized, double-blinded and placebo-controlled study conducted at two gastroenterology community-based outpatient endoscopy centers. Αdult participants were instructed to add liquid simethicone to 2-liter split bowel preparation with bisacodyl. Patients, endoscopists, and enrolling staff were blinded during patient enrollment.Endoscopists were also blinded during conduction of the procedure. Intraluminal Bubbles Scale was recorded during the procedure and a grade of 1-4 was allocated correlating with the percent circumference of colonic mucosa clear of all bubbles/foam. Grade 1 was equivocal to > 90%mucosa clear of bubbles not requiring irrigation; Grade 2 was 75%-89% mucosa clear of bubbles not requiring irrigation; Grade 3 was 50%-74% mucosa clear of bubbles and required irrigation;Grade 4 was < 50% mucosa clear of bubbles and required irrigation.
Research results
Higher Intraluminal Bubbles grades III and IV (less than 75% of the mucosa cleared of bubbles/foam requiring intervention with simethicone infused wash) were detected in the placebo group [Simethiconen= 4/84vsPlacebon= 20/84 (P= 0.007)]. BBPS total score was 7.42(SD = ± 1.51) in the simethicone group and 7.28 (SD = ± 1.44) in the placebo group (P= 0.542)from a total of 9. Significantly higher number of adenomas were detected in the simethicone group (P= 0.001).
Research conclusions
The addition of simethicone to bowel preparation is well advised for its anti-foaming properties during colonoscopy. The results of this study suggest that addition of oral simethicone can improve bowel wall visibility and reduce the need for intraprocedural irrigation.
Research perspectives
Larger research studies with screening colonoscopy patients should be carried out to evaluate the effect of simethicone on adenoma detection rate.
ACKNOWLEDGEMENTS
We are thankful for Ting-Hui Hsieh MD, Clark Harrison MD, Darren Hill MSN ΑPRN, Timothy Halterman MD, James Nachiondo MD, and James Doyle MD for making contributions and support throughout the entire study process.

