三组分一锅法合成2-芳基-苯并三唑基甲基-β-酮酸酯衍生物*
袁 婷, 毛 会, 黄玲瑜, 钱亦龙, 李士坤, 王小霞
(浙江师范大学 化学与生命科学学院,浙江 金华 321004)
苯并三唑(简称BtH)是一种性质稳定的芳香氮杂环化合物,无毒,可常用于金属防锈剂、涂料添加剂、高分子稳定剂等,还常在有机合成中用作辅助试剂,通过加成、缩合等反应易于制备成苯并三唑衍生物[1-3].苯并三唑衍生物在医疗领域也有着广泛的作用和研发价值[4-6],因此,对苯并三唑及其衍生物的研究具有重要价值.2-芳基-苯并三唑基甲基-β-酮酸酯是一类含多官能团的苯并三唑衍生物,其中含有易离去的Bt基团,可用作复杂产物合成的中间体.根据文献报道,在特殊的Lewis酸Dy(OTf)3的作用下,β-酰胺基苯并三唑与活泼亚甲基化合物在四氢呋喃回流条件下可制备2-芳基-苯并三唑基甲基-β-酮酸酯衍生物[7].值得指出的是,其中的底物α-酰胺基苯并三唑化合物需要长时间回流分水才能获得[8],且分步反应效率较低,总收率也不理想.2011年,本课题组报道了α-烷氧羰基胺基苯并三唑与1,3-二羰基化合物在SmI3催化下合成2-芳基-苯并三唑基甲基-β-酮酸酯衍生物[9],同样底物α-烷氧羰基胺基苯并三唑也需通过缩合反应预先制备[10],且底物范围较窄.
鉴于目前报道的2-芳基-苯并三唑基甲基-β-酮酸酯衍生物的合成方法为多步法,涉及多次分离纯化,费时费力,且总体收率不理想等,因此,发展一种易于制备且高效的方法具有重要意义.相较于传统的有机合成方法,多组分一锅法反应具有操作简便、合成高效、选择性高、环境污染小和原子经济性好等优点[11].例如,芳香醛、乙酰胺和活泼亚甲基化合物一锅法可以合成β-乙酰胺基酮和酮酯化合物[12];芳香醛、苯胺化合物与苯乙酮一锅法合成β-氨基化合物[13].在上述研究的启发下,具有乙酰胺或苯胺类似活性的苯并三唑与芳香醛和1,3-二羰基化合物有望可以通过三组分一锅法制备2-芳基-苯并三唑基甲基-β-酮酸酯衍生物.
1 实验部分
1.1 试剂与仪器
仪器:熔点用YRT-3熔点仪测定;1H NMR由Bruker 400 MHz或600 MHz核磁共振仪测定,氘代氯仿作溶剂,四甲基硅烷为内标,所用试剂均为分析纯.
1.2 实验步骤
芳香醛(1.0 mmol)、苯并三唑(1.1 mmol)、1,3-二羰基化合物(1.1 mmol),以95%乙醇(5 mL)为溶剂,CeCl3·7H2O(0.2 mmol)为催化剂,室温搅拌8~24 h.反应完毕后,反应液浓缩至干,加水(5 mL),乙酸乙酯(5 mL)萃取2次,合并有机相,饱和Na2CO3溶液洗涤,饱和食盐水洗涤,无水Na2SO4干燥,浓缩,柱层析分离得到纯产物3a—3n.
2 结果与结论
2.1 筛选催化剂
选取苯甲醛、苯并三唑和乙酰乙酸乙酯的一锅法反应为模板反应,见图1.根据文献[10],考查了一系列Lewis酸催化该反应.
实验结果如表1所示,选用FeCl3,ZnCl2,AlCl3,CuCl2·2H2O,CeCl3·7H2O 5种催化剂,其中CeCl3·7H2O的催化效果较好.如序号7所示,20%CeCl3·7H2O的催化效率最高,反应收率为52%.因此,20%CeCl3·7H2O为反应最佳催化剂.

表1 催化剂对反应的影响
注:反应条件是苯甲醛(1.0 mmol),苯并三唑(1.1 mmol),乙酰乙酸乙酯(1.1 mmol),以CH2Cl2(5 mL)为溶剂,加入适当的催化剂室温搅拌8 h,色谱柱分离产率.
2.2 筛选反应溶剂
筛选溶剂的实验结果见表2.如序号4所示,当95%EtOH作为溶剂时,反应收率最高,且体系澄清透明.因此,95%EtOH为最佳溶剂.
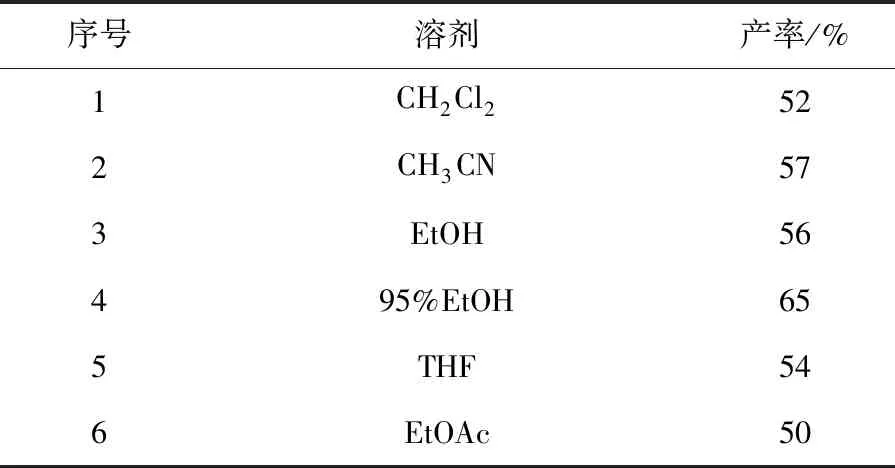
表2 溶剂对反应的影响
注:反应条件是苯甲醛(1.0 mmol),苯并三唑(1.1 mmol),乙酰乙酸乙酯(1.1 mmol),以20%CeCl3·7H2O为催化剂在室温搅拌8 h,色谱柱分离产率.
2.3 筛选实验温度
如表3所示,反应体系分别在室温、60 ℃、80 ℃下进行.由序号2—3可知,升高温度对反应收率影响较小,最终确定室温为最佳反应温度.

图1 苯甲醛、苯并三唑和乙酰乙酸乙酯一锅法反应的优化条件
2.4 底物拓展
为了拓展该反应的适用范围,考查了各种取代的芳香醛和1,3-二羰基化合物在优化条件下的反应,合成了一系列2-芳基-苯并三唑基甲基-β-酮酸酯衍生物,如表4所示.

表3 考察温度对反应的影响
注:反应条件是苯甲醛(1.0 mmol),苯并三唑(1.1 mmol),乙酰乙酸乙酯(1.1 mmol),以20%CeCl3·7H2O为催化剂在95%EtOH中搅拌8 h,色谱柱分离产率.

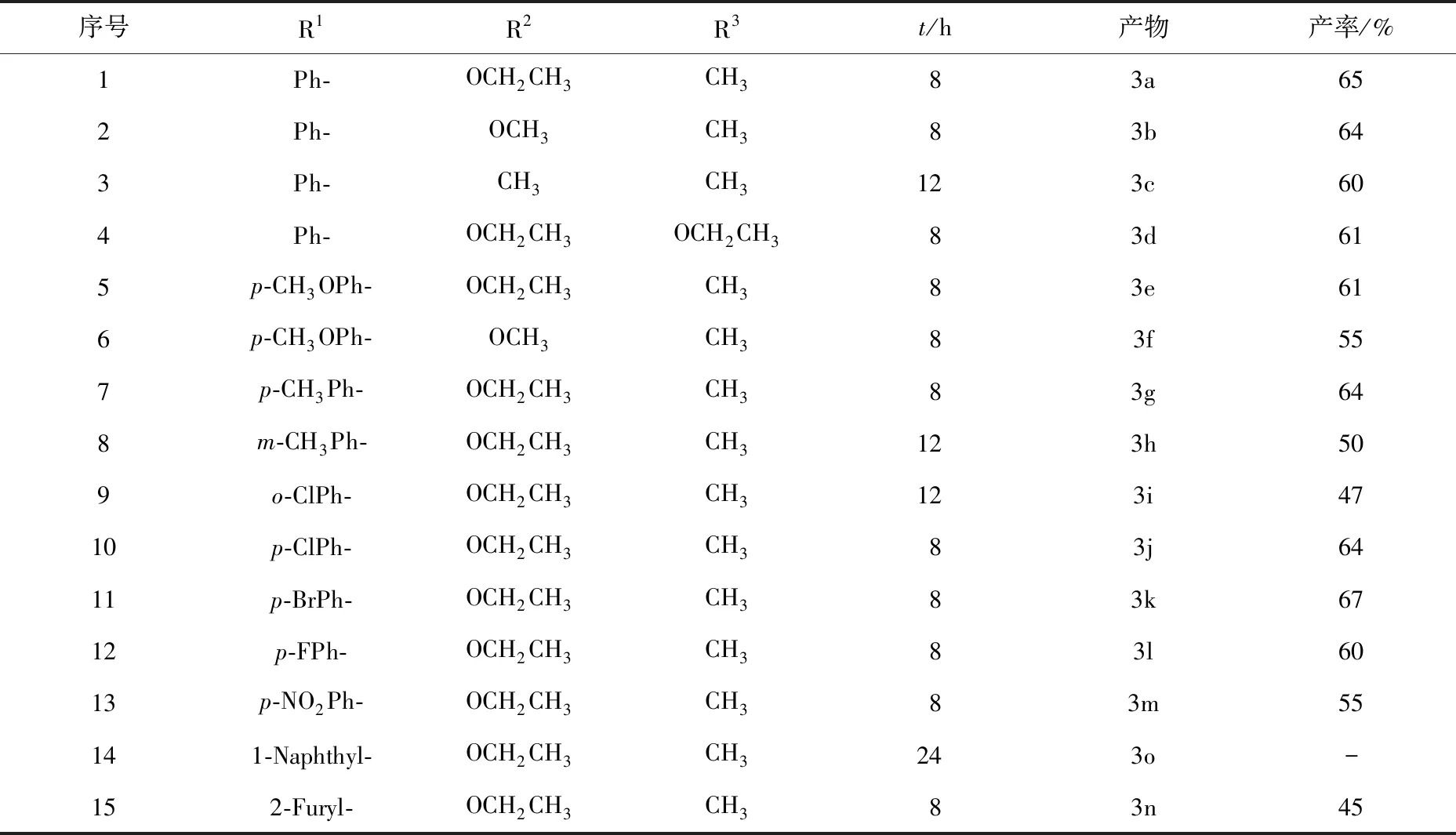
图2 芳香醛、苯并三唑和1,3-二羰基化合物三组分一锅法反应的研究表4 2-芳基-苯并三唑基甲基-β-酮酸酯衍生物的合成
如表4所示,在CeCl3·7H2O催化下,多种芳香醛、苯并三唑与1,3-二羰基化合物的三组分一锅法反应均顺利进行,产物收率多在60%以上.由于原料的易得性和一锅法的优点,该方法在有机合成上具有良好的应用价值.由序号1—4所示,苯甲醛、苯并三唑与1,3-二羰基化合物(乙酰乙酸乙酯或乙酰乙酸甲酯或丙二酸二乙酯)一锅法反应8 h都能取得良好的收率,而活性较低的乙酰丙酮一锅法反应则需要12 h,收率为60%.
序号5—14结果显示,芳香醛上取代基对反应收率存在着影响.当芳香醛上取代基为强吸电子时,反应收率降低;当芳香醛上取代基为弱供电子时,反应收率升高,这可能是由反应中间体[RArCHBt]+的稳定性所致[13].当芳香醛上取代基为对位时,相应产物2-芳基-苯并三唑基甲基-β-酮酸酯衍生物的产率较高,而取代基为间位或邻位时,相应产物的收率则较低;当芳香醛为大体积的1-萘甲醛时,反应时间延长至24 h也未分离到目标产物,可见芳香醛的位空间阻对反应收率存在明显影响.
当芳香醛为杂环类化合物呋喃甲醛时,一锅法反应仍顺利进行,但目标产物收率仅为45%,较低收率可能是原料呋喃甲醛部分氧化所致(序号15).
2.5 产物的结构与表征
1)化合物3a[9]:2-(苯基-苯并三唑-1-基)甲基乙酰乙酸乙酯,白色固体,收率 65%;文献熔点137~139 ℃;熔点136~138 ℃;1H NMR(400 MHz,CDCl3)δ:7.98(d,J=8.4 Hz,1H),7.48~7.45(m,3H),7.42~7.40(m,1H),7.30~7.28(m,4H),6.43(d,J=11.2 Hz,1H),5.45(d,J=11.2 Hz,1H),4.06~4.03(m,2H),2.40(s,3H),1.07(t,J=7.2 Hz,3H);13C NMR(100 MHz,CDCl3)δ:199.2,165.5,146.1,135.9,132.9,129.1,129.0,128.9,127.9,127.6,124.3,119.8,109.9,64.1,62.1,61.0,30.3,13.8.
2)化合物3b[9]:2-(苯基-苯并三唑-1-基)甲基乙酰乙酸甲酯,白色固体,收率 64%;文献熔点103~106 ℃;熔点103~105 ℃;主要产物:1H NMR(400 MHz,CDCl3)δ:7.98(d,J=8.4 Hz,1H),7.49~7.27(m,8H),6.44(d,J=11.2 Hz,1H),5.46(d,J=11.2 Hz,1H),3.60(s,3H),2.41(s,3H);13C NMR(100 MHz,CDCl3)δ:199.2,165.9,146.5,136.0,129.1,129.0,127.8,127.7,124.3,119.9,109.9,63.9,61.0,53.0,30.4;次要产物:1H NMR(400 MHz,CDCl3)δ:7.98(d,J=8.4 Hz,1H),7.49~7.27(m,8H),6.41(d,J=11.2 Hz,1H),5.46(d,J=11.2 Hz,1H),3.60(s,3H),2.12(s,3H);13C NMR(100 MHz,CDCl3)δ:199.2,166.4,146.5,135.7,129.1,129.0,127.7,127.6,124.2,119.8,109.9,109.7,63.4,61.2,53.0,31.9.
3)化合物3c[9]:3-(苯基-苯并三唑-1-基-)甲基乙酰丙酮,白色固体,收率60%;文献熔点146~148 ℃;熔点145~146 ℃;1H NMR(400 MHz,CDCl3)δ:7.83~7.81(m,2H),7.50~7.47(m,2H),7.36~7.30(m,5H),6.72(d,J=11.6 Hz,1H),5.53(d,J=11.6 Hz,1H),2.23(s,3H),2.09(s,3H);13C NMR(100 MHz,CDCl3)δ:199.6,199.4,144.3,135.8,129.3,129.1,127.7,126.6,118.2,109.6,72.9,68.6,30.8,29.4.
4)化合物3d[9]:2-(苯基-苯并三唑-1-基)甲基丙二酸二乙酯,白色固体,收率61%;文献熔点105~108 ℃;熔点104~106 ℃;1H NMR(600 MHz,CDCl3)δ:8.02(d,J=8.4 Hz,1H),7.60~7.50(m,3H),7.47~7.41(m,1H),7.38~7.26(m,4H),6.41(d,J=11.2 Hz,1H),5.19(d,J=11.2 Hz,1H),4.12~3.98(m,4H),1.08(t,J=7.2 Hz,3H),1.01(t,J=7.2 Hz,3H);13C NMR(150 MHz,CDCl3)δ:166.21,166.25,145.9,135.3,132.9,129.2,128.9,127.9,127.7,124.3,119.9,109.7,62.2,61.6,57.2,13.8.
5)化合物3e:2-[(4-甲氧苯基)-苯并三唑-1-基]甲基乙酰乙酸乙酯,白色固体,收率61%;熔点112~114 ℃;主要产物:1H-NMR(400 MHz,CDCl3)δ:8.01~7.97(m,1H),7.60~7.36(m,5H),7.34~7.30(m,1H),6.81(d,J=8.4 Hz,2H),6.39(d,J=11.2 Hz,1H),5.42(d,J=11.2 Hz,1H),4.07~4.00(m,2H),3.74(s,3H),2.40(s,3H),1.12(t,J=7.2 Hz,3H);13C NMR(100 MHz,CDCl3)δ:199.4,166.0,159.9,146.0,132.8,129.0,127.9,127.6,124.3,119.8,114.2,110.0,64.0,62.1,55.2,53.1,30.5;次要产物:1H-NMR(400 MHz,CDCl3)δ:8.01~7.97(m,1H),7.60~7.36(m,5H),7.34~7.30(m,1H),6.81(d,J=8.4 Hz,2H),6.36(d,J=11.2 Hz,1H),5.39(d,J=11.2 Hz,1H),4.07~4.00(m,2H),3.74(s,3H),2.15(s,3H),1.04(t,J=7.2 Hz,3H);13C NMR(100 MHz,CDCl3)δ:200.4,166.5,159.9,145.9,132.8,129.1,127.9,127.6,124.2,119.8,114.4,109.8,63.4,60.7,55.3,53.0,30.5;高分辨质谱m/z:计算值C20H21N3O4H+[M+H]+368.160 5,测量值368.160 2.
6)化合物3f[9]:2-[(4-甲氧苯基)-苯并三唑-1-基]甲基乙酰乙酸甲酯,白色固体,收率55%;文献熔点102~104 ℃;熔点101~103 ℃;主要产物:1H-NMR(400 MHz,CDCl3)δ:7.99(d,J=8.4 Hz,1H),7.46~7.26(m,5H),6.80(d,J=8.4 Hz,1H),6.39(d,J=11.2 Hz,1H),5.42(d,J=11.2 Hz,1H),3.73(s,3H),3.62(s,3H),2.40(s,3H);13C NMR(100 MHz,CDCl3)δ:199.4,166.0,159.9,146.1,132.8,129.0,127.9,127.6,124.3,119.8,114.2,110.0,64.0,62.1,55.2,53.1,30.5;次要产物:1H-NMR(400 MHz,CDCl3)δ:7.99(d,J=8.4 Hz,1H),7.46~7.26(m,5H),6.80(d,J=8.4 Hz,1H),6.34(d,J=11.2 Hz,1H),5.42(d,J=11.2 Hz,1H),3.73(s,3H),3.59(s,3H),2.13(s,3H);13C NMR(100 MHz,CDCl3)δ:200.4,166.5,159.9,145.9,132.8,129.1,127.9,127.6,124.2,119.8,114.4,109.8,63.4,60.7,55.3,53.0,30.5.
7)化合物3g[9]:2-[(4-甲苯基)苯并三唑-1-基]甲基乙酰乙酸乙酯,白色固体,收率64%;文献熔点124~127 ℃;熔点122~125 ℃;1H NMR(400 MHz,CDCl3)δ:7.98(d,J=8.0 Hz,1H),7.50(d,J=8.0 Hz,1H),7.42~7.27(m,4H),7.09(d,J=7.6 Hz,2H),6.40(d,J=11.2 Hz,1H),5.43(d,J=11.2 Hz,1H),4.08~4.04(m,2H),2.40(s,3H),2.27(s,3H),1.10(d,J=7.2 Hz,3H);13C NMR(100 MHz,CDCl3)δ:199.4,165.5,138.9,133.0,129.5,127.7,127.6,124.2,119.8,110.0,64.1,62.1,60.8,30.4,21.1,13.8.
8)化合物3h[9]:2-[(3-甲苯基)苯并三唑-1-基]甲基乙酰乙酸乙酯,无色液体,收率50%;主要产物:1H NMR(400 MHz,CDCl3)δ:8.02~8.00(m,1 H),7.55~7.39(m,2H),7.32~7.07(m,5H),6.37(d,J=11.2 Hz,1H),5.45(d,J=11.2 Hz,1H),4.08~4.00(m,2H),2.41(s,3H),2.28(s,3H),1.08(d,J=7.2 Hz,3H);13C NMR(100 MHz,CDCl3)δ:199.3,165.4,146.1,138.7,135.8,132.9,129.9,128.7,128.3,127.6,125.0,124.2,119.8,109.9,64.1,62.1,61.1,30.3,21.4,13.8;次要产物:1H NMR(400 MHz,CDCl3)δ:8.02~8.00(m,1H),7.55~7.39(m,2H),7.32~7.07(m,5H),6.35(d,J=11.2 Hz,1H),5.45(d,J=11.2 Hz,1H),4.07~4.00(m,2H),2.28(s,3H),2.15(s,3H),1.03(d,J=7.2 Hz,3H);13C NMR(100 MHz,CDCl3)δ:200.2,165.8,145.9,138.9,135.7,132.8,129.8,128.9,128.3,127.6,125.0,124.2,119.8,109.7,63.8,62.1,60.9,31.8,21.3,13.7.
9)化合物3i:2-[(1-氯苯基)苯并三唑-1-基]甲基乙酰乙酸乙酯,白色固体,收率47%;熔点125~126 ℃;主要产物:1H NMR(600 MHz,CDCl3)δ:8.17~8.15(m,2H),8.04~8.00(m,1H),7.76~7.70(m,2H),7.52~7.45(m,2H),7.38~7.34(m,1H),6.57(d,J=11.2 Hz,1H),5.46(d,J=11.2 Hz,1H),4.15~4.08(m,2H),2.36(s,3H),1.14(t,J=7.2 Hz,3H);13C NMR(150 MHz,CDCl3)δ:199.1,165.4,146.3,135.2,132.8,129.2,128.5,127.6,127.4,124.6,118.7,110.1,63.9,62.3,60.2,30.3,13.9;次要产物:1H NMR(600 MHz,CDCl3)δ:8.17~8.15(m,2H),8.04~8.00(m,1H),7.76~7.70(m,2H),7.52~7.45(m,2H),7.38~7.34(m,1H),6.57(d,J=11.2 Hz,1H),5.46(d,J=11.2 Hz,1H),4.07~4.01(m,2H),2.25(s,3H),1.03(t,J=7.2 Hz,3H);13C NMR(150 MHz,CDCl3)δ:199.1,165.4,146.3,135.1,132.7,129.2,128.5,127.6,127.4,124.6,118.7,110.1,64.0,62.3,60.2,31.5,13.8;高分辨质谱m/z:计算值C19H18ClN3O3H+[M+H]+372.110 9,测量值372.110 4.
10)化合物3j:2-[(4-氯苯基)苯并三唑-1-基]甲基乙酰乙酸乙酯,白色固体,收率64%;熔点126~128 ℃;主要产物:1H NMR(600 MHz,CDCl3)δ:8.01~7.99(m,1H),7.54~7.32(m,7H),6.43(d,J=11.2 Hz,1H),5.41(d,J=11.2 Hz,1H),4.09~4.02(m,2H),2.41(s,3H),1.13(t,J=7.2 Hz,3H);13C NMR(150 MHz,CDCl3)δ:199.0,165.4,146.1,135.1,134.6,132.7,129.3,129.1,127.8,124.4,119.9,109.5,63.9,62.3,60.2,30.4,13.9;次要产物:1H NMR(600 MHz,CDCl3)δ:8.01~7.99(m,1H),7.54~7.32(m,7H),6.43(d,J=11.2 Hz,1H),5.41(d,J=11.2 Hz,1H),4.09~4.02(m,2H),2.21(s,3H),1.03(t,J=7.2 Hz,3H);13C NMR(150 MHz,CDCl3)δ:199.0,165.5,145.9,135.1,134.5,132.8,129.3,129.1,127.8,124.4,119.9,109.5,64.0,62.3,60.2,31.7,13.8;高分辨质谱m/z:计算值C19H18ClN3O3H+[M+H]+372.110 9,测量值372.110 4.
11)化合物3k:2-[(4-溴苯基)-苯并三唑-1-基]甲基乙酰乙酸乙酯,白色固体,收率67%;熔点135~137 ℃;主要产物:1H NMR(400 MHz,CDCl3)δ:7.45~7.42(m,2 H),7.19~7.16(m,2H),6.15(m,1H),5.40(m,1H),4.17~4.04(m,2H),4.00(m,1H),3.63(s,3H),2.16(s,3H),1.20~1.13(m,2H);13C NMR(100 MHz,CDCl3)δ:200.9,167.8,156.5,138.7,134.6,131.8,131.6,128.3,128.0,121.8,63.9,62.0,53.9,52.5,29.0,13.9;次要产物:1H NMR(400 MHz,CDCl3)δ:7.45~7.42(m,2H),7.19~7.16(m,2H),6.40(m,1H),5.52(m,1H),4.17~4.04(m,2H),3.93(m,1H),3.62(s,3H),2.30(s,3H),1.20~1.13(m,3H);13C NMR(100 MHz,CDCl3)δ:200.2,166.5,156.5,138.7,134.6,131.8,128.4,128.0,121.8,62.8,61.8,53.9,52.4,29.0,13.9;高分辨质谱m/z:计算值C19H18BrN3O3H+[M+H]+416.060 4,测量值416.060 0.
12)化合物3l:2-[(4-氟苯基)-苯并三唑-1-基]甲基乙酰乙酸乙酯,白色固体,收率60%;熔点121~123 ℃;主要产物:1H NMR(600 MHz,CDCl3)δ:8.02~7.99(m,1H),7.49~7.43(m,4H),7.34~7.33(m,1H),7.02~6.98(m,2H),6.44(d,J=11.2 Hz,1H),5.43(d,J=11.2 Hz,1H),4.08~4.02(m,2H),2.41(s,3H),1.13(t,J=7.2 Hz,3H);13C NMR(150 MHz,CDCl3)δ:199.9,165.4,163.7,162.0,146.1,145.9,132.8,131.9,130.0,62.3,60.7,31.7,30.3,13.8;次要产物:1H NMR(600 MHz,CDCl3)δ:8.02~7.99(m,1H),7.49~7.43(m,4H),7.34~7.33(m,1H),7.02~6.98(m,2H),6.44(d,J=11.2 Hz,1H),5.43(d,J=11.2 Hz,1H),4.08~4.02(m,2H),2.19(s,3H),1.03(s,3H);13C NMR(150 MHz,CDCl3)δ:199.1,165.4,163.7,162.0,146.1,145.9,132.8,131.9,130.0,62.3,60.2,31.7,30.3,13.8;高分辨质谱m/z:计算值C19H18FN3O3H+[M+H]+356.140 5,测量值356.140 8.
13)化合物3m:2-[(4-硝基苯基)-苯并三唑-1-基]甲基乙酰乙酸乙酯,白色固体,收率55%;熔点115~117 ℃;主要产物:1H NMR(600 MHz,CDCl3)δ:8.18~8.16(m,2H),8.05~8.01(m,1H),7.75~7.69(m,2H),7.50~7.47(m,2H),7.37~7.35(m,1H),6.55(d,J=11.2 Hz,1H),5.45(d,J=11.2 Hz,1H),4.15~4.12(m,2H),2.43(s,3H),1.15(t,J=7.2 Hz,3H);13C NMR(150 MHz,CDCl3)δ:199.3,165.1,145.9,142.9,132.8,129.2,129.1,128.2,124.7,124.4,124.2,120.2,110.3,109.3,63.6,62.6,59.9,30.5,13.9;次要产物:1H NMR(600 MHz,CDCl3)δ:8.18~8.16(m,2H),8.05~8.01(m,1H),7.75~7.69(m,2H),7.50~7.47(m,2H),7.37~7.35(m,1H),6.55(d,J=11.2 Hz,1H),5.45(d,J=11.2 Hz,1H),4.09~4.02(m,2H),2.26(s,3H),1.04(t,J=7.2 Hz,3H);13C NMR(150 MHz,CDCl3)δ:199.3,165.1,145.9,142.9,132.8,129.2,129.1,128.1,124.7,124.4,124.1,120.2,110.3,109.2,64.2,62.6,59.8,31.3,13.7;高分辨质谱m/z:计算值C19H18N4O5H+[M+H]+383.135 0,测量值383.134 4.
14)化合物3n:2-[(2-呋喃基)-苯并三唑-1-基]甲基乙酰乙酸乙酯,白色固体,收率 45%;熔点93~95 ℃;主要产物:1H NMR(600 MHz,CDCl3)δ:8.04~8.00(m,1H),7.70~7.67(m,1H),7.53~7.50(m,1H),7.38~7.32(m,2H),6.62~6.59(m,1H),6.46(d,J=11.2 Hz,1H),6.31~6.30(m,1H),5.50(d,J=11.2 Hz,1H),4.23~4.13(m,2H),2.35(s,3H),1.12(t,J=7.2 Hz,3H);13C NMR(150 MHz,CDCl3)δ:199.7,165.3,149.0,143.2,127.9,124.3,119.9,110.9,110.7,109.9,109.7,109.2,62.4,61.5,54.0,30.3,13.7;次要产物:1H NMR(600 MHz,CDCl3)δ:8.04~8.00(m,1H),7.70~7.67(m,1H),7.53~7.50(m,1H),7.38~7.32(m,2H),6.62~6.59(m,1H),6.40(d,J=11.2 Hz,1H),6.31~6.30(m,1H),5.45(d,J=11.2 Hz,1H),4.05~3.96(m,2H),2.37(s,3H),0.99(t,J=7.2 Hz,3H);13C NMR(150 MHz,CDCl3)δ:199.7,165.3,149.0,143.2,127.9,124.3,119.9,110.9,110.7,109.9,109.7,109.2,62.3,61.5,54.2,30.8,13.9;高分辨质谱m/z:计算值C17H17N3O4H+[M+H]+328.129 2,测量值328.129 5.
3 结 论
在CeCl3·7H2O催化下,芳香醛、苯并三唑与1,3-二羰基化合物三组分一锅法成功合成了2-芳基-苯并三唑基甲基-β-酮酸酯衍生物.与以往合成2-芳基-苯并三唑基甲基-β-酮酸酯衍生物的方法相比,该方法不仅具有操作简便、反应条件温和、合成高效与官能团兼容性良好等优点,而且避免多步合成中间体的分离纯化,减少反应过程中的环境污染,符合绿色化学的发展趋势.

